Abstract
Blue cod (Parapercis colias) support important fisheries around New Zealand, but limited mixing over small spatial scales renders subpopulations vulnerable to depletion and highlights the importance of resolving fine-scale population structure. Fiordland represents a rare opportunity to achieve this in a relatively pristine environment and intact ecosystem. Between 2000 and 2004, spatial population structure in three fjords was used to test how spatial variability in growth between inner and outer fjord subpopulations can be statistically explained by diet, demography and site-specific factors. Sex ratios and size distributions vary between habitats, with a female bias and a higher frequency of individuals in the larger size classes within the inner fjords. Stomach content analysis shows that in the inner fjords the diet of blue cod includes a diverse array of benthic species while in the outer fjords P. colias is able to utilise a less diverse array of pelagic prey that may be of a higher nutritional quality. Stable isotope analysis reveals that in two of the three outer fjord habitat sites the Laminarian Ecklonia radiata accounts for a large component of basal organic matter to the local population (66–95%). Consequently, fish condition and individual growth rates are high in these kelp-bed fuelled outer fjord habitats. Our findings demonstrate that baseline variability in the nutritional landscape can result in spatially structured populations, highlighting the importance of spatially-explicit management measures in the sustainable exploitation of reef fishes.
Introduction
Demography and vital rates, such as individual growth and fecundity, interact to influence a population's productivity. For exploited species an understanding of spatial variability in these processes is essential to understand population dynamics, predict sustainable yields and to ensure the efficacy of management. To interpret spatial variability in population structure and dynamics in terms of underlying mechanisms requires an understanding of the role of the nutritional landscape in determining a population's trophic ecology. Recognising that exploited species form integral parts of, and rely on provision of organic matter from, complex food webs is therefore essential from an ecosystem management and fisheries management perspective. It has been well recognised that spatial variability in vital rates and productivity constitutes a major management and conservation issue for invertebrate populations (Orensanz & Jamieson Citation1998). For example, population structure of grazing and suspension feeding invertebrates is subject to gradients in productivity across ecotone boundaries (Wing Citation2009; Wing & Leichter Citation2011), but the effects of such extrinsic structuring processes at higher trophic levels have rarely been considered. Here we investigate these issues in an exploited temperate reef fish.
Blue cod (Parapercis colias: Pinguipedidae, Forster 1801) are endemic to New Zealand where they support major commercial and recreational fisheries. Studies into the fine-scale movement of adults have shown them to be largely sedentary (Cole et al. Citation2000) and territorial (Mutch Citation1983) with evidence to suggest a decoupling of populations and limited mixing over relatively small spatial scales in enclosed waterways (Cole et al. Citation2000; Carbines & McKenzie Citation2004). In Dusky Sound—one of a series of 14 glacially-carved fjords in southwestern New Zealand, collectively known as Fiordland—65% of tagged adults moved less than 1 km from their release site during 17 months at liberty; larger movements were generally in an up-fjord direction, generating the hypothesis that the more mobile and interactive outer fjord and open coast populations act as a source draining up to 10% annually into the inner fjord sink population, where residency was estimated at 100% (Carbines & McKenzie Citation2004). There is some evidence to suggest a decoupling of population structure between habitats in Fiordland, with inner fjord populations typically female-dominated and including a higher proportion of larger, older individuals than the male-dominated outer fjord populations (Rodgers Citation2005). Inner fjord habitats are generally less productive than the wave-washed outer coast, resulting in nutritional stress and depressed growth rates in some species. For example, sea urchin populations in the inner fjords have relatively low growth rates and are subject to periodic mass mortality, resulting in greater demographic variability than those on the open coast (Wing Citation2009). Similarly, plasticity in growth and morphology among adjacent populations of sea perch in Fiordland reflects local habitat quality and food resources (Lawton et al. Citation2010), demonstrating the potential for the environment to strongly influence the productivity of higher trophic level vertebrates in this system.
Ranging over 200 km of remote coastline, the World Heritage and National Park protection afforded to the Fiordland region has ensured that anthropogenic impacts have been minimal and the natural environment has been preserved in a remarkably pristine condition. The steep topography, dense Nothofagus beech forest cover and high orographic rainfall result in substantial inputs of terrigenous organic material into the fjords, representing an unusually high degree of connectivity between terrestrial and marine ecosystems (McLeod & Wing Citation2009). The estuarine circulation pattern and entrance sills act to retain a significant portion of this material within the deep basins (Glasby Citation1978; Pickrill Citation1987) where it has been found to fuel a chemoautotrophic food web (Wing et al. Citation2008; McLeod et al. Citation2010). This is particularly prevalent in the inner fjord basins where terrestrial inputs are greatest. In Doubtful Sound the outflow of freshwater from a hydroelectric power station has altered the salinity regime and reduced or eradicated populations of filter-feeding bivalves, thereby reducing the availability of more labile heterotrophic carbon (Tallis et al. Citation2004; Rutger & Wing Citation2006; McLeod & Wing Citation2008) and increasing the importance of recycled terrestrially-derived organic matter to higher order consumers including blue cod (Rodgers & Wing Citation2008), red rock lobsters (Jack et al. Citation2009) and hagfish (McLeod & Wing Citation2007). It is not yet known to what extent this chemosynthetically-derived energy features in the food webs of more pristine fjords, although recent evidence suggests that in marginal inner fjord habitats it may provide a mechanism for the persistence of blue cod populations (Wing et al. Citation2012). In the inner fjords the low light and flow environment restricts macrophyte growth to a thin band of freshwater/estuarine species in the upper few metres, while in the wave-exposed outer fjord habitats, productive and diverse phytoplankton and macroalgal assemblages support kelp forest communities (Goebel et al. Citation2005; Miller et al. Citation2006; Wing et al. Citation2007). These processes result in a large difference in productivity and composition of basal organic matter sources within food webs between inner and outer fjord habitats.
Stomach content analysis has shown that P. colias, a demersal species, feeds on a wide vertebrate and invertebrate prey base, with a small direct intake of macroalgae and detritus (Thomson Citation1891; Graham Citation1939; Mutch Citation1983; Jiang & Carbines Citation2002). One of the major limitations of dietary studies based on stomach content analysis is that one cannot determine whether diet composition at the time of sampling reflects the availability of different prey types or a strong selectivity for a particular prey type. Integrating across several sampling events and employing stable isotope analysis in parallel helps resolve this issue by giving an indication of the longer-term importance of broad dietary components to a population. In the Doubtful–Bradshaw Sound complex, stable isotopic evidence for differences in diet and organic matter sources in blue cod from inner and outer fjord habitats lends support to the hypothesis that adult blue cod in Fiordland form discrete subpopulations at the scale of tens of kilometres, with a high degree of residency over months to years (Rodgers & Wing Citation2008). δ13C and δ15N are more deplete in 13C and 15N and more variable among the inner fjord subpopulation than the outer fjord and open coast populations, suggesting a significant assimilation of recycled carbon and wider individual variability in trophic position among fish in inner fjord habitats (Rodgers & Wing Citation2008).
