Abstract
The Australasian sea cucumber (Australostichopus mollis) has attracted commercial attention for aquaculture development, partly due to its potential for co-culture with shellfish and finfish species. However, minimal attention has been given to the possibility of co-culturing this species with oysters. In this study we evaluated the growth of juvenile sea cucumbers (36.7 ± 0.9 g, wet weight) caged underneath Pacific oyster farms in northern New Zealand. Co-culture started at the end of the summer, and after 304 days the juveniles had doubled in size (79.8 ± 3.3 g, wet weight), but their subsequent growth appeared to be constrained by overstocking of the cages and summer water temperatures, reaching a carrying capacity of 720 g m−2. Overall, the results of this study indicate that the co-culture of juvenile sea cucumbers with Pacific oysters is feasible, if sea cucumber losses are reduced (between 33% and 52% lost in this study) and careful attention is given to stocking rates and the water temperature regimes of oyster farms in order to maintain adequate growth rates.
Introduction
Increasing market demand and prices for sea cucumbers has led to overexploitation of wild stocks worldwide and triggered the development of commercial aquaculture of sea cucumbers (Toral-Granda et al. Citation2008). However, large scale commercial aquaculture has only been developed for the most valuable species; especially the temperate Japanese sea cucumber (Apostichopus japonicus) and the tropical sandfish (Holothuria scabra) (Huiling et al. Citation2004; Renbo & Yuan Citation2004; Xiyin et al. Citation2004; Duy Citation2012; Gamboa et al. Citation2012). The ongoing market demand for sea cucumbers has led to increased research efforts focused on hatchery and grow-out technology for other species with commercial aquaculture potential, including the Australasian sea cucumber Australostichopus mollis (Paltzat et al. Citation2008; Guisado et al. Citation2012; Jimmy et al. Citation2012; Mercier et al. Citation2012; Nelson et al. Citation2012; Zamora & Jeffs Citation2013). Australostichopus mollis is an aspidochirotid sea cucumber that is very similar in appearance to Apostichopus japonicus, and as a consequence fetches good market prices of over US$275 kg−1 dry weight (Purcell et al. Citation2012). This species can be found all along the coast of New Zealand and southern Australia, including the island of Tasmania (Pawson Citation1970). Since A. mollis is a deposit-feeding sea cucumber that utilises the waste of other animals, most of the current aquaculture efforts are directed towards co-culturing them with species that occupy different trophic levels, such as filter-feeding bivalves and abalone (Slater & Carton Citation2007; Stenton-Dozey Citation2007; Maxwell et al. Citation2009).
Co-culture of deposit-feeding and suspension-feeding sea cucumbers together with mussels, oysters, scallops, abalone, shrimps and finfish in both land-based and open water culture systems has been tested throughout the world, with varying degrees of success (Ahlgren Citation1998; Kang et al. Citation2003; Zhou et al. Citation2006; Bell et al. Citation2007; Paltzat et al. Citation2008; Ren et al. Citation2012; Yokoyama Citation2013). In Australasia the significant mussel farming industry (Perna canaliculus and Mytilus galloprovincialis) is pursuing the possibility of growing A. mollis under mussel farms with the benefits being extensively researched (Slater & Carton Citation2007, Citation2009, Citation2010; Slater et al. Citation2009; Zamora & Jeffs Citation2011, Citation2012a,Citationb; MacTavish et al. Citation2012). This research has shown that sea cucumbers grow well beneath mussel farms, whilst also reducing the organic enrichment of the benthic sediment due to the mussel farming activity (Zamora & Jeffs Citation2013). Despite the finfish and oyster aquaculture industries being of considerable size in Australasia, they are yet to fully examine the potential for co-culturing sea cucumbers (Nell Citation2001; Forrest et al. Citation2007; King & Lake Citation2013). The co-culture of oysters and sea cucumbers has shown considerable promise for both Apostichopus japonicus and Parastichopus californicus (Zhou et al. Citation2006; Paltzat et al. Citation2008; Yokoyama Citation2013). Moreover, in some initial experimental work, it has been shown that caged A. mollis grow faster in sites in close proximity to oyster farms than those located at some distance away (Slater & Jeffs Citation2010). Therefore, the aim of this study was to evaluate the possibility of growing caged juvenile A. mollis together with Pacific oysters (Crassostrea gigas) in northern New Zealand, by determining the retention and growth of the sea cucumbers under the oyster farms.
Materials and methods
Site description
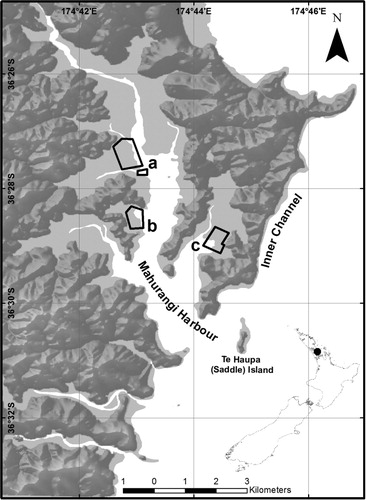
The current study was conducted under three oyster farms located in the Mahurangi Harbour, a 25 km2 shallow (> 15 m) and narrow harbour on the northeastern coast of New Zealand (). The Mahurangi Harbour encompasses a wide variety of benthic habitats, such as sand, mud and shell dominated sediments. The oyster farms selected for this study are located on intertidal mudflats in Dyers (36°27′17.6″ S, 174°42′57.10″ E), Brownes Bay (36°28′26.74″ S, 174°42′57.01″ E) and Te Kapa (36°28′58.53″ S, 174°44′12.01″ E) ().
The oyster farms at Te Kapa and Dyers grow oysters attached to horizontal wooden sticks that are held within the intertidal range on permanent wooden stands, which together is commonly known as the rack and rail oyster farming system. The oyster farm at Brownes Bay uses the same permanent wooden stands, but the oysters were held in the intertidal range in large plastic mesh bags. All three oyster farms have a continual cycle of harvesting larger oysters (> 90 mm shell height) and seeding out juvenile oysters (20–30 mm shell height), although within farms this activity is staggered so that there are always oysters of different sizes in the farms.
Collection of experimental juvenile sea cucumbers
In January 2011 around 100 juvenile sea cucumbers were collected by divers from the Mahurangi Harbour, near the oyster farms (). The sea cucumbers were transferred immediately in a 100 L plastic tank half-filled with fresh seawater from the collection site to the nearby Leigh Marine Laboratory. Once in the laboratory the juveniles were held in tanks with flowing 100 µm filtered seawater at ambient temperature. The collected juveniles were unfed for 48 h to ensure that the gut contents were fully evacuated. The sea cucumbers were then weighed to the nearest gram, after the excess water from the respiratory tree was removed by gently squeezing the posterior half of each animal and the external water was blot dried (Sewell Citation1990). Only individuals weighing between 30 and 40 g were selected, since they were unlikely to be able to pass through the mesh of the experimental culture cages. The selected sea cucumbers were photo-identified so that individuals could continue to be recognised throughout the experiment from their pattern of body markings (Raj Citation1998).
Co-culture conditions
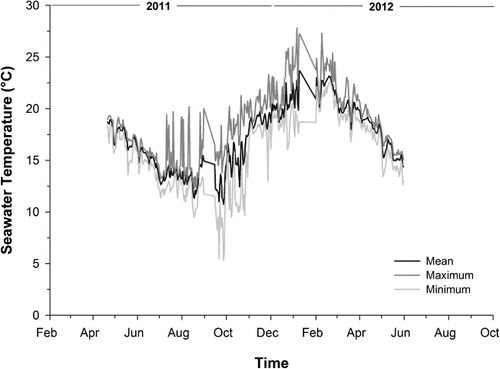
The juveniles were randomly allocated into groups of four animals and assigned to 18 plastic cages (1.0 × 0.5 × 0.15 m, L × W × H). The cages consisted of plastic mesh (10 mm squares) formed into a cuboid shape, but with the base buried into the benthic sediments. Six cages were deployed directly beneath each of the three oyster farms (Dyers, Brownes Bay and Te Kapa) in subtidal locations. The mean vertical distance between the farmed oysters and the sea cucumber cages was 1.5 m. The experiment started the second week of February 2011 and finished at the end of October 2012 (i.e. 619 days). The sea cucumbers were identified, reweighed and the cages cleaned and repaired after 61, 194, 304, 432 and 619 days from the start of the experiment. Any missing sea cucumbers were replaced by individuals of similar size in order to maintain the stocking biomass of each cage. Seawater temperature loggers (HOBO pro-V2) were placed on the cages in order to have better understanding of the temperature regime to which the juveniles were exposed during the experimental period.
At day 469, the sea cucumbers from one of the plastic cages at each of the three farm sites were separated into two equivalent size mesh cages (i.e. two sea cucumbers in each cage) in order to determine if growth would be affected by halving the stocking biomass in the cages.
Sea cucumber growth
Measurements of sea cucumber wet weight were used to measure the individual daily specific growth rate (DSGR) of the sea cucumbers at each farm site as per Ricker (Citation1979):
Statistical analyses
Differences in wet weight and growth rates within farms at different times and among farms within a specific sampling time were tested with a Kruskal-Wallis ANOVA on ranks, since data were found to be nonparametric after testing for normality and homogeneity of variance using a Shapiro-Wilk and a Levene's test, respectively. When significant differences were detected, considering unequal sample sizes, a Dunn's post-hoc comparison of means was performed to identify the source of the differences. A Mann-Whitney test was performed to compare the mean wet weight change of the juveniles from day 469 to 619 for those that were exposed to a density reduction within each farm. A Mann-Whitney test was also used to compare the mean wet weight of the juveniles that were exposed to a density reduction with those without a density reduction for each oyster farm.
Results
Sea cucumbers weight and growth rates
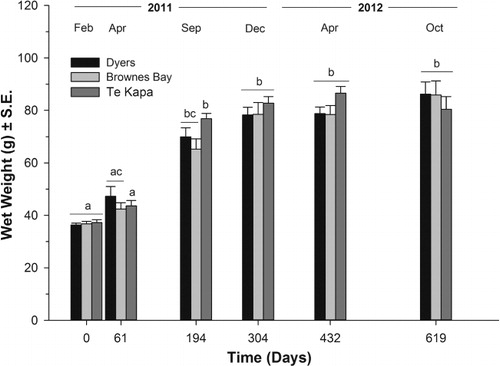
Overall, the sea cucumbers showed significant weight gains when co-cultured in all three oyster farms for the duration of the experiment (Kruskal-Wallis, H = 67.97, df = 5, P < 0.01 for Dyers; Kruskal-Wallis, H = 76.83, df = 5, P < 0.01 for Brownes Bay; and Kruskal-Wallis, H = 74.58, df = 5, P < 0.01 for Te Kapa) (). However, the growth of sea cucumbers beyond day 304 was not significant (Dunn's, P > 0.05 for all oyster farms) (). There were no significant differences in the wet weight of the sea cucumbers among farms, except at day 194 (Kruskal-Wallis, H = 6.15, df = 2, P = 0.04) when the sea cucumbers held under the Te Kapa farm were slightly heavier than those placed under the Brownes Bay farm (Dunn's, P < 0.05) ().
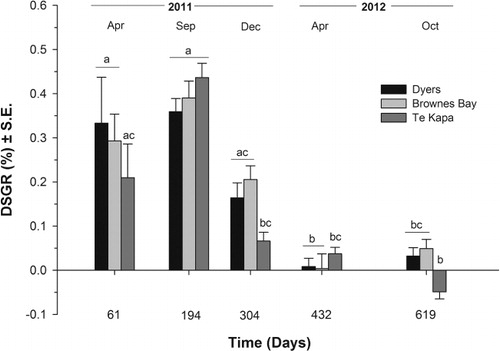
The DSGR of the juvenile sea cucumbers changed significantly over time at all three farm sites (Kruskal-Wallis, H = 34.42, df = 4, P < 0.01 for Dyers; Kruskal-Wallis, H = 38.63, df = 4, P < 0.01 for Brownes Bay; and Kruskal-Wallis, H = 34.42, df = 4, P < 0.01 for Te Kapa) (). In all oyster farms the DSGR was significantly higher during the first half of the experiment (until day 304) than in the second half (day 304–619), reaching a peak of growth during the period between day 61 and 194 (Dunn's, P < 0.05 for all cases) (). After day 304 any changes in DSGR were marginal, with sea cucumbers losing weight during the last sampled period (day 432–619), especially those under the Te Kapa farm (). There were significant differences in DSGR of the sea cucumbers among farms only at day 304 (Kruskal-Wallis, H = 7.69, df = 2, P < 0.02) and at day 619 (Kruskal-Wallis, H = 16.12, df = 2, P < 0.01). At day 304 the DSGR of the juveniles under the Te Kapa farm was higher than under the Brownes Bay farm (Dunn's, P < 0.05), and at day 619 the DSGR of the juveniles under the Te Kapa farm was significantly lower than for the other two oyster farms (Dunn's, P < 0.05 in both cases) ().
Reduction of density results
Halving the stocking biomass of sea cucumbers in the cages at each farm site resulted in an increase of the wet weight of the sea cucumbers from day 469 to day 619, although this increase was significant only for the juveniles under the Te Kapa oyster farm (Mann-Whitney, U: 0.0, P = 0.03) (). However, there were no significant differences in the DSGR among all the farm sites for only the cages where the density had been reduced (Kruskal-Wallis, H = 0.93, df = 2, P = 0.67) ().
Table 1 Mean wet weight (WW) and daily specific growth rate (DSGR) of the sea cucumbers Australostichopus mollis that were provided with a reduction in culture density during the last period of the experiment (day 469–619) in all three Pacific oyster farms (Dyers, Brownes Bay, Te Kapa).
At day 619 the sea cucumbers that were exposed to a biomass reduction had a higher wet weight than those that were not exposed to the reduction; however, significant higher biomass were only found for sea cucumbers at the Te Kapa farm (Mann-Whitney, U: 4.0, P = 0.02). Similarly, the overall DSGR of the juveniles that were exposed to a reduction in stocking biomass tended to be higher than the DSGR of the juveniles without a reduction at day 619; however, significant differences between cages with the two different stocking rates were only detected at the Te Kapa farm (Kruskal-Wallis, H = 26.75, df = 3, P < 0.01, Dunn's, P < 0.05).
Losses of juvenile sea cucumbers
It was not possible to measure survival in the experiment since no sea cucumber carcasses were found at any time in any cage when an individual animal was found to be missing. Over the course of the experiment (619 days) 52% of the total number of sea cucumbers used (including replacements) were lost at Dyers, 38% at Brownes Bay and 33% at Te Kapa. Most of the sea cucumbers (80% for Dyers, 50% for Brownes Bay and 100% for Te Kapa of the total lost sea cucumbers) went missing during the first 61 days of the experiment, during which time four entire cages were lost from the experiment, two cages in Dyers and two cages in Te Kapa, which were not replaced and corresponded to 50% and 100% of the sea cucumbers lost in each farm respectively.
Discussion
In this study we demonstrated that juvenile A. mollis can grow and survive when caged beneath Pacific oyster farms in northern New Zealand. Although this study does not provide direct evidence of the sea cucumbers actually being able to use the oyster farm waste, our results indicate that the sediments enriched by the organic waste from the farms are suitable for the sea cucumbers growth, depending on variables such as seawater temperature and stocking biomass.
Sea cucumber losses under co-culture conditions
Considerable numbers of juvenile sea cucumbers were lost from the experimental cages for reasons that were not clear. With the current cage design it is impossible to determine whether losses were due to escapes, predation or mortalities due to stress, injury or diseases. This species does not have many natural predators, and no diseases or predators were observed in the remaining sea cucumbers at any time (Sewell Citation1990). Escapes could have been measured if outer cages were placed around the experimental cages; however, this was not practical as it would have interfered with the operation of the oyster farm. Many of the juvenile losses were associated with the disappearance of the entire experimental cages, which was likely to be caused by oyster farm operations such as shallow water vessel movements snagging on the cages. This suggests that future co-culture efforts may need changes to oyster farming operations in order to take into account the presence of the sea cucumber cages beneath the oyster farm. The losses of juveniles from intact experimental cages (21%–38%) was comparatively higher than for the sea cucumbers P. californicus (0%) and A. japonicus (4%) co-cultured on suspended trays in Pacific oyster farms in Canada and Japan respectively (Paltzat et al. Citation2008; Yokoyama Citation2013). These two other species were in suspended culture systems so that not having the cages in contact with the sediments may have helped to reduce escapes of the juveniles, although it could create other issues such as agitation due to the influence of tidal flows on the cages. The majority of the juvenile sea cucumbers in the current study were lost from cages during the first two months of the experiment, which could be due to the stress of handling and transfer into the experiment or due to an ability of these smaller sized animals to escape through the plastic enclosures onto the surrounding sediment. Once the juvenile sea cucumbers reached over 60 g of wet weight there were almost no further losses from the experiment. This indicates that cage design and maintenance would be very important in order to avoid sea cucumber escapes for a commercial aquaculture operation of this type (Purcell & Agudo Citation2013).
The importance of seawater temperature for sea cucumber growth
The juvenile sea cucumbers grew very well under the experimental conditions, almost doubling in size from an average of 36.8 ± 0.5 g (mean ± SEM) to 70.1 ± 1.9 g (mean ± SEM) over six months of experimental culture. After this time their growth slowed dramatically, with the DSGR of sea cucumbers across all farms declining from nearly 0.4% d−1 (between day 61 and 194) to 0.01% d−1 (between day 304 and 432). This marked decline in growth rates could be related to seasonal increase in seawater temperatures, which increased from a low around 13 °C in winter to around 21 °C in summer (). It has been previously shown that seawater temperature affects the feeding behaviour and growth of sea cucumbers under co-culture conditions with bivalves (Zhou et al. Citation2006; Paltzat et al. Citation2008). In the case of A. mollis, seawater temperature has been found to be particularly important in the growth of juveniles growing in both the wild and in the laboratory (Slater et al. Citation2009; Slater & Jeffs Citation2010). Maxwell et al. (Citation2009) showed that seawater temperature also affects the energy available for growth depending on the food given to the juveniles. Furthermore, subsequent studies on juvenile A. mollis proved that the feeding behaviour, nutrient absorption, physiology and growth become negatively affected at higher seawater temperatures typically found during summer (i.e. > 15 °C) in northern New Zealand (Zamora & Jeffs Citation2012b, Citation2014a). The negative effect of elevated seawater temperatures on the growth of A. mollis is not as extreme as in A. japonicus and P. californicus which suffer greater physiological and behavioural consequences due to seasonal changes in seawater temperature (Ji et al. Citation2008; Paltzat et al. Citation2008). Nonetheless, this information is useful for the selection of suitable aquaculture sites that should be naturally exposed to cooler seawater temperature regimes throughout the year in order to enhance the growth of juvenile A. mollis, for which growth rates can be as high as 0.7% d−1 at 15 °C (Zamora & Jeffs Citation2012b). Pacific oysters are not only cultured in the warmer coastal waters of northern New Zealand, but also in the colder water regions of southern New Zealand and Australia, which could be more suitable for co-culture of sea cucumbers (Nell Citation2001; Forrest et al. Citation2007). Perhaps oyster farms located in deeper waters should be chosen for co-culture of sea cucumbers because shallow water farms would be exposed to more daily and seasonal variations in surface seawater temperatures, which would be detrimental to juvenile sea cucumbers. Paying attention to the natural seasonal seawater temperature variation would also be useful to determine the best time of year to transfer the juveniles for grow-out in order to optimise growth (Zamora & Jeffs Citation2014b). For example, in this study, the growth of the juveniles during the first two months of co-culture across all of the farms was not as good (0.27% d−1) as in the next period (day 61–194) in which seawater temperatures were considerably lower (i.e. > 5 °C lower) ().
Sea cucumber stocking densities and growth
According to our results, temperature was not the only factor affecting growth of the sea cucumbers, since the DSGR of the sea cucumbers did not improve greatly after the summer, only recovering to around 0.02% d−1 (between day 432 and 619). This suggested that the growth limitation may have been due to the depletion of food resources within the experimental cages, possibly as a result of the stocking biomass exceeding the rate of replenishment of the organic matter from the overlying oyster farm (Forrest & Creese Citation2006). In the current study, the mean biomass of the juvenile A. mollis for all experimental cages went from 294 g m−2 at the outset of the experiment to 560 g m−2 after six months of co-culture, and reaching 720 g m−2 four months later. After this time (day 304) the sea cucumbers almost stopped growing, most likely because food and/or space became limiting factors for growth and thus reaching the carrying capacity in terms of biomass of sea cucumber per unit of area of the cages used under the oyster farms. This explanation is supported by the increased growth of sea cucumbers in cages where the density of animals was halved at the end of the experiment (for day 469 to 619). Estimations of carrying capacity have been reported for H. scabra and they appear to vary widely (100 to 692 g m−2) due to the holding conditions (tanks, sea pens or ponds) and also the location and time of the year (Battaglene et al. Citation1999; Purcell & Simutoga Citation2008; Lavitra et al. Citation2010; Robinson & Pascal Citation2012). Even higher values of carrying capacity have been reported for adult A. mollis (109 g wet weight) being caged under a mussel farm, with their growth being compromised and losing weight at a stocking biomass around 1500 g m−2 (Slater & Carton Citation2007). Also, juvenile A. mollis kept in tanks and fed mussel waste under near ideal holding conditions in the laboratory can generate growth from an initial biomass of 857 g m−2 to 1885 g m−2 in three months without compromising their growth rates (Zamora & Jeffs Citation2012b). Conversely, when the biomass of juvenile A. mollis reached 165 g m−2 after being caged for six months in locations near to the oyster farms used in this experiment their growth rate (0.5% d−1) started to decrease, most likely due to overstocking (Slater & Jeffs Citation2010). Therefore, Pacific oyster farms appear capable of supporting higher stocking biomass of juvenile sea cucumbers while delivering similar growth rates when compared with nearby sites that are not affected by the presence of the oyster aquaculture activity (Slater & Jeffs Citation2010). However, stocking biomass and growth rates of the juvenile sea cucumbers beneath Pacific oyster farms appear lower than have been observed for mussel farms, most probably due to differences in the organic loadings from the two different shellfish farming activities (Slater & Carton Citation2007; Zamora & Jeffs Citation2012a). These differences in food availability between oyster and mussel farms could also be partly due to the effect of stronger tidal water movements to which the oyster farms are exposed on a daily basis, which would reduce the organic matter accumulation under these farms (Forrest & Creese Citation2006).
Conclusions
This is the first study reporting the growth of juveniles of the Australasian sea cucumber Australostichopus mollis under Pacific oyster farms. Our results suggest that co-culturing juvenile sea cucumbers with oysters is feasible, with juvenile sea cucumbers (starting at around 40 g) potentially reaching harvestable sizes (i.e. > 95 g) after two years. However, optimum stocking densities, variation in growth response to water temperature and more effective culture holding systems for commercial-scale aquaculture need to be established through further research. In addition, it needs to be determined whether or not this co-culture system with Pacific oysters can be used to grow larger adult sea cucumbers (> 95 g) at the densities required for commercial aquaculture.
Acknowledgements
This research was supported by the University of Auckland in New Zealand, the Glenn Family Foundation and the Comisión Nacional de Investigación Científica y Tecnológica of Chile. We would like to thank the staff members of Biomarine Ltd for helping with access to the oyster farms, lending equipment and helping out during the data collection. We are also grateful to Fabio Picinato and Natali Delorme who helped with setting up the experiment and during the data collection.
References
- Ahlgren MO 1998. Consumption and assimilation of salmon net pen fouling debris by the red sea cucumber Parastichopus californicus: implications for polyculture. Journal of the World Aquaculture Society 29: 133–139. 10.1111/j.1749-7345.1998.tb00972.x
- Battaglene SC, Seymour JE, Ramofafia C 1999. Survival and growth of cultured juvenile sea cucumbers Holothuria scabra. Aquaculture 178, 293–322. 10.1016/S0044-8486(99)00130-1
- Bell JD, Agudo NN, Purcell SW, Blazer P, Simutoga M, Pham D et al. 2007. Grow-out of sandfish Holothuria scabra in ponds shows that co-culture with shrimp Litopenaeus stylirostris is not viable. Aquaculture 273: 509–519. 10.1016/j.aquaculture.2007.07.015
- Duy NDQ 2012. Large scale sandfish production from pond culture in Vietnam. In: Hair CA, Pickering TD, Mills DJ eds. Asia–Pacific tropical sea cucumber aquaculture. Proceedings of an international symposium held in Noumea, New Caledonia. Canberra, ACIAR Proceedings No. 136. Pp. 34–39.
- Forrest BM, Creese RG 2006. Benthic impacts of intertidal oyster culture with consideration of taxonomic sufficiency. Environmental Monitoring and Assessment 112: 159–176. 10.1007/s10661-006-0359-3
- Forrest B, Keeley N, Gillespie P, Hopkins G, Knight B, Govier D 2007. Review of the ecological effects of marine finfish aquaculture: final report. Prepared for Ministry of Fisheries. Cawthron Report No. 1285. Nelson, Cawthron Institute. 71 p.
- Gamboa RU, Aurelio RM, Ganad DA, Concepcion LB, Abreo NA 2012. Small-scale hatcheries and simple technologies for sandfish (Holothuria scabra) production. In: Hair CA, Pickering TD, Mills DJ eds. Asia–Pacific tropical sea cucumber aquaculture. Proceedings of an international symposium held in Noumea, New Caledonia. Canberra, ACIAR Proceedings No. 136. Pp. 63–74.
- Guisado C, Carrasco SA, Diaz-Guisado D, Maltrain R, Rojas H 2012. Embryonic development, larval morphology and juvenile growth of the sea cucumber Athyonidium chilensis (Holothuroidea: Dendrochirotida). Revista de Biologia Marina y Oceanografia 47: 65–73. 10.4067/S0718-19572012000100006
- Huiling S, Mengqinq L, Jingping Y, Bijuan C 2004. Nutrient requirements and growth of the sea cucumber, Apostichopus japonicus. In: Lovatelli A, Conand C, Purcell S, Uthicke S, Hamel JF, Mercier A eds. Advances in sea cucumber aquaculture and management. FAO Fisheries technical paper No. 463. Rome, Food and Agriculture Organization of the United Nations. Pp. 327–331.
- Ji T, Dong Y, Dong S 2008. Growth and physiological responses in the sea cucumber, Apostichopus japonicus Selenka: aestivation and temperature. Aquaculture 283: 180–187. 10.1016/j.aquaculture.2008.07.006
- Jimmy RA, Pickering TD, Hair CA 2012. Overview of sea cucumber aquaculture and stocking research in the Western Pacific region. In: Hair CA, Pickering TD, Mills DJ eds. Asia–Pacific tropical sea cucumber aquaculture. Proceedings of an international symposium held in Noumea, New Caledonia. Canberra, ACIAR Proceedings No. 136. Pp. 12–21.
- Kang KH, Kwon JY, Kim YM 2003. A beneficial coculture: charm abalