ABSTRACT
We present molecular data demonstrating that the New Zealand endemic Plagiochila caducifolia represents an isolated population of Plagiochila (sect. Arrectae) spinulosa, a species previously thought confined to the eastern Holarctic but which also occurs in Lesotho, southern Africa. In New Zealand, the wide distribution of P. spinulosa in undisturbed and relatively isolated habitats throughout the South Island is consistent with an indigenous, rather than adventive, species. Plagiochila spinulosa is the first reported bipolar disjunct species within the Plagiochilaceae. Although other bipolar disjunctions are known in vascular and non-vascular plants, they usually involve species with wider circumboreal or circumarctic distributions, presumably indicating an origin in the Northern Hemisphere. Most species of Plagiochila sect. Arrectae reproduce asexually by caducous leaves and have disjunct distributions; the significance of this correlation and biogeographic patterns in this lineage both require testing against additional data.
Introduction
Phylogenetic relationships among Southern Hemisphere plants are dominated by long-distance dispersal events (Muñoz et al. Citation2004; Sanmartín & Ronquist Citation2004; Viljoen et al. Citation2013). The resulting trans-oceanic disjunctions are a recurrent theme within the New Zealand flora, whose affinities with other southern landmasses show concordant patterns in frequency and direction of long-distance dispersal events in many different lineages (Sanmartín & Ronquist Citation2004). Trans-Tasman disjunctions in particular are a prominent feature of the New Zealand flora. Many genera, and even species, traverse this sea, and plant dispersal in both directions has been inferred (Perrie et al. Citation2010; Birch et al. Citation2012). Trans-Pacific disjunctions are fewer, but species in the genera Jovellana Ruiz and Pav. (Nylinder et al. Citation2012), Griselinea J.R.Forst. and G.Forst. (Allan Citation1961), Ourisia Comm. ex Juss. (Meudt & Simpson Citation2006), Myosotidium Hook. (Weigend et al. Citation2013; Holstein et al. Citation2016) and Phyllothallia E.A.Hodgs. (Crandall-Stotler & Stotler Citation2012) have nearest relatives in South America, as have New Zealand representatives of the family Gesneriaceae Rich. and Juss. (Woo et al. Citation2011; Perret et al. Citation2013). Several species distributions span the Southern Pacific, including Blechnum blechnoides (Bory) Keyserl. (Chambers & Farrant Citation1996), Notogrammitis patagonica (C.Chr.) Parris, Veronica elliptica G.Forst. and V. salicifolia G.Forst. (Allan Citation1961), and species in the genus Menegazzia A.Massal. (Kantvilas Citation2012). Again, dispersal events in both directions have been inferred (Wagstaff et al. Citation2000, Citation2002; Meudt & Simpson Citation2006; Emadzade & Hörandl Citation2011; Sun et al. Citation2014).
New Zealand’s flora also has links with the Northern Hemisphere in the form of bipolar disjunctions—distributions encompassing some or all of the temperate zones in both Northern and Southern Hemispheres but not the intervening tropics. The ranges of some 56 circum-North Temperate genera extend across the tropics into southern temperate regions, including Empetrum L., Euphrasia L. and Geum L. (Thorne Citation1972). Many of these genera have dispersed from north to south, including Euphrasia (Gussarova et al. Citation2008), Myosotis L. (Winkworth et al. Citation2005; Meudt et al. Citation2015) and Ranunculus L. (Emadzade & Hörandl Citation2011). In a review of plant distributions in North and South America, Raven (Citation1963) reported 26 vascular plant species or species pairs having bipolar disjunctions, all but two of which had circumarctic or circumboreal distributions in the Northern Hemisphere. This distributional pattern is a theme common to bipolar disjunctions across land plants, including cryptogams, for example Hylocomium splendens (Hedw.) Schimp., Plagiomnium rugicum (Laurer) T.J.Kop. and Cinclidium stygium Sw. (Koponen Citation1972; Schofield Citation1974). The indigenous New Zealand liverwort flora includes nine bipolar disjunct species (). These occur alongside species belonging to many globally distributed lineages having high species diversity in New Zealand such as Chiloscyphus Corda, and Plagiochila (Dumort.) Dumort. In both of these genera most of the species that occur in New Zealand or Australasia are endemics. Others are shared with southern South America. Few species are actually shared with the Northern Hemisphere, and these often have cosmopolitan or discontinuous pantropical, rather than bipolar disjunct distributions, for example Chiloscyphus muricatus (Lehm.) J.J.Engel and R.M.Schust., Lejeunea flava (Sw.) Nees, Cephaloziella divaricata (Sm.) Schiffn. and Anthelia juratzkana (Limpr.) Trevis. (Engel & Glenny Citation2008).
Table 1. Indigenous New Zealand liverworts with bipolar disjunct distributions, and their island of occurrence within New Zealand.
Trans-oceanic bryophyte distributions are primarily the result of dispersal (Shaw et al. Citation2003; Vanderpoorten et al. Citation2008; Heinrichs et al. Citation2009; Sun et al. Citation2014; Scheben et al. Citation2016; Bechteler et al. Citation2017), and have been inferred within Plagiochila (Rycroft et al. Citation2001; Groth et al. Citation2003; Heinrichs et al. Citation2004, Citation2005a). Although some Neotropical Plagiochila species extend into northern temperate regions, for example Plagiochila stricta Lindenb. (Rycroft et al. Citation2002) and P. punctata (Taylor) Taylor (Davison et al. Citation2006), no bipolar disjunct species are currently known within this genus, or even within the family Plagiochilaceae. However, three accessions of the New Zealand endemic Plagiochila caducifolia Inoue and R.M.Schust. formed a well-supported clade within Plagiochila sect. Arrectae Carl sister to a single accession of the western European Plagiochila spinulosa (Dicks.) Dumort. in the phylogeny of Renner et al. (Citation2017), suggesting the existence of an unusual bipolar disjunct sister-species pair. We expanded the sampling of species within sect. Arrectae based on published sequences and obtained new sequences from an additional individual of P. spinulosa to test the relationship between P. caducifolia and P. spinulosa. Contrary to expectation, molecular and morphological evidence showed that P. caducifolia is conspecific with P. spinulosa, which also occurs in Lesotho, southern Africa. We conclude that P. spinulosa is native to New Zealand, and report the first bipolar disjunct distribution within the Plagiochilaceae Müll. Frib.
Materials and methods
Taxon sampling
A representative nrITS-cpDNA rps4-rbcL dataset for Plagiochila sect. Arrectae was downloaded from GenBank based on the phylogenetic hypotheses of Patzak et al. (Citation2016) and Renner et al. (Citation2017). Accessions of P. sect. Rutilantes Carl were chosen as outgroup based on the tree topology in both those studies. The dataset was completed with newly generated sequences of P. spinulosa, P. retrorsa Gottsche and P. sticticola Mont. and Gottsche. DNA extraction and sequencing followed Patzak et al. (Citation2016, ‘Munich protocol’). All sequences were aligned manually in BioEdit v7.0.5.2 (Hall Citation1999). Ambiguous positions were excluded and parts of sequences that were lacking were coded as missing. Specimen and GenBank data are provided in .
Table 2. DNA voucher specimen details and associated GenBank accession numbers for sequence data used in this study.
Phylogeny reconstruction
Substitution models were estimated for the combined dataset with jModeltest 2.1.7 (Guindon & Gascuel Citation2003; Darriba et al. Citation2012). The Akaike information criterion (AIC) as well as the AIC corrected for finite sample size (AICc) supported the GTR+Γ+I model. The Bayesian information criterion (BIC) supported the TIM1+Γ model. This model was not available in RAxML, hence the model selected with AIC and AICc was applied. Maximum likelihood analyses were conducted with RAxML 8.2.9 (Stamatakis Citation2014) on the CIPRES Science Gateway (Miller et al. Citation2010; https://www.phylo.org/) using the thorough-bootstrap-option, 1000 replicate runs, bootstrap replicates estimated with the autoMRE option, saving branch lengths and GTRGAMMA.
Bayesian analysis was performed by MrBayes 3.2 (Ronquist & Huelsenbeck Citation2003; Ronquist et al. Citation2012) with separate unlinked GTR+G+I models with six gamma categories for each partition. Two runs, each with four chains of 2 million generations length were sampled every 2000 generations. Runs were checked for convergence with Tracer 1.6 (Rambaut et al. Citation2014). The majority-rule tree with median node heights summarising samples from the posterior probability distribution was calculated after excluding trees from the first 200,000 generations from each run.
Results
The nrITS alignment contained 48 sequences, was 815 base pairs (bp) long and had 137 parsimony informative sites (16.8%); two accessions of Plagiochila caducifolia from New Zealand were missing nrITS. The rps4 alignment contained 20 sequences, was 700 bp long and had 190 parsimony informative sites (27.1%); the rbcL alignment contained 19 sequences, was 1297 bp long and had 89 parsimony informative sites (6.9%).
In the most likely phylogram, the three individuals of the New Zealand Plagiochila caducifolia formed a supported clade nested within a fully supported but largely polytomous lineage containing individuals of P. spinulosa from Madeira, western Europe and one individual from Lesotho in southern Africa (). The Lesotho individual was sister to the remainder, without support. Specimens from New Zealand, Lesotho and western Europe share morphological features diagnostic of P. spinulosa. Sister to P. spinulosa was a lineage corresponding to P. punctata, whose individuals from North America, Africa, Madagascar, the Neotropics and Europe exhibited greater phylogenetic divergence than among individuals of P. spinulosa. Sister to these two species was P. stricta, whose individuals exhibited geographically-correlated phylogenetic relationships.
Figure 1. Most likely phylogeny returned by RAxML. Numbers above branches are ML bootstrap values, those below are Bayesian posterior probabilities.
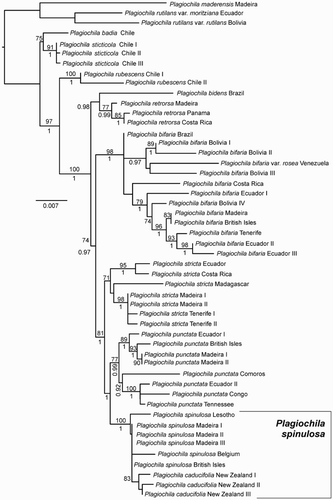
The topology of the majority-rule tree from Bayesian analysis was the same as the maximum likelihood tree from RAxML. Effective sample sizes for all parameters sampled by MrBayes were greater than 1300, and mean standard deviation of splits frequencies was less than 0.005.
Discussion
Plagiochila spinulosa is known from three geographic regions, with occurrences growing on literally opposite sides of the planet, and the third in southern Africa. This is the first recognised bipolar disjunct distribution within the Plagiochilaceae ().
Figure 2. Generalised global distribution of Plagiochila spinulosa. The black dot represents the single specimen of Plagiochila spinulosa from Lesotho.
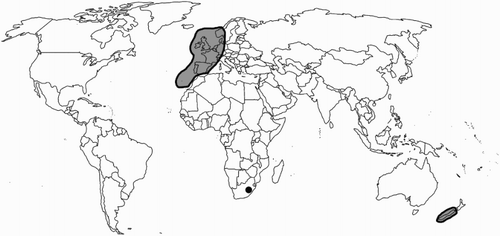
In contrast with most bipolar disjunct plant species, Plagiochila spinulosa has a restricted distribution within the Northern Hemisphere where it occurs in the Faroe Islands, Great Britain, Ireland, Norway, Belgium, Luxembourg, France, Spain and Macaronesia on the island of Madeira (Blockeel et al. Citation2014). Previous confusion between Macaronesian accessions of P. spinulosa and P. stricta was clarified by Rycroft et al. (Citation2002), and Sim-Sim et al. (Citation2005) demonstrated the presence of both species on Madeira. Plagiochila spinulosa, along with P. britannica Paton and P. norvegica H.H.Blom and Holten, was one of only three Plagiochila species restricted to the eastern Holarctic (Paton Citation1999; Barbulescu et al. Citation2017). The specimen from Lesotho was originally identified as Plagiochila lunata S.W.Arnell and published as such (Groth Citation2006). This particular specimen, however, represents depauperate P. spinulosa, and is similar to forms found in New Zealand and poorly developed shoots within European stands. Such plants would hardly be aligned with P. spinulosa without molecular evidence (). Currently, in the absence of further information, P. lunata remains a species distinct from P. spinulosa.
Figure 3. Plagiochila spinulosa from Lesotho (A–G) and Scotland (H–K). A–D, H–I, shoots in dorsal view; E, medial leaf cells; F, dislocated leaf; G, shoot segment in ventral view; J, medial leaf cells showing oil-bodies; K, perianth in dorsal and lateral views showing subfloral innovations. A–G, from Lesotho, Duckett et al. 34026 (E); H–K, from Scotland, Rycroft 17001 (M).

Despite the magnitude of these disjunctions, it is hard to judge how surprised by them we should be. The rate of dispersal is predicted to decline with increasing distance (MacArthur & Wilson Citation1967), but in danthonioid grasses this decline ceased at around 5000 km and the same rate was inferred for dispersal events over distances of 5000 to 14,000 km (Linder et al. Citation2013). Whether a similar erosion of the distance effect manifests in bryophyte spore or fragment dispersal systems is unknown.
Plagiochila spinulosa is one of the few liverworts known only in female condition (Paton Citation1999; reports of male plants refer to misidentified specimens) and may therefore have formed its range by dispersal of the frequently produced caducous leaves. The important role of vegetative diaspores for range formation over large distances has recently been discussed by Laenen et al. (Citation2016); however, it still needs to be tested whether gametophyte fragments of members of P. sect. Arrectae survive air transport over larger distances. Arrectae may have originated in southern South America and diversified in tropical America before colonising other regions of the world (Heinrichs et al. Citation2005b). Plagiochila spinulosa is unusual among Arrectae in its apparent absence from the Neotropics, given the considerable attention paid to Plagiochilaceae from this region. The molecular data currently available are insufficient to reconstruct the area of origin of P. spinulosa.
Two dispersal models may explain the disjunct distribution of P. spinulosa: stepwise versus direct long-distance dispersal. These two models describe either end of a distribution of potential dispersal distances and dispersal event numbers required for dissemination of propagules between two points, defined by the distribution of intervening suitable habitats. Stepwise dispersal along the Andes mountain chain may have contributed to the trans-tropical range expansion in the lichen Cetraria aculeata (Schreb.) Fr. (Fernández-Mendoza & Printzen Citation2013). In contrast, the shared haplotypes in Cinclidium stygium Sw. from Tierra del Fuego and Iceland suggest a single direct long-distance dispersal event (Piñeiro et al. Citation2012), as do the widely disjunct distributions of lineages within Tetraplodon Bruch and Schimp. (Lewis et al. Citation2014a). In a similar fashion, the disjunct populations of Ptilidium ciliare (L.) Hampe in New Zealand and Chile each shared different haplotypes with Northern Hemisphere populations, consistent with two independent direct long-distance dispersal events from the Northern Hemisphere (Kreier et al. Citation2010). Discriminating direct from stepwise dispersal is dependent, in part, on adequate knowledge of distributions. Ptilidium Nees is relatively conspicuous, and should be detected within intervening habitats if its presence and bryophyte collecting coincided. That it has not been collected in suitable habitats within the tropics, and the fact that known Himalayan populations belonged to a separate lineage, support direct long-distance dispersal to New Zealand. However, the ongoing turnover in the list of known bipolar disjunct bryophyte taxa without intermediate occurrences in the Neotropics (Schofield Citation1974; Ochyra Citation1992; Ochyra & Buck Citation2003) demonstrates our ever increasing, but still incomplete, knowledge of global bryophyte distributions. As collecting effort has increased, new bipolar disjuncts have been identified and added to the list, and others removed when Neotropical occurrences for previously bipolar disjunct taxa were detected. Active bryophyte collection and research programmes are ongoing within the Neotropics and southern South America (Larraín Citation2016). The paucity of bryological collections from many regions of Malesia and Africa leaves open the possibility that P. spinulosa occurs elsewhere.
Disjunctions between two of the three regions where Plagiochila spinulosa occurs are mirrored in other plant groups. African–European disjunctions are known in Anemone L. sect. Anemone (Hoot et al. Citation2012) and Schismus P.Beauv. (Linder et al. Citation2013), with the latter associated with a south–north dispersal. An African–New Zealand disjunction is exhibited by Chionochloa Zotov, which dispersed out of Africa (Linder et al. Citation2013). Few disjunctions between Europe and New Zealand have been proposed, for example Myosotidium hortensia (Decne.) Baill. for whom closest relatives were identified from the Mediterranean region (Heenan et al. Citation2010). However, subsequent studies with wider species sampling have resolved Myosotidium Hook. sister to the southern South American species of Selkirkia Hemsl. with strong support (Weigend et al. Citation2013; Holstein et al. Citation2016), entirely consistent with the broader patterns of southern hemispheric affinities within the New Zealand flora (e.g. Linder et al. Citation2013).
Regardless of the distribution of Plagiochila spinulosa, the disjunctions themselves still must be explained. What might have mediated the dispersal events? Wind dispersal is an obvious possibility in organisms reproducing sexually by spores, but trans-hemispheric wind dispersal would have to contend with the intra-tropical convergence zone and a general lack of exchange of air masses between Northern and Southern Hemispheres. Regardless, there is molecular evidence for transequatorial north–south dispersal in the lichens Flavocetraria nivalis (L.) Kärnefelt and A.Thell (Geml et al. Citation2010) and Cladonia P.Browne (Myllys et al. Citation2003) and the flowering plants Empetrum (Popp et al. Citation2011) and Carex L. (Escudero et al. Citation2010). The disjunction in Empetrum could have been achieved with birds (or a bird) as the vector. Birds may also transport bryophyte and lichen propagules, at least over short distances as demonstrated by Bailey & James (Citation1979), and perhaps over much greater distances if propagules become embedded within the bird’s feather matrix (Lewis et al. Citation2014b). The correlation between transequatorial migratory bird routes and bipolar disjunct bryophyte distributions, and the presence of bryophyte and fungal propagules embedded within the breast feather matrix of migratory birds, is highly suggestive of bird-mediated dispersal of bipolar disjunct taxa in North and South America (Lewis et al. Citation2014b).
An unusual tendency among New Zealand bipolar disjunct bryophytes is their single-island occupancy. Known occurrences of the moss Hylocomium splendens and liverwort Scapania nemorea (L.) Grolle are on the North Island only. Seven of the eight remaining liverwort species are found on the South Island only, the exception being Marsupella sparsifolia (Lindb.) Dumort. which also occurs on Stewart Island. After dispersing across literally thousands of kilometres of open ocean, it is paradoxical that these species have not yet traversed Cook Strait. Perhaps there is no local equivalent to the globally operative dispersal vectors that brought the species to New Zealand in the first instance. Perhaps these species are more widely distributed within New Zealand, but have not yet been detected beyond known stations. Perhaps colonisation in each case is in its early stages, and range expansion is happening.
In bryophytes many trans-oceanic disjunctions have been based on morphological circumscriptions incorrectly ascribing populations from two or more regions to the same species (Shaw Citation2001; Fernandez et al. Citation2006). Integrative studies have resulted in the rejection of some disjunctions, for instance in Orthotrichum tenellum Bruch ex Brid. (Medina et al. Citation2013), and the refinement of others, for example Orthotrichum consimile Mitt. (Medina et al. Citation2012). New disjunctions have been confirmed by integrative revision in Orthotrichum acuminatum H.Philib. (Vigalondo et al. Citation2016). Likewise, in this study, two isolated populations ascribed to different species are in fact geographically disjunct occurrences of the same species. However, the taxonomic decision made by Inoue & Schuster (Citation1971) can hardly be criticised, as Plagiochila caducifolia was distinct among Australasian species and it is unrealistic to expect globally comprehensive consideration of type material, or even species circumscriptions, within the context of a regional revision within this species-rich, challenging genus. As a testable hypothesis of relationships among individuals, P. caducifolia has served its purpose.
Taxonomic treatment
Plagiochila spinulosa (Dicks.) Dumort. Recueil d’Observations sur les Jungermanniacées: 15. 1835.
≡ Martinellius spinulosus (Dicks.) Gray A Natural Arrangement of British Plants 1: 692. 1821.
≡ Radula spinulosa (Dicks.) Dumort. Sylloge Jungermannidearum Europae Indigenarum: 43. 1831.
≡ Jungermannia spinulosa Dicks. Fasciculus Plantarum Cryptogamicarum Britanniae 2: 14. 1790.
Type citation. Scotland ‘in alpibus Scoticis’. Wales, Snowdon.
Type specimen. n.v.
= Plagiochila caducifolia Inoue et R.M.Schust. Journal of the Hattori Botanical Laboratory 34: 71. 1971. syn. nov.
Type citation. New Zealand: South Island: Fiordland Natl. Park, trail from Eglinton-Hollyford Divide to Lake Howden, in Nothofagus menziesii forest, RMS 51964 (MASS; duplicate in TNS).
Type specimen. n.v.
Description (from New Zealand material). Plants with branched sprawling leafy shoots forming loose, untidy, predominantly pure turfs; bronze-green to dull tan, shoot systems without fixed hierarchical structure, while stolons and leafy shoots are sharply differentiated they are completely intermixed; leafy shoots to 50 mm long and 1510–2536 μm wide, leafy shoots dimorphic, stature reduced at base close to stolons; flagellae absent. Branching within stolons and leafy shoots exclusively lateral-intercalary. Stems of stolons and primary shoots in leaf sectors reddish-brown, to 220 μm diameter, transversely elliptic, surfaces smooth; cortical cells in 2 layers dorsally and ventrally, cortical cells smaller than medullar, with continuous brown thickenings on cell walls, medullar cell walls brown-pigmented, with small concave trigones at cell junctions and thin continuous thickening on medial walls. Rhizoids on stolons scattered, produced from lateral and ventral merophytes. Leaves on leafy shoots remote to contiguous, obliquely orientated, ovate to deltoid, 775–1940 μm long by 486–1515 μm wide, dorsal margin shallowly curved or in large leaves straight, entire; when dry antical and postical margin in-rolled along the long axis; 2 teeth prominent at the leaf apex, both 5–8 cells broad at base, narrow triangular, straight, uniseriate in upper part for around 4 cells, capped by a single acicular, hyaline cell; ventral margin straight or weakly curved in outer third, broadly curved and ampliate in basal two thirds, with 1–12 teeth on postical margin, variable in stature, from 2 or 3 cells only with a hyaline acicular apical cell hyaline up to 33 μm long, to 4 cells broad at the base and 7 or 8 cells long, uniseriate for most of the length, spacing between teeth fairly regular; insertion J-shaped, oblique, decurrent dorsally, with a low wing of tissue extending down the stem; slightly recurved at ventral end, not attaining ventral stem midline leaving, variably along a shoot, 1 to 3 cortical cell rows leaf-free, attaining the dorsal stem midline, stem visible between leaves. Marginal cells quadrate to rectangular, 14.8–16.7 μm long by 8.0–13.1 μm wide; cells in median portion more or less isodiametric to elliptic, 16.2–21.8 μm long by 11.5–16.9 μm wide, walls with discrete cordate to convex trigones, medial wall thickenings absent; cells in leaf base rectangular to long rectangular, 31.5–42.0 μm long by 10.5–12.9 μm wide, trigones bulging, low medial wall thickenings present, occasionally confluent with adjacent trigones; leaves on stolons bifid, weakly spreading, ovate. Cell surfaces striolate in basal cells, typically pronounced on smaller leaves and faint on large leaves, grading to faintly papillose on medial cell surfaces. Oil-bodies (4)5–7(8) per cell, tan pigmented, ovoid, granular, arranged in a loose submarginal ring. Underleaves absent or present, narrow triangular up to 4 cells broad at base and 6 cell tiers high. Asexual reproduction by caducous leaves that fragment in entirety. Sexual reproductive structures not seen.
Recognition. In New Zealand, Plagiochila spinulosa is a distinctive species characterised by: 1. caducous leaves which fragment from the stem in their entirety; 2. leaves with a bifid apex and smaller spinulose teeth on the margin, particularly the postical margin; 3. exclusively lateral-intercalary branching; and 4. the striolate-papillose leaf cell surface, particularly on the basal leaf cells ().
Figure 4. Plagiochila spinulosa from New Zealand. A, plant habit; B, hydrated shoots showing sectors denuded by caducous leaf dislocation; C, dorsal stem surface showing long decurrency down dorsal stem midline; D, stem section; E, striolate papillae on surfaces of medial-basal leaf cells; F, ventral stem surface; G, marginal leaf cells showing oil-bodies in partly degraded state; H, basal leaf cells showing striolate papillae; I, leafy shoot showing lateral-intercalary vegetative branch; J, marginal leaf cells showing spinose teeth whose cell surfaces bear papillae; K, medial leaf cells, showing oil-bodies in partly degraded state; L, basal leaf cells, showing striolate papillae on the cell surfaces; M–P, leaves showing variation in size, shape and dentition. All from J.J. Engel & M. von Konrat 27071B, C0311977F (F).
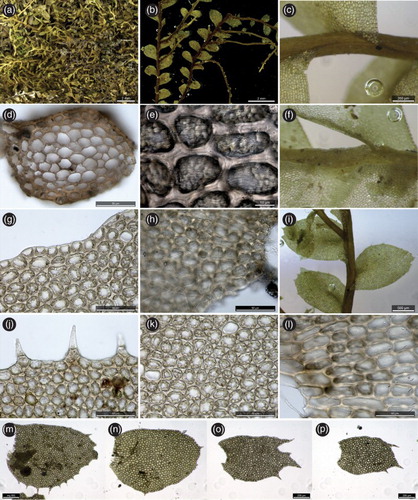
The decurrent wing of leaf tissue extending to the dorsal stem mid-line is an unusual feature among Australasian species.
Plagiochila spinulosa could be confused with species of the P. fasciculata Lindenb. complex, but differs from all in its lack of terminal Frullania-type vegetative branching. Frullania-type vegetative branches occur within shoot systems of all species of the P. fasciculata complex, either as the dominant branching mode or mixed with lateral-intercalary branching. Plagiochila spinulosa is also similar to P. incurvicolla (Hook.f. and Taylor) Gottsche, Lindenb. and Nees, but again the latter possesses vegetative Frullania-type branches, though these occur infrequently, with lateral-intercalary vegetative branching dominant in this species. No species of the P. fasciculata complex nor P. incurvicolla have the conspicuously striolate-papillose ornamentation on the basal leaf cell surfaces.
Among Australasian and Malesian Plagiochila, P. spinulosa shares the striolate-verrucose cuticle and caducous leaves with plants from Papua New Guinea collected by Schuster and described as P. decidua Inoue and Grolle (Inoue Citation1970). On the basis of the protologue of P. decidua, these plants differ from P. spinulosa in two critical features. Firstly, the dorsal leaf insertion does not reach the stem mid-line, whereas it does in P. spinulosa; and, secondly, the dorsal leaf insertion is not long decurrent, whereas it is in P. spinulosa. Plagiochila decidua Inoue and Grolle was listed in synonymy of P. sciophila Nees ex Lindenb. by So & Grolle (Citation1999).
Distribution and ecology. In New Zealand Plagiochila spinulosa has been collected at a range of locations in the South Island from Western Nelson to Fiordland in a variety of habitats including Nothofagus Blume dominated forest and alpine herbfield. On the Thousand Acre Plateau, near Matiri, P. spinulosa formed a small mass within a sheltered hollow on a steep slope dominated by Dracophyllum filifolium Hook.f., Chionochloa pallens Zotov, Oreobolus R.Br. and Donatia J.R.Forst. and G.Forst. At Bridal Veil Falls and Rainbow skifield, P. spinulosa grew as a lithophyte on cliff faces.
Some of the locations P. spinulosa has been collected from are infrequently visited and isolated sites, notably the Thousand Acres Plateau. The species is not associated with anthropogenic disturbance or habitats, and though collected in the vicinity of a skifield it was there within the head of a ravine system below the ski tow area. These features are consistent with P. spinulosa being an indigenous species, rather than a recent accidental introduction.
Specimens examined. New Zealand: South Island: Western Nelson Province, Kahurangi National Park, Matiri Range, Thousand Acres Plateau, 41°37.5′S 172°17.0′E, 1100–1200 m, 22 Feb. 2006, J.J. Engel & M. von Konrat 27071B, C0311977F; Nelson Province, St Arnaud Range, Rainbow skifield just below ski tow area, E of S end of Lake Rotoiti, S of St Arnaud, 41°53′S 172°51′E, 1210 m, 2 Mar. 1997, J.J. Engel 22807, F 1141636; Westland Province, Arthurs Pass National Park, Bridal Veil Track, E side of Bealey River and just N of town of Arthurs Pass, 42°56′S 171°33′E, 760–825 m, 7 Mar. 1997, J.J.Engel 22932, F 1141635; Tributary of Siberia River, opposite Hut, 2500 ft, 17 Jan. 1976, J. Child 2889, BM.
Acknowledgements
We thank the curators at E, F, FH, GOET, HO, JE, M, MEL, S for loans, access to specimens and permission for destructive sampling; and John Engel, Matt von Konrat and David Glenny for their support of the project. Associate Editor: Dr Leon Perrie.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Allan HH. 1961. Flora of New Zealand. Volume 1. Wellington, New Zealand: Government Printer.
- Bailey RH, James PW. 1979. Birds and the dispersal of lichen propagules. The Lichenologist. 11:105–106. doi: 10.1017/S0024282979000141
- Barbulescu EVI, Patzak SDF, Feldberg K, Schäfer-Verwimp A, Rycroft DS, Renner MAM, Heinrichs J. 2017. Allopolyploid origin of the leafy liverwort Plagiochila britannica (Plagiochilaceae). Botanical Journal of the Linnean Society. 183:250–259. doi: 10.1093/botlinnean/bow005
- Bechteler J, Schäfer-Verwimp A, Lee GE, Feldberg K, Pérez-Escobar OA, Pócs T, Peralta DF, Renner MAM, Heinrichs J. 2017. Geographical structure, narrow species ranges, and Cenozoic diversification in a pantropical clade of epiphyllous leafy liverworts. Ecology and Evolution. 7:638–653. doi: 10.1002/ece3.2656
- Bednarek-Ochyra H, Váňa J, Ochyra R, Lewis Smith RI. 2000. The liverwort flora of Antarctica. Cracow: Polish Academy of Sciences, Institute of Botany.
- Birch J, Keeley SC, Morden CW. 2012. Molecular phylogeny and dating of Asteliaceae (Asparagales):Astelia s.l. evolution provides insight into the Oligocene history of New Zealand. Molecular Phylogenetics and Evolution. 65:102–115. doi: 10.1016/j.ympev.2012.05.031
- Blockeel TL, Bosanquet SDS, Hill MO, Preston CD, editors. 2014. Atlas of British & Irish Bryophytes. Volume 1. Newbury: Pisces Publications.
- Chambers TC, Farrant PA. 1996. Blechnum blechnoides (Bory) Keys, (Blechnaceae), formerly B. banksii (Hook. f.) Mett. ex Diels, a fern from salt-spray habitats of New Zealand and Chile. New Zealand Journal of Botany. 34:441–445. doi: 10.1080/0028825X.1996.10410125
- Crandall-Stotler BJ, Stotler RE. 2012. Ontogenetic studies, sporophyte anatomy and phylogenetic affinities of Phyllothallia nivicola (Phyllothalliaceae, Marchantiophyta). Nova Hedwigia. 95: 277–294. doi: 10.1127/0029-5035/2012/0065
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. JModelTest 2: more models, new heuristics and parallel computing. Nature Methods. 9:772. doi: 10.1038/nmeth.2109
- Davison PG, Smith DK, Feldberg K, Lindner M, Heinrichs J. 2006. Plagiochila punctata (Jungermanniopsida: Plagiochilaceae) in Tennessee, new to North America. The Bryologist. 109:242–246. doi: 10.1639/0007-2745(2006)109[242:PPPITN]2.0.CO;2
- Emadzade K, Hörandl E. 2011. Northern Hemisphere origin, transoceanic dispersal, and diversification of Ranunculeae DC. (Ranunculaceae) in the Cenozoic. Journal of Biogeography. 38:517–530. doi: 10.1111/j.1365-2699.2010.02404.x
- Engel JJ. 2006. Austral Hepaticae 40. Tritomaria (Jungermanniaceae subfam. Lophozioideae) new to the south temperate, together with a new subspecies, T. exsecta subsp. novaezelandiae subsp. nov. The Bryologist. 109:60–67. doi: 10.1639/0007-2745(2006)109[0060:AHTJSL]2.0.CO;2
- Engel JJ, Glenny D. 2008. A flora of the liverworts and hornworts of New Zealand. Volume 1. St Louis, Missouri: Missouri Botanical Garden Press.
- Escudero M, Valcárcel V, Vargas P, Luceño M. 2010. Bipolar disjunctions in Carex: long-distance dispersal, vicariance, or parallel evolution? Flora - Morphology, Distribution, Functional Ecology of Plants. 205:118–127. doi: 10.1016/j.flora.2009.01.005
- Fernandez CC, Shevock JR, Glazer AN, Thompson JN. 2006. Cryptic species within the cosmopolitan desiccation-tolerant moss Grimmia laevigata. Proceedings of the National Academy of Sciences. 103:637–642. doi: 10.1073/pnas.0510267103
- Fernández-Mendoza F, Printzen C. 2013. Pleistocene expansion of the bipolar lichen Cetraria aculeata into the Southern Hemisphere. Molecular Ecology. 22:1961–1983. doi: 10.1111/mec.12210
- Geml J, Kauff F, Brochmann C, Taylor DL. 2010. Surviving climate changes: high genetic diversity and transoceanic gene flow in two arctic-alpine lichens, Flavocetraria cucullata and F. nivalis (Parmeliaceae, Ascomycota). Journal of Biogeography. 37:1529–1542.
- Groth H. 2006. Molecular phylogeny and morphological reconstructions of Plagiochilaceae (Jungermanniopsida) with hypotheses on biogeography and divergence times. Thesis. Available from: Göttingen, http://hdl.handle.net/11858/00-1735-0000-0006-AC2F-E.
- Groth H, Lindner M, Wilson R, Hartmann FA, Schmull M, Gradstein SR, Heinrichs J. 2003. Biogeography of Plagiochila (Hepaticae): natural species groups span several floristic kingdoms. Journal of Biogeography. 30:965–978. doi: 10.1046/j.1365-2699.2003.00916.x
- Guindon S, Gascuel O. 2003. A simple, fast and accurate method to estimate large phylogenies by maximum likelihood. Systematic Biology. 52:696–704. doi: 10.1080/10635150390235520
- Gussarova G, Popp M, Vitek E, Brochmann C. 2008. Molecular phylogeny and biogeography of the bipolar Euphrasia (Orobanchaceae): recent radiations in an old genus. Molecular Phylogenetics and Evolution. 48:444–460. doi: 10.1016/j.ympev.2008.05.002
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 41:95–98.
- Heenan PB, Mitchell AD, de Lange PJ, Keeling J, Paterson AM. 2010. Late-Cenozoic origin and diversification of Chatham Islands endemic plant species revealed by analyses of DNA sequence data. New Zealand Journal of Botany. 48:83–136. doi: 10.1080/0028825X.2010.494337
- Heinrichs J, Groth H, Lindner M, Feldberg K, Rycroft DS. 2004. Molecular, morphological, and phytochemical evidence for a broad species concept of Plagiochila bifaria (Hepaticae). The Bryologist. 107:28–40. doi: 10.1639/0007-2745(2004)107[28:MMAPEF]2.0.CO;2
- Heinrichs J, Hentschel J, Feldberg K, Bombosch A, Schneider H. 2009. Phylogenetic biogeography and taxonomy of disjunctly distributed bryophytes. Journal of Systematics and Evolution. 47:497–508. doi: 10.1111/j.1759-6831.2009.00028.x
- Heinrichs J, Lindner M, Gradstein SR, Groth H, Buchbender V, Solga A, Fischer E. 2005a. Origin and subdivision of Plagiochila (Jungermanniidae: Plagiochilaceae) in tropical Africa based on evidence from nuclear and chloroplast DNA sequences and morphology. Taxon. 54:317–333. doi: 10.2307/25065360
- Heinrichs J, Lindner M, Groth H, Renker C. 2005b. Distribution and synonymy of Plagiochila punctata (Taylor) Taylor, with hypotheses on the evolutionary history of Plagiochila sect. Arrectae (Plagiochilaceae, Hepaticae). Plant Systematics and Evolution. 250:105–117. doi: 10.1007/s00606-004-0220-5
- Holstein N, Chacón J, Hilger HH, Weigend M. 2016. No longer shipwrecked—Selkirkia (Boraginaceae) back on the mainland with generic rearrangements in South American “Omphalodes” based on molecular data. Phytotaxa. 270:231–251. doi: 10.11646/phytotaxa.270.4.1
- Hoot SB, Meyer KM, Manning JC. 2012. Phylogeny and reclassification of Anemone (Ranunculaceae), with an emphasis on Austral species. Systematic Botany. 37:139–152. doi: 10.1600/036364412X616729
- Inoue H. 1970. Novae Guineae Hepaticae Schusteranae, II. Plagiochilae Species Novae. Journal of the Hattori Botanical Laboratory. 33:317–330.
- Inoue H, Schuster RM. 1971. A monograph of the New Zealand and Tasmanian Plagiochilaceae. Journal of the Hattori Botanical Laboratory. 34:1–225.
- Kantvilas G. 2012. The genus Menegazzia (Lecanorales: Parmeliaceae) in Tasmania revisited. The Lichenologist. 44:189–246. doi: 10.1017/S0024282911000685
- Koponen T. 1972. Speciation on the Mniaceae. Journal of the Hattori Botanical Laboratory. 35:142–154.
- Kreier HP, Feldberg K, Mahr F, Bombosch A, Schmidt AR, Zhu RL, von Konrat M, Shaw B, Shaw AJ, Heinrichs J. 2010. Phylogeny of the leafy liverwort Ptilidium: cryptic speciation and shared haplotypes between the Northern and Southern Hemispheres. Molecular Phylogenetics and Evolution. 57:1260–1267. doi: 10.1016/j.ympev.2010.10.002
- Laenen B, Machac A, Gradstein SR, Shaw B, Patiño J, Désamoré A, Goffinet B, Cox CJ, Shaw J, Vanderpoorten A. 2016. Geographical range in liverworts: does sex really matter? Journal of Biogeography. 43:627–635. doi: 10.1111/jbi.12661
- Larraín J. 2016. The mosses (Bryophyta) of Capitan Prat Province, Aisen Region, southern Chile. PhytoKeys. 68:91–116. doi: 10.3897/phytokeys.68.9181
- Lewis LR, Behling E, Gousse H, Qian E, Elphick CS, Lamarre JF, Bêty J, Liebezeit J, Rozzi R, Goffinet B. 2014b. First evidence of bryophyte diaspores in the plumage of transequatorial migrant birds. PeerJ. 2:e424. doi: 10.7717/peerj.424
- Lewis LR, Rozzi R, Goffinet B. 2014a. Direct long-distance dispersal shapes a new world amphitropical disjunction in the dispersal-limited dung moss Tetraplodon (Bryopsida: Splachnaceae). Journal of Biogeography. 41:2385–2395. doi: 10.1111/jbi.12385
- Linder HP, Antonelli A, Humphreys AM, Pirie MD, Wüest RO. 2013. What determines biogeographical ranges? Historical wanderings and ecological constraints in the danthonioid grasses. Journal of Biogeography. 40:821–834. doi: 10.1111/jbi.12070
- MacArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press.
- Medina R, Lara F, Goffinet B, Garilleti R, Mazimpaka V. 2012. Integrative taxonomy successfully resolves the pseudo-cryptic complex of the disjunct epiphytic moss Orthotrichum consimile s.l. (Orthotrichaceae). Taxon. 61:1180–1198.
- Medina R, Lara F, Goffinet B, Garilleti R, Mazimpaka V. 2013. Unnoticed diversity within the disjunct moss Orthotrichum tenellum s.l. validated by morphological and molecular approaches. Taxon. 62:1133–1152. doi: 10.12705/626.15
- Meudt H, Prebble JM, Lehnebach CA. 2015. Native New Zealand forget-me-nots (Myosotis, Boraginaceae) comprise a pleistocene species radiation with very low genetic divergence. Plant Systematics and Evolution. 301:1455–1471. doi: 10.1007/s00606-014-1166-x
- Meudt H, Simpson BB. 2006. The biogeography of the austral, subalpine genus Ourisia (Plantaginaceae) based on molecular phylogenetic evidence: South American origin and dispersal to New Zealand and Tasmania. Biological Journal of the Linnean Society. 87:479–513. doi: 10.1111/j.1095-8312.2006.00584.x
- Miller, MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, Lousiana, USA (pp. 1-8).
- Muñoz J, Felicísimo ÁM, Cabezas S, Burgaz AR, Martínez I. 2004. Wind as a long-distance dispersal vehicle in the Southern Hemisphere. Science. 304:1144–1147. doi: 10.1126/science.1095210
- Myllys L, Stenroos S, Thell A, Ahti T. 2003. Phylogeny of bipolar Cladonia arbuscula and Cladonia mitis (Lecanorales, Euascomycetes). Molecular Phylogenetics and Evolution. 27:58–69. doi: 10.1016/S1055-7903(02)00398-6
- Nylinder S, Swenson U, Persson C. 2012. A dated species-tree approach to the trans-Pacific disjunction of the genus Jovellana (Calceolariaceae, Lamiales). Taxon. 61:381–391.
- Ochyra R. 1992. Amblyodon dealbatus (Musci, Meesiaceae)—a bipolar disjunct. Fragmenta Floristica et Geobotanica. 37:7–12.
- Ochyra R, Buck WR. 2003. Arctoa fulvella, new to Tierra del Fuego, with notes on trans-American bipolar bryogeography. The Bryologist. 106:532–538. doi: 10.1639/0007-2745(2003)106[532:AFNTTD]2.0.CO;2
- Paton JA. 1999. The liverwort flora of the British Isles. Colchester: Harley Books.
- Patzak SDF, Renner MAM, Schäfer-Verwimp A, Feldberg K, Heslewood MM, Peralta DF, Souza A, Schneider H, Heinrichs J. 2016. A phylogeny of Lophocoleaceae-Plagiochilaceae-Brevianthaceae and a revised classification of Plagiochilaceae. Organisms Diversity and Evolution. 16:481–495. doi: 10.1007/s13127-015-0258-y
- Perret M, Chautems A, De Araujo AO, Salamin N. 2013. Temporal and spatial origin of Gesneriaceae in the New World inferred from plastid DNA sequences. Botanical Journal of the Linnean Society. 171:61–79. doi: 10.1111/j.1095-8339.2012.01303.x
- Perrie LR, Ohlsen DJ, Shepherd LD, Garrett M, Brownsey PJ, Bayly MJ. 2010. Tasmanian and Victorian populations of the fern Asplenium hookerianum result from independent dispersals from New Zealand. Australian Systematic Botany. 23:387–392. doi: 10.1071/SB10028
- Piñeiro R, Popp M, Hassel K, Listl D, Westergaard KB, Flatberg KI, Stenøien HK, Brochmann C. 2012. Circumarctic dispersal and long-distance colonization of South America: the moss genus Cinclidium. Journal of Biogeography. 39:2041–2051. doi: 10.1111/j.1365-2699.2012.02765.x
- Popp M, Mirré V, Brochmann C. 2011. A single mid-Pleistocene long-distance dispersal by a bird can explain the extreme bipolar disjunction in crowberries (Empetrum). Proceedings of the National Academy of Sciences. 108:6520–6525. doi: 10.1073/pnas.1012249108
- Rambaut A, Suchard MA, Xie D, Drummond AJ. 2014. Tracer v1.6. Available from http://beast.bio.ed.ac.uk/Tracer [Verified 20 May 2016]
- Raven PH. 1963. Amphitropical relationships in the floras of North and South America. The Quarterly Review of Biology. 38:151–177. doi: 10.1086/403797
- Renner MAM, Heslewood MM, Patzak SDF, Schäfer-Verwimp A, Heinrichs J. 2017. By how much do we underestimate species diversity of liverworts using morphological evidence? An example from Australasian Plagiochila (Plagiochilaceae: Jungermanniopsida). Molecular Phylogenetics and Evolution. 107:576–593. doi: 10.1016/j.ympev.2016.12.018
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19:1572–1574. doi: 10.1093/bioinformatics/btg180
- Ronquist F, Teslenko M, van der Mark P, Ayres D, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 61:539–542. doi: 10.1093/sysbio/sys029
- Rycroft DS, Cole WJ, Heinrichs J, Groth H, Renker C, Pröschold T. 2002. Phytochemical, morphological and molecular evidence for the occurrence of the Neotropical liverwort Plagiochila stricta in the Canary Islands, new to Macaronesia. The Bryologist. 105:363–372. doi: 10.1639/0007-2745(2002)105[0363:PMAMEF]2.0.CO;2
- Rycroft DS, Heinrichs J, Cole WJ, Anton H. 2001. A phytochemical and morphological study of the liverwort Plagiochila retrorsa Gottsche, new to Europe. Journal of Bryology. 23:23–34. doi: 10.1179/jbr.2001.23.1.23
- Sanmartín I, Ronquist F. 2004. Southern hemisphere biogeography inferred by event-based models: plant versus animal patterns. Systematic Biology. 53:216–243. doi: 10.1080/10635150490423430
- Scheben A, Bechteler J, Lee GE, Pócs T, Schäfer-Verwimp A, Heinrichs J. 2016. Multiple transoceanic dispersals and geographical structure in the pantropical leafy liverwort Ceratolejeunea (Lejeuneaceae, Porellales). Journal of Biogeography. 43:1739–1749. doi: 10.1111/jbi.12779
- Schofield WB. 1974. Bipolar disjunctive mosses in the Southern Hemisphere, with particular reference to New Zealand. Journal of the Hattori Botanical Laboratory. 38:13–32.
- Schuster RM. 1969. Problems of antipodal distribution in lower land plants. Taxon. 18:46–91. doi: 10.2307/1218591
- Schuster RM. 1974. The Hepaticae and Anthocerotae of North America. Volume 3. New York: Columbia University Press.
- Schuster RM. 1980. The Hepaticae and Anthocerotae of North America. Volume 4. New York: Columbia University Press.
- Schuster RM. 1984. Phytogeography of the Bryophyta. In Schuster RM, editor. New manual of Bryology. Volume 1. Nichinan: Hattori Botanical Laboratory; p. 463–626.
- Schuster RM. 1996. Studies in Antipodal Hepaticae XII. Gymnomitriaceae. Journal of the Hattori Botanical Laboratory. 80:1–147.
- Shaw AJ. 2001. Biogeographic patterns and cryptic speciation in bryophytes. Journal of Biogeography. 28:253–261. doi: 10.1046/j.1365-2699.2001.00530.x
- Shaw AJ, Werner O, Ros RM. 2003. Intercontinental Mediterranean disjunct mosses: morphological and molecular patterns. American Journal of Botany. 90:540–550. doi: 10.3732/ajb.90.4.540
- Sim-Sim M, Esquível MD, Fontinha S, Stech M. 2005. The genus Plagiochila (Dumort.) Dumort. (Plagiochilaceae, Hepaticophytina) in Madeira Archipelago - Molecular relationships, ecology, and biogeographic affinities. Nova Hedwigia. 81:449–462. doi: 10.1127/0029-5035/2005/0081-0449
- So ML, Grolle R. 1999. Studies on Plagiochila in Asia: supplements to sections Abietinae, Annotinae, Ciliatae, Contiguae, Cucullatae, Poeltiae, Subtropicae and Zonatae. Cryptogamie Bryologie. 20:167–179. doi: 10.1016/S1290-0796(99)80014-5
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30:1312–1313. doi: 10.1093/bioinformatics/btu033
- Sun Y, He XL, Glenny D. 2014. Transantarctic disjunctions in Schistochilaceae (Marchantiophyta) explained by early extinction events, post Gondwanan radiations and palaeoclimatic changes. Molecular Phylogenetics and Evolution. 76:189–201. doi: 10.1016/j.ympev.2014.03.018
- Thorne RF. 1972. Major disjunctions in geographic ranges of seed plants. The Quarterly Review of Biology. 47:365–411. doi: 10.1086/407399
- Vanderpoorten A, Devos N, Goffinet B, Hardy OJ, Shaw AJ. 2008. The barriers to oceanic island radiation in bryophytes: insights from the phylogeography of the moss Grimmia montana. Journal of Biogeography. 35:654–663. doi: 10.1111/j.1365-2699.2007.01802.x
- Vigalondo B, Lara F, Draper I, Valcarcel V, Garilleti R, Mazimpaka V. 2016. Is it really you, Orthotrichum acuminatum? Ascertaining a new case of intercontinental disjunction in mosses. Botanical Journal of the Linnean Society. 180:30–49. doi: 10.1111/boj.12360
- Viljoen JA, Muasya MA, Barrett RL, Bruhl JJ, Gibbs AK, Slingsby JA, Wilson KL, Verboom GA. 2013. Radiation and repeated transoceanic dispersal of Schoeneae (Cyperaceae) through the southern hemisphere. American Journal of Botany. 100:2494–2508. doi: 10.3732/ajb.1300105
- Wagstaff SJ, Bayly MJ, Garnock-Jones PJ, Albach DC. 2002. Classification, origin and diversification of the New Zealand Hebes (Scrophulariaceae). Annals of the Missouri Botanical Garden. 89:38–63. doi: 10.2307/3298656
- Wagstaff SJ, Martinsson K, Swenson U. 2000. Divergence estimates of Tetrachondra hamiltonii and T. patagonica (Tetrachondraceae) and their implications for austral biogeography. New Zealand Journal of Botany. 38:587–596. doi: 10.1080/0028825X.2000.9512707
- Weigend M, Luebert F, Selvi F, Brokamp G, Hilger HH. 2013. Multiple origins for hound’s tongues (Cynoglossum L.) and navel seeds (Omphalodes Mill.)—the phylogeny of the borage family (Boraginaceae s. str.). Molecular Phylogenetics and Evolution. 68:604–618. doi: 10.1016/j.ympev.2013.04.009
- Winkworth RC, Wagstaff SJ, Glenny D, Lockhart PJ. 2005. Evolution of the New Zealand mountain flora: origins, diversification and dispersal. Organisms Diversity and Evolution. 5:237–247. doi: 10.1016/j.ode.2004.12.001
- Woo VL, Funke MM, Smith JF, Lockhart PJ, Garnock-Jones PJ. 2011. New World origins of Southwest Pacific Gesneriaceae: multiple movements across and within the South Pacific. International Journal of Plant Sciences. 172:434–457. doi: 10.1086/658183