Since 2005, the marine environment of Fiordland has been managed using a network of marine protected areas introduced under the Fiordland Marine Management Act 2005; the internal waters of all fjords are closed to commercial fishing and Doubtful Sound and Milford Sound—the two most accessible fjords—are closed to recreational fishing for blue cod. In addition, 10 marine reserves distributed among eight of the fjords afford full no-take protection to areas representative of the major habitat types.
The observed spatial separation in habitat quality, blue cod population structure and trophic ecology between subpopulations in inner and outer fjord habitats raises interesting questions regarding the resilience of subpopulations to localised depletion and the productivity of inner fjord populations. Previous ecological studies in Fiordland have tended to focus on Doubtful Sound, where the modified salinity regime is known to have altered carbon routing through the inner fjord ecosystem, or have compared between sites subject to differential management practices (Jack et al. Citation2009; Jack & Wing Citation2010; Wing Citation2011; Wing et al. Citation2012). Data collected before the introduction of the Fiordland Marine Management Act 2005 allow us to investigate the baseline ecological processes that influence population structure and dynamics. Focusing on the more pristine southern fjords, we employ an orthogonal sampling design with paired inner and outer fjord sites in Breaksea Sound, Dusky Sound and Long Sound–Preservation Inlet to, firstly, identify and characterise differences in growth rate between inner and outer fjord subpopulations of P. colias individuals and, secondly, to ask: in the absence of habitat modification and differential management, are these differences best statistically explained by diet, demographics or site-specific factors? By testing ecological processes independently of anthropogenic influences, greater insight can be gained into the forces that structure natural populations—forces that fisheries management models must account for if yields are to be sustainable. Furthermore, efforts to regenerate exploited stocks and allow populations to recover can only be successful if these baseline ecological processes are understood.
Materials and methods
Sample collection
Blue cod were sampled between 2000 and 2004 at paired inner and outer fjord sites in Breaksea Sound (inner: n=33; outer: n=33), Dusky Sound (inner: n=33; outer: n=38) and Long Sound–Preservation Inlet (inner: n=50; outer: n=50) (). A standardised experimental design was employed with all samples collected during austral spring (October–November), using hand lines with artificial lures and size 6/0 hooks to target adults and reduce by-catch (Carbines Citation1999). Fish were euthanised before being measured (±1mm total length, TL), weighed (whole blotted wet weight) and macroscopically sexed. Sagittal otoliths were removed, cleaned of adhering cerebral tissue and left to air dry for 48 h in unsealed centrifuge tubes. Stomachs were removed by abdominal dissection, blotted and weighed before the contents were transferred to labelled screw-top sample jars and fixed in a 10% seawater-buffered formalin solution. As very few fish sampled had empty stomachs (<1%) these were excluded from stomach content analyses without further consideration. Gutted weight was calculated as (wet weight – visceral cavity contents weight). Fulton's condition factor (Ricker Citation1975) was calculated as:
where W is the gutted weight (g) and L is the total length (mm).
Stomach content analysis
Fixed stomach contents were rinsed over a 500 µm mesh and transferred to a 70% ethanol solution. Prey items were sorted into broad taxonomic groups (pisces, zooplankton, Echinodermata, Mollusca, Crustacea, other benthic invertebrates, macroalgae and detritus) under a dissecting stereomicroscope and the blotted wet weight of each group was recorded. This was then expressed as a percentage of the total wet body weight of the fish (% BW). Groups were classified as representing either a ‘pelagic’ (zooplankton) or ‘benthic’ feeding strategy (Echinodermata, Mollusca, Crustacea, other benthic invertebrates, detritus). Pisces remains were not identified to a lower taxonomic level and could not therefore be assigned to either feeding strategy, as both demersal and pelagic species are common in the fjords; macroalgal matter was not assigned to either strategy due to the possibility of drifting macroalgal debris being consumed from the water column or anchored macroalgae being encountered during benthic grazing.
Multivariate stomach content data were pooled across years to provide a time-integrated overview of the diet rather than a ‘snapshot’ of one moment in time. Data were presence–absence transformed and a Bray–Curtis resemblance matrix was constructed. A presence–absence transformation was selected as variability in the weight of items in each taxonomic group and differing degrees of digestion would lead to bias in the inferred importance of each group to an individual's diet. Sampling of stomach contents was opportunistic and no consideration was given to the time of day, state of the tide, etc. Stage of digestion would therefore likely be an issue when comparing stomach content weights between sampling events. With presence–absence data the metric of abundance becomes the incidence of occurrence of a prey type among individuals within a population (Hynes Citation1950) and reflects population-wide feeding habits (Cailliet Citation1977), although small, highly abundant prey items are likely to be over-emphasised relative to less abundant, larger prey items.
The % BW of each dietary group was scaled to a proportion of the total stomach % BW before Shannon–Wiener diversity, H′ (Shannon Citation1948) was calculated for each individual as:
where S is the number of dietary groups, p i is the relative contribution of each group to the total stomach weight, n i is the % BW of group i and N is the total % BW of all groups.
Stable isotope analysis
In 2004, a subsample of fish from each site were selected for stable isotope analysis (n=68); dorsal muscle tissue was sampled from behind the head, rinsed in deionised water and frozen at − 20 °C in sterile centrifuge tubes. Muscle tissue samples were defrosted and oven-dried at 60 °C for 72 h. Dry samples were homogenised using a pestle and mortar, which was rinsed between samples with deionised water and dried with lint-free tissue. Rodgers and Wing (Citation2008) found that lipids do not need to be extracted prior to stable isotope analysis of blue cod muscle tissue. However, an independent check was conducted for our data set given the concerns raised by Post et al. (Citation2007) that failure to account for differences in lipid content among consumer populations can introduce considerable bias when interpreting patterns based on δ13C, particularly when variation is <10‰–12‰. As individual blue cod C:N ratios did not differ between fjords, habitats or sites (P>0.1), stable isotopic data were not normalised for lipid content. 0.1mg samples were weighed into 5×3.5 mm tin capsules and analysed using a Europa Scientific Hydra 20/20 continuous flow isotope ratio mass spectrometer coupled to a Carlo Erba NC2500 elemental analyser by Iso-Trace New Zealand Ltd (Dunedin, New Zealand). The natural isotope abundance of 13C and 15N was expressed in delta (δ) notation, or parts per thousand difference from a standard, PeeDee Belemnite for δ13C and air for δ15N (precision: 0.2‰):
where X is the heavier isotope and R is the corresponding isotopic ratio in the sample or standard. Half-lives of δ13C and δ15N in fish muscle tissue may be many months (Hesslein et al. Citation1993); turnover rates of blue cod muscle tissue result in a stable isotopic signature that integrates across a similar time period to that represented by stomach content data (Rodgers & Wing Citation2008; Suring & Wing Citation2009).
Mass balance mixing models
The proportional contributions of n + 1 sources to the diet of a consumer can be estimated with n isotope tracers using a linear mixing model based on mass balance equations (Phillips & Gregg Citation2003). Stable isotopic signatures of the major organic matter source pools in the system have previously been characterised for Doubtful Sound (Cornelisen et al. Citation2007; Wing et al. Citation2008): a suspended particulate organic matter (SPOM) signature was derived from vertical plankton tow samples collected during a phytoplankton bloom; signatures for Ulva pertusa and Ecklonia radiata (respectively the dominant estuarine/freshwater and marine macroalgal producers in the system) were integrated from samples taken along the fjord axis to control for spatial variability in environment and oceanography that could result in heterogeneous δ13C values (Cornelisen et al. Citation2007), and a terrigenous organic matter (TOM) signature was obtained from forest litter sampled from inner Doubtful Sound. Source pool isotopic signatures (mean±standard error) were: SPOM δ13C=− 21.3±0.18, δ15N=6.6±0.36; estuarine macroalgae (U. pertusa) δ13C=− 15.7±0.09, δ15N=3.5±0.06; marine macroalgae (E. radiata) δ13C=− 18.6±0.05, δ15N=5.9±0.12 and TOM δ13C=− 13.2±1.14, δ15N=− 2.8±0.75. Two-source mixing models were constructed using average carbon isotopic ratios for each site with IsoError (Phillips & Gregg Citation2001a, Citationb). To estimate the basal δ15N for each site, source pools were plotted in carbon–nitrogen space and a linear regression fitted between the two basal organic matter sources adjacent to the site-average consumer signature. The equation of this regression was used to calculate trophic level based on the interpolated difference in δ15N between source and consumer; source pool δ13C values were then adjusted with trophic discrimination factors for each site model to align them with the site-average consumer signature. As Parapercis colias feeds at a relatively high trophic level (4 to 5) and Δ15N is averaged over four to five steps, uncertainty in this estimate was reduced (sensu Vander Zanden & Vadeboncoeur Citation2002). In the absence of a specific trophic discrimination factor for blue cod muscle tissue, average aquatic poikilotherm values of 2.3‰ for δ15N and 0.4‰ for δ13C were used (McCutchan et al. Citation2003). Two-source, single-isotope models are limited by the assumption that it is the combination of proximal rather than more distal sources which results in the intermediate carbon isotopic signature of the consumer (Fry & Sherr Citation1984). The validity of this assumption can be assessed given prior biological and ecological knowledge of the study system (Post Citation2002). For inner fjord habitats, E. radiata was not considered an ecologically plausible carbon source pool and was rejected in favour of more distal sources; elsewhere, the two adjacent source pools were used. Using a single value (e.g. mean) to describe source pool contributions to consumer isotopic signatures is not advised (Phillips & Gregg Citation2003), therefore the range of feasible solutions (95% confidence interval, CI) is reported for all models.
Age estimation
One of each pair of otoliths was embedded in K142 epoxy resin (NUPLEX Construction Products, Auckland, New Zealand). It is not expected that the left and right otoliths of a pair would yield different age estimates; however, for consistency and ease of sectioning, whenever possible the right otolith was selected for age estimation. Transverse sections (c. 0.7 mm thick) were cut through the primordium using a Buehler Isomet low speed diamond-tipped saw and the sections mounted on glass slides using a small amount of resin. Ages were estimated from photomicrographs of sectioned otoliths taken under transmitted light. Only opaque (winter growth) zones bordered on both sides by translucent (summer growth) zones were counted. Image editing software (ImageJ, NIH, USA) was used to enhance the images and allow more accurate identification of annual growth rings, in particular those nearer the core. Ages were recorded by two independent agers and the readability of the sections was scored from 1 (easy to determine all growth bands) to 5 (unreadable); any otoliths graded as unreadable by either reader were excluded from growth rate analyses. The coefficient of variance, CV (Campana Citation2001) was estimated for each otolith as:
where CV j is the age precision estimate for the jth fish, X ij is the ith age determination of the jth fish, X j is the mean age estimate of the jth fish and R is the number of times each fish is aged. As the mean CV for all otoliths was<5%, ager bias was deemed to be within acceptable limits and the age estimates of the more experienced ager (SRW) were used for all further analyses.
Modelling of growth
Size-at-age data were used to construct von Bertalanffy growth curves (von Bertalanffy Citation1934) for each site, pooled across years to increase sample sizes and maximise model fit:
where L t is length at time t, L ∞ is the asymptotic length, k is the growth constant expressing the rate at which length approaches the asymptote and t 0 is the theoretical age of an individual at 0 size. Optimal values for L ∞ and k were obtained by minimising residual sums of squares with the Solver application (Frontline Systems Inc., USA) for Excel 2008 (Microsoft Corporation 2007). No fish were sampled from the youngest age classes (<3 yrs) so t 0 was set to 0 for all models. As blue cod are protogynous hermaphrodites (Mutch Citation1983; Carbines Citation2004), growth was modelled separately for males and females.
Statistical analyses
Diet composition was compared between habitats (fixed factor, two levels) using permutational (multivariate) analysis of variance (PERMANOVA) in PERMANOVA + for PRIMER v6 (PRIMER-E Ltd, Plymouth, UK). This generates a pseudo-F statistic but as P values are calculated under permutation, the assumptions of normal distribution and homogeneity of variance inherent in traditional ANOVA methods are avoided (Anderson Citation2001). Multivariate diet composition was unaffected by fish sex or size (P<0.05) so spatial tests were pooled across sexes and size classes. Canonical analysis of principal coordinates (CAP) was used to calculate leave-one-out reclassification to habitat success rates (Anderson & Willis Citation2003). Permutational ANOVA based on Euclidean distance between samples was used to identify the dietary groups driving overall habitat effects.
Diet diversity (H′), fish condition and size-at-age were compared between habitats using one-way PERMANOVA based on Euclidean distance between samples. Size-at-age data were first normalised (mean=0, SD=1) to account for differences in variable range; spatial variability was investigated for males and females separately.
The ratio of males to females in the population at each study site was compared using Chi-square tests; Chi-square contingency tables were then used to compare the sex ratio at paired inner and outer fjord sites. Male and female size frequency distributions were compared between inner and outer fjord habitats using PERMANOVA based on Euclidean distance between samples.
Model selection
An information theoretic approach (Akaike Citation1974; Burnham & Anderson Citation1998; Anderson et al. Citation2001) with the corrected Akaike Information Criterion (AICc) was used to rank nested models and to quantify the explanatory power of dietary (proportion benthic versus pelagic), demographic (sex, condition) and site-specific factors (habitat, fjord) on individual size-at-age. Of the site-specific factors, ‘habitat’ refers to either inner or outer fjord locations, which tend to be environmentally and ecologically different, while ‘fjord’ incorporates any variability that might arise due to latitudinal or geomorphological differences among the three fjord basins included in this study. Formulae for AICc are provided in Anderson et al. (2001). A permutation-based least squares linear model framework (DistLM, PERMANOVA + for PRIMER v6) was used to test for interactions and collinearity among the explanatory variables.
Results
Stomach contents analysis
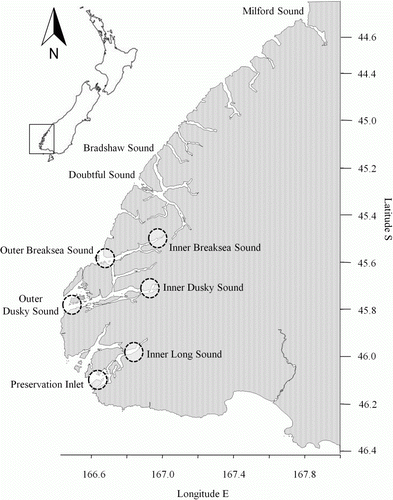
Diet composition differed between inner and outer fjord habitats overall (PERMANOVA, pseudo-F 1,232 =19.813, P=0.0001) with 72% of fish correctly reclassified to habitat; a reclassification success rate of 49–51% would be expected by chance alone. Pair-wise tests showed that this habitat effect was driven by pronounced differences in diet between inner and outer fjord populations in Dusky Sound (PERMANOVA, pseudo-F 1,69 =32.513, P=0.0001, 94% reclassification success) and Long Sound–Preservation Inlet (PERMANOVA, pseudo-F 1,97 =9.7097, P=0.0001, 74% reclassification success) and a marginal difference in Breaksea Sound (PERMANOVA, pseudo-F 1,64 =2.6247, P=0.0425, 62% reclassification success). In all fjords the occurrence of pelagic prey resources in stomach contents was significantly (PERMANOVA, P<0.05) higher among outer fjord fish, while the occurrence of benthic prey resources was frequently higher among inner fjord populations ().
Figure 2 Mean P. colias stomach contents occurrence at each site of A, Zooplankton, B, Pisces, C, Echinodermata, D, Crustacea, E, Mollusca, F, Benthic invertebrates, G, Algae and H, Detritus. Data have been pooled across sexes and years. Standard error bars are shown. A significant difference (P < 0.05) between paired inner and outer fjord sites is denoted by an asterisk. Dark bars indicate inner fjord sites and light bars indicate outer fjord sites.
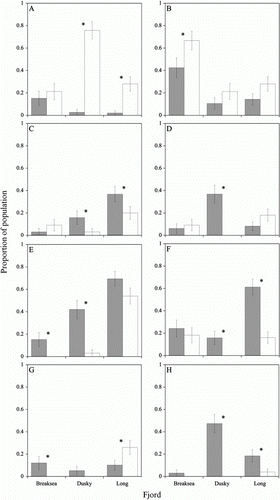
Diet diversity (H′) was higher among inner fjord populations (PERMANOVA, pseudo-F 1,235 =9.0892, P=0.0023). This pattern was upheld in Dusky Sound and Long Sound–Preservation Inlet, but in Breaksea Sound diversity did not differ between habitats (a). Fish condition was lower among inner fjord subpopulations overall (PERMANOVA, pseudo-F 1,229 =6.6353, P=0.0067) and in Breaksea Sound and Dusky Sound, but in Long Sound–Preservation Inlet condition did not differ between habitats (b).
Figure 3 A, Mean Shannon–Wiener diversity (H′) at each site. Data have been pooled across sexes and years. Standard error bars are shown. Dark bars indicate inner fjord sites and light bars indicate outer fjord sites. A significant difference (P<0.05) between paired inner and outer fjord sites is denoted by an asterisk. B, Mean condition at each site. Data have been pooled across sexes and years. Standard error bars are shown. Dark bars indicate inner fjord sites and light bars indicate outer fjord sites. A significant difference (P < 0.05) between paired inner and outer fjord sites is denoted by an asterisk.
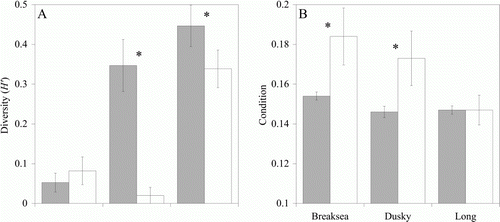
Figure 4 Sex ratio of Parapercis colias from each study site recorded as % male in the population. Dark bars indicate inner fjord sites and light bars indicate outer fjord sites; total sample size at each site is given. A significant deviation from a 50:50 sex ratio (P<0.05) is denoted by an asterisk; underlined fjord labels indicate a significant difference (P<0.05) in the sex ratio between inner and outer fjord subpopulations.
Stable isotope analysis and mass balance mixing models
Spatial variability in the δ13C signatures of P. colias was minimal (). Despite the presence of the chemoautotrophic clam Solemya parkinsoni in the stomach contents of a few individuals sampled from inner Breaksea Sound (n=3), inner Dusky Sound (n=7) and Preservation Inlet (n=1), no evidence was found to support long-term chemoautotrophic carbon use by any individuals (δ13C range:−20.7– − 15.0). Mass balance analyses demonstrate that in the inner fjords, on average 40–60% of organic matter is derived from SPOM with the remainder contributed by estuarine algal production (). Similar contributions to blue cod nutrition are made by SPOM in outer Dusky Sound and Preservation Inlet, but in outer Breaksea Sound SPOM contributions are minimal and the majority of organic matter is derived from marine macroalgal production (E. radiata). Marine macroalgae are also an important organic matter source for blue cod in outer Dusky Sound, but in Preservation Inlet freshwater/estuarine macroalgal production constitutes the major organic matter source.
Table 1 δ13C and δ15N of Parapercis colias muscle tissue from each study site and results of mass balance mixing models estimating carbon contributions from each basal organic matter source pool. Data shown are the 95% confidence interval (mean±standard error).
Population structure
Inner fjord subpopulations were female-dominated in Breaksea Sound (Chi-square test, χ2=23.310, P<0.0001, d.f.=1), Dusky Sound (Chi-square test, χ2=40.076, P<0.0001, d.f.=1) and Long Sound–Preservation Inlet (Chi-square test, χ2=9.6240, P=0.0030, d.f.=1). Outer fjord subpopulations were female-dominated in Dusky Sound (Chi-square test, χ2=16.004, P<0.0001, d.f.=1) and male-dominated in Long Sound–Preservation Inlet (Chi-square test, χ2=51.062, P<0.0001, d.f.=1) but in Breaksea Sound the outer fjord sex ratio did not differ from 50:50 (Chi-square test, χ2=3.2140, P=0.0776, d.f.=1). Significant differences were observed between inner and outer fjord sex ratios in Breaksea Sound (Chi-square test, χ2=4.5920, P=0.0321, d.f.=1) and Long Sound–Preservation Inlet (Chi-square test, χ2=40.030, P<0.0001, d.f.=1), but in Dusky Sound sex ratio did not differ between habitats (Chi-square test, χ2=1.6300, P=0.2018, d.f.=1).
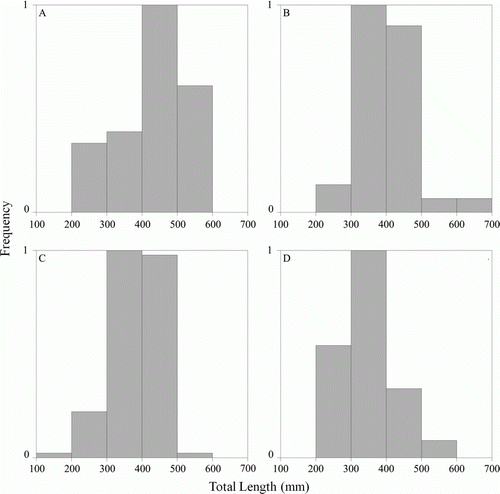
Size distributions differed between habitats overall (PERMANOVA, pseudo-F 1,267 =4.4185, P=0.0387) with a higher proportion of individuals in the larger size classes in the inner fjord habitat for both sexes ( and ).
Figure 5 A, Size distributions of male Parapercis colias in the inner fjords (n=39). B, Size distributions of male Parapercis colias in the outer fjords (n=60). C, Size distributions of female Parapercis colias in the inner fjords (n=94). D, Size distributions of female Parapercis colias in the outer fjords (n=44).
Growth models
Both male and female blue cod achieved greater asymptotic lengths (L ∞ ) in inner fjord habitats while the curvature of modelled growth trajectories (k) was higher among outer fjord subpopulations (); in Breaksea Sound the male growth model was compromised by the small sample which contained very few younger individuals (<9 yrs), particularly from the inner fjord. Overall, size-at-age was lower among inner fjord than outer fjord subpopulations for both males (PERMANOVA, pseudo-F 1,116 =12.037, P=0.0003) and females (PERMANOVA, pseudo-F 1,149 =18.755, P=0.0001). This pattern was upheld in Long Sound–Preservation Inlet (PERMANOVA, males: pseudo-F 1,51 =22.017, P=0.0002; females: pseudo-F 1,44 =30.626, P=0.0001) and for males in Breaksea Sound (PERMANOVA, pseudo-F 1,33 =5.5131, P=0.0134). Size-at-age did not differ between habitats for females in Breaksea Sound (PERMANOVA, pseudo-F 1,46 =1.6919, P=0.1858) or for either sex in Dusky Sound (PERMANOVA, males: pseudo-F 1,28 =0.8953, P=0.3699; females: pseudo-F 1,55 =2.3147, P=0.1146).
Table 2 Von Bertalanffy growth model parameters (L ∞ , k), residual sum of squares (RSS) and degrees of freedom (d.f.) for male and female fish sampled in inner and outer fjord habitats (pooled across years). t 0 was set at 0 for all models.
Model selection
Model selection was used to assess the statistical explanatory weighting of dietary (benthic versus pelagic feeding strategy), demographic (sex, condition) and site-specific factors (fjord, habitat) in determining individual size-at-age. Significant correlations were found between size-at-age and dietary (r 2 =0.03, P=0.0215), demographic (r 2 =0.04, P=0.0014) and site-specific factors (r 2 =0.18, P=0.0001). Diet explained a significant portion of the variability in size-at-age given that explained by demographics (r 2 =0.02, P=0.0415). Site-specific factors explained a significant portion of the variability in size-at-age given that explained by demographics and diet (r 2 =0.16, P=0.0001). Model selection revealed that site-specific factors (summed Akaike weight: 1.000) were collectively more important drivers of size-at-age than demographics (summed Akaike weight: 0.988) or diet (summed Akaike weight: 0.138), but the best model explained just 23% of the total variability in size-at-age ().
Table 3 Details of log-likelihood, number of parameters (K), AIC c , delta values, Akaike weights and variance explained (R 2 ) for models explaining variability in size-at-age.
Discussion
The results presented here provide direct evidence that spatial variability in growth trajectories of a coastal reef fish are strongly linked to differences in habitat and the nutritional landscape at a scale of 10–20 km. Spatial variability in composition and nutritional quality of diet as well as composition of basal organic matter sources supporting food webs was observed between multiple pairs of inner fjord and outer fjord/open coastal sites in Fiordland. Spatial patterns in these data largely corresponded with variability in condition and growth trajectories within subpopulations of blue cod observed among sites. In the inner fjords, the diet of blue cod typically comprised diverse, benthic resources, while in the outer fjords diets were more pelagic in nature and less diverse. Although the energy equivalent of sampled stomach contents was not measured, a review of published values for similar dietary components suggests that pelagic resources (i.e. zooplankton) are likely to be of a higher nutritional quality than benthic food sources such as detritus and invertebrates (Steimle & Terranova Citation1985; Dudgeon et al. Citation1990; Zoufal & Taborsky Citation1991). This spatial separation in diet composition was reflected by higher condition among individuals from outer fjord subpopulations in two of the fjords. Similar correspondence between blue cod trophic ecology and fish condition has been observed in Bradshaw–Thompson Sound (Beer Citation2011). In Long Sound–Preservation Inlet, no difference was observed between samples from inner and outer fjord habitats; here, diets in both habitats were more diverse than in the inner waters in Breaksea and Dusky Sounds, and fish condition was lower than elsewhere. This may be linked to the generally higher incidence of benthic resources (in particular gastropod molluscs) in stomach contents of fish from Preservation Inlet than those from the other outer fjord sites, as this benthic diet is likely to be nutritionally poorer than the more pelagic diet typical of other outer fjord sites. No evidence was found to suggest that growth of P. colias in the inner fjords is limited as a result of their diet.
Although isotopic mixing models are limited by the relevance of the input source values, they can provide some general insight into broadscale patterns of basal resource utilisation at higher trophic levels (Fry et al. Citation1999; Vander Zanden & Vadeboncoeur Citation2002). Analysis of the basal organic matter sources supporting blue cod prey suggest that Preservation Inlet also lacks significant inputs from common kelp, E. radiata, indicating a basic difference in the food web in this region. The unusually shallow entrance sill that separates the deeper waters of Preservation Inlet from the open Tasman Sea restricts wave action within the inlet and low-salinity surface water is retained within the shallow entrance sill (Stanton & Pickard Citation1981; Wing et al. Citation2003). As a consequence, the habitats near the entrance of Preservation Inlet are relatively wave-sheltered and have low density of kelps, particularly E. radiata (Wing et al. Citation2007). In contrast, semi wave-exposed habitats in Breaksea Sound and Dusky Sound host food webs dominated by E. radiata production typical of exposed coastal kelp forest habitats. Analysis of inputs of pelagic organic matter sources into food webs supporting blue cod indicates the importance of benthic–pelagic coupling, particularly in the inner fjord basins where phytoplankton production is relatively high (Goebel et al. Citation2005). These basic differences observed in the organic matter sources supporting local populations of blue cod propagate through the food web resulting in large differences in the nutritional quality of the prey base and correspond with observed spatial variability in growth and population structure among habitats.
Both males and females in outer fjord subpopulations approached asymptotic size (L ∞ ) faster than those in inner fjord subpopulations. Nevertheless, in the inner fjord habitats we observed a larger proportion of large, old individuals in the populations, and the asymptotic size was slightly larger in the inner fjord habitats. The proportion of individuals in the larger size classes is a product of the size frequency distribution, whereas the size-at-age is a result of the growth trajectory; the pattern observed indicates that inner fjord subpopulations had relatively fewer small recruits in the size distribution, but the asymptotic size was smaller. Therefore, although in the inner fjords individual growth is slow (and average size-at-age is low), there is a higher proportion of old growth in the population since individuals survive to populate the older age/larger size classes, albeit slowly. This may reflect differences in adult mortality between the two environments or differences in the allocation of resources to growth and reproduction among the female-dominated inner fjord subpopulations. Commercial and recreational fishing pressure, whilst relatively low throughout the study area compared with elsewhere around the South Island of New Zealand, has historically been concentrated along the open coast and in the entrances to Dusky Sound and Preservation Inlet (Davey & Hartill Citation2008; Starr & Kendrick Citation2008). Increased fishing pressure can result in a population structure that is biased towards faster growing, early maturing individuals, and in blue cod the constant removal of large males is thought to increase the incidence of sex change among females, resulting in a male-dominated population (Beentjes & Carbines Citation2005).
These observed patterns in population structure have important implications for local population productivity within each habitat and for yield-per-recruit estimation within the fishery management area. The adoption of spatial management of the blue cod fishery within Fiordland in 2005 highlighted the need to incorporate information on vital rates of subpopulations into management planning. In this case commercial fishing has been excluded from the inner fjord habitats, recreational take has been reduced and a series of 10 no-take marine reserves provides refugia for spawning stock in the inner fjord environments.
The present study demonstrates that baseline variability in the nutritional landscape can result in spatially structured populations with substantial variability in growth evident at the mesoscale (10–20 km) in the absence of significant anthropogenic disturbance. Management models for invertebrate fisheries such as abalone (Shepherd et al. Citation2001) and sea urchins (Quinn et al. Citation1993) commonly incorporate spatial variability in vital rates. Our findings highlight the importance of resolving spatial variability in vertebrate growth rates and of spatially-explicit management measures in the sustainable exploitation of temperate and tropical reef fishes, which typically form highly resident local populations. Failure to account for spatial variability in growth and other vital rates could result in overestimation of the productivity of an exploited population and runs the serious risk of localised depletion.
Ethical standards
This research complies with the current laws of New Zealand and the requirements of the University of Otago's Animal Ethics Committee.
Acknowledgements
We thank S. Lusseau, S. Rutger, K. Rodgers, B. Dickson, P. Hesseltine and P. Meredith for their assistance with sample collection. Monetary support was provided by the Royal Society of New Zealand's Hutton Fund (NAB), the Commonwealth Scholarship and Fellowship Plan (NAB), a University of Otago Postgraduate Publishing Bursary (NAB), and by a Royal Society of New Zealand Marsden Grant to SRW (UoO–00213).
References
- Akaike , H . 1974 . A new look at statistical model identification . IEEE Transactions on Automatic Control , 19 : 716 – 723 . doi: 10.1109/TAC.1974.1100705
- Anderson , DR , Link , WA , Johnson , DH and Burnham , KP . 2001 . Suggestions for presenting the results of data analyses . Journal of Wildlife Management , 65 : 373 – 378 . doi: 10.2307/3803088
- Anderson , MJ . 2001 . Permutation tests for univariate or multivariate analysis of variance and regression . Canadian Journal of Fisheries and Aquatic Sciences , 58 : 626 – 639 . doi: 10.1139/f01-004
- Anderson , MJ and Willis , TJ . 2003 . Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology . Ecology , 84 : 511 – 525 . doi: 10.1890/0012-9658(2003)084[0511:CAOPCA]2.0.CO;2
- Beentjes , MP and Carbines , G . 2005 . Population structure and relative abundance of blue cod (Parapercis colias) off Banks Peninsula and in Dusky Sound, New Zealand . New Zealand Journal of Marine and Freshwater Research , 39 : 77 – 90 . doi: 10.1080/00288330.2005.9517293
- Beer NA 2011 . Blue cod (Parapercis colias) population structure and connectivity in the New Zealand fjords . Unpublished thesis, Dunedin, University of Otago . 353
- Burnham KP , Anderson DR 1998 . Model selection and inference . New York , Springer-Verlag . 349
- Cailliet GM 1977 . Several approaches to the feeding ecology of fishes . In : Simenstad CA , Lipovsky SJ . Fish food habits studies . Proceedings of the 1st Pacific Northwest Technical Workshop , 13–15 October . Seattle , USA , Washington Sea Grant Program, University of Washington 1 – 13 .
- Campana , SE . 2001 . Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods . Journal of Fish Biology , 59 : 197 – 242 . doi: 10.1111/j.1095-8649.2001.tb00127.x
- Carbines , G . 1999 . Large hooks reduce catch-and-release mortality of blue cod Parapercis colias in the Marlborough Sounds of New Zealand . North American Journal of Fisheries Management , 19 : 992 – 998 . doi: 10.1577/1548-8675(1999)019%3C0992:LHRCAR%3E2.0.CO;2
- Carbines , G . 2004 . Age determination, validation, and growth of blue cod Parapercis colias, in Foveaux Strait, New Zealand . New Zealand Journal of Marine and Freshwater Research , 38 : 201 – 214 . doi: 10.1080/00288330.2004.9517231
- Carbines G , McKenzie J 2004 . Movement patterns and stock mixing of blue cod in Dusky Sound in 2002 . New Zealand Fisheries Assessment Report . 28
- Cole , RG , Villouta , E and Davidson , RJ . 2000 . Direct evidence of limited dispersal of the reef fish Parapercis colias (Pinguipedidae) within a marine reserve and adjacent fished areas . Aquatic Conservation: Marine and Freshwater Ecosystems , 10 : 421 – 436 . doi: 10.1002/1099-0755(200011/12)10:6%3C421::AID-AQC423%3E3.0.CO;2-E
- Cornelisen , CD , Wing , SR , Clark , KL , Bowman , MH , Frew , RD and Hurd , CL . 2007 . Patterns in the delta 13C and delta 15N signature of Ulva pertusa: interaction between physical gradients and nutrient source pools . Limnology and Oceanography , 52 : 820 – 832 . doi: 10.4319/lo.2007.52.2.0820
- Davey NK , Hartill B 2008 . A characterisation of recreational fisheries in the Fiordland Marine Area monitoring between 2006 and 2008 . Final Research Report . 42
- Dudgeon , D , Ma , HHT and Lam , PKS . 1990 . Differential palatability of leaf litter to four sympatric isopods in a Hong Kong forest . Oecologia , 84 : 398 – 403 .
- Fry , B , Mumford , P , Tam , F , Fox , D , Warren , G , Havens , K and Steinman , A . 1999 . Trophic position and individual feeding history of fish Lake Okeechobee, Florida . Canadian Journal of Fisheries and Aquatic Sciences , 56 : 590 – 600 . doi: 10.1139/f98-204
- Fry , B and Sherr , EB . 1984 . δ13C measurement as indicators of carbon flow in marine and freshwater ecosystems . Contributions in Marine Science , 27 : 13 – 47 .
- Glasby , GP . 1978 . Sedimentation and sediment geochemistry of Caswell, Nancy and Milford Sounds . New Zealand Oceanographic Institute Memoir , 79 : 19 – 37 .
- Goebel , NL , Wing , SR and Boyd , PW . 2005 . A mechanism for onset of diatom blooms in a fjord with persistent salinity stratification . Estuarine Coastal and Shelf Science , 64 : 546 – 560 . doi: 10.1016/j.ecss.2005.03.015
- Graham , DH . 1939 . Food of the fishes of Otago Harbour and adjacent seas . Transactions of the Royal Society of New Zealand , 68 : 421 – 436 .
- Hesslein , R , Hallard , K and Ramlal , P . 1993 . Replacement of sulfur, carbon, and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C and δ15N . Canadian Journal of Fisheries and Aquatic Sciences , 50 : 2071 – 2076 . doi: 10.1139/f93-230
- Hynes , HBN . 1950 . The food of freshwater sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a review of methods used in the study of food of fishes . Journal of Animal Ecology , 19 : 36 – 58 . doi: 10.2307/1570
- Jack , L and Wing , SR . 2010 . Maintenance of old-growth size structure and fecundity of the red rock lobster Jasus edwardsii among marine protected areas in Fiordland, New Zealand . Marine Ecology Progress Series , 404 : 161 – 172 . doi: 10.3354/meps08499
- Jack , L , Wing , SR and McLeod , RJ . 2009 . Prey base shifts in red rock lobster Jasus edwardsii in response to habitat conversion in Fiordland marine reserves: implications for effective spatial management . Marine Ecology Progress Series , 381 : 213 – 222 . doi: 10.3354/meps07971
- Jiang , WM and Carbines , G . 2002 . Diet of blue cod, Parapercis colias, living on undisturbed biogenic reefs and on seabed modified by oyster dredging in Foveaux Strait, New Zealand . Aquatic Conservation: Marine and Freshwater Ecosystems , 12 : 257 – 272 . doi: 10.1002/aqc.495
- Lawton , RJ , Wing , SR and Lewis , AM . 2010 . The New Zealand fjords contain discrete subpopulations of sea perch (Helicolenus percoides) . New Zealand Journal of Marine and Freshwater Research , 44 : 309 – 322 . doi: 10.1080/00288330.2010.519777
- McCutchan , JH , Lewis , WM , Kendall , C and McGrath , CC . 2003 . Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur . Oikos , 102 : 378 – 390 . doi: 10.1034/j.1600-0706.2003.12098.x
- McLeod , RJ and Wing , SR . 2007 . Hagfish in the New Zealand fjords are supported by chemoautotrophy of forest carbon . Ecology , 88 : 809 – 816 . doi: 10.1890/06-1342
- McLeod , RJ and Wing , SR . 2008 . Influence of an altered salinity regime on the population structure of two infaunal bivalve species . Estuarine Coastal and Shelf Science , 78 : 529 – 540 . doi: 10.1016/j.ecss.2008.01.019
- McLeod , RJ and Wing , SR . 2009 . Strong pathways for incorporation of terrestrially derived organic matter into benthic communities . Estuarine Coastal and Shelf Science , 82 : 645 – 653 . doi: 10.1016/j.ecss.2009.02.025
- McLeod , RJ , Wing , SR and Skilton , JE . 2010 . High incidence of invertebrate-chemoautotrophic symbioses in benthic communities of the New Zealand fjords . Limnology and Oceanography , 55 : 2097 – 2106 . doi: 10.4319/lo.2010.55.5.2097
- Miller , SM , Wing , SR and Hurd , CL . 2006 . Photoacclimation of Ecklonia radiata (Laminariales, Heterokontophyta) in Doubtful Sound, Fiordland, Southern New Zealand . Phycologia , 45 : 44 – 52 . doi: 10.2216/04-98.1
- Mutch PG 1983 . Factors influencing the density and distribution of blue cod (Parapercis colias) (Pisces: Mugiloidae) . Unpublished thesis , Auckland New Zealand University of Auckland . 76
- Orensanz JM , Jamieson GS 1998 . The assessment and management of spatially structured stocks: an overview of the North Pacific Symposium on Invertebrate Stock Assessment and Management . In : Jamieson GS , Campbell A . Proceedings of the North Pacific Symposium on Invertebrate Stock Assessment and Management . Ottawa , Canada , Canadian Science Publishing (NRC Research Press) . 441 – 459 .
- Phillips DL , Gregg JW 2001a . Uncertainty in source partitioning using stable isotopes (vol 172, pg 171, 2001) . Oecologia 128 : 304 – 304 . doi: 10.1007/s004420100723
- Phillips , DL and Gregg , JW . 2001b . Uncertainty in source partitioning using stable isotopes . Oecologia , 127 : 171 – 179 . doi: 10.1007/s004420000578
- Phillips , DL and Gregg , JW . 2003 . Source partitioning using stable isotopes: coping with too many sources . Oecologia , 136 : 261 – 269 . doi: 10.1007/s00442-003-1218-3
- Pickrill , RA . 1987 . Circulation and sedimentation of suspended particulate matter in the New Zealand fjords . Marine Geology , 74 : 21 – 39 . doi: 10.1016/0025-3227(87)90003-X
- Post , DM . 2002 . Using stable isotopes to estimate trophic position: models, methods, and assumptions . Ecology , 83 : 703 – 718 . doi: 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
- Post , DM , Layman , CA , Arrington , DA , Takimoto , G , Quattrochi , J and Montana , CG . 2007 . Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses . Oecologia , 152 : 179 – 189 . doi: 10.1007/s00442-006-0630-x
- Quinn , JF , Wing , SR and Botsford , LW . 1993 . Harvest refugia in marine invertebrate fisheries: models and applications to the red sea urchin, Strongylocentrotus fransiscanus . American Zoologist , 33 : 537 – 550 .
- Ricker , WE . 1975 . Computation and interpretation of biological statistics of fish populations . Bulletin of the Fisheries Research Board of Canada , 191 : 1 – 382 .
- Rodgers KL 2005 . Spatial structure of blue cod (Parapercis colias) populations in Doubtful Sound, Fiordland inferred from growth patterns and stable isotope signatures . Unpublished thesis , Dunedin University of Otago . 164
- Rodgers , KL and Wing , SR . 2008 . Spatial structure and movement of blue cod Parapercis colias in Doubtful Sound, New Zealand, inferred from delta 13C and delta 15N . Marine Ecology Progress Series , 359 : 239 – 248 . doi: 10.3354/meps07349
- Rutger , SM and Wing , SR . 2006 . Effects of freshwater input on shallow-water infaunal communities in Doubtful Sound, New Zealand . Marine Ecology Progress Series , 314 : 35 – 47 . doi: 10.3354/meps314035
- Shannon , CE . 1948 . A mathematical theory of communication . Bell System Technology Journal , 27 : 379 – 403 .
- Shepherd , SA , Rodda , KR and Vargas , KM . 2001 . A chronicle of collapse in two abalone stocks with proposals for precautionary management . Journal of Shellfish Research , 20 : 843 – 856 .
- Stanton , BR and Pickard , GL . 1981 . Physical oceanography of the New Zealand fiords . New Zealand Oceanographic Institute Memoir , 88 : 3 – 37 .
- Starr PJ , Kendrick TH 2008 . Review of the BCO 5 fishery . Report to South East Finfish Management Ltd . 51
- Steimle , FW and Terranova , RJ . 1985 . Energy equivalents of marine organisms from the continental shelf of the temperate northwest Atlantic . Journal of Northwest Atlantic Fishery Science , 6 : 117 – 124 . doi: 10.2960/J.v6.a11
- Suring , E and Wing , SR . 2009 . Isotopic turnover rate and fractionation in multiple tissues of red rock lobster (Jasus edwardsii) and blue cod (Parapercis colias): consequences for ecological studies . Journal of Experimental Marine Biology and Ecology , 370 : 56 – 63 . doi: 10.1016/j.jembe.2008.11.014
- Tallis , HM , Wing , SR and Frew , RD . 2004 . Historical evidence for habitat conversion and local population decline in a New Zealand fjord . Ecological Applications , 14 : 546 – 554 . doi: 10.1890/03-5104
- Thomson , GM . 1891 . Notes on sea-fishes . Transactions and Proceedings of the New Zealand Institute , 24 : 202 – 215 .
- Vander Zanden , MJ and Vadeboncoeur , Y . 2002 . Fishes as integrators of benthic and pelagic food webs in lakes . Ecology , 83 : 2152 – 2161 . doi: 10.1890/0012-9658(2002)083[2152:FAIOBA]2.0.CO;2
- von Bertalanffy , L . 1934 . Investigations on the rules of growth. Part I. General principles of the theory: mathematical and physiological laws of growth in aquatic animals . Wilhelm Roux Archiv Fűr Entwicklungsmechanik Der Organismen , 131 : 613 – 652 . doi: 10.1007/BF00650112
- Wing , SR . 2009 . Decadal-scale dynamics of sea urchin population networks in Fiordland, New Zealand are driven by juxtaposition of larval transport against benthic productivity gradients . Marine Ecology Progress Series , 378 : 125 – 134 . doi: 10.3354/meps07878
- Wing SR 2011 . Population networks with sources and sinks along productivity gradients in the Fiordland Marine Area, New Zealand: a case study on the sea urchin Evechinus choroticus . In : Liu J , Hull V , Morzillo A , Weins J . Sources, sinks, and sustainability . Cambridge , , UK , Cambridge University Press . 382 – 398 .
- Wing , SR and Leichter , JJ . 2011 . Variation in environmental conditions in a subtidal prey refuge: effects of salinity stress, food availability and predation on mussels in a fjord system . Marine Ecology Progress Series , 422 : 201 – 210 . doi: 10.3354/meps08911
- Wing , SR , Beer , NA and Jack , L . 2012 . Resource base of blue cod Parapercis colias subpopulations in marginal fjordic habitats is linked to chemoautotrophic production . Marine Ecology Progress Series , 466 : 205 – 214 . doi: 10.3354/meps09929
- Wing SR , Bowman MH , Smith F , Vennell R 2003 . Analysis of biodiversity and management in Fiordland: a case study . Report 1 of 3 to the Ministry for the Environment , New Zealand . 200
- Wing , SR , McLeod , RJ , Clark , KL and Frew , RD . 2008 . Plasticity in the diet of two echinoderm species across an ecotone: microbial recycling of forest litter and bottom-up forcing of population structure . Marine Ecology Progress Series , 360 : 115 – 123 . doi: 10.3354/meps07434
- Wing , SR , Leichter , JJ , Perrin , C , Rutger , SM , Bowman , MH and Cornelisen , CD . 2007 . Topographic shading and wave exposure influence morphology and ecophysiology of Ecklonia radiata (C. Agardh 1817) in Fiordland, New Zealand . Limnology and Oceanography , 52 : 1853 – 1864 . doi: 10.4319/lo.2007.52.5.1853
- Zoufal , R and Taborsky , M . 1991 . Fish foraging periodicity correlates with daily changes of diet quality . Marine Biology , 108 : 193 – 196 . doi: 10.1007/BF01344333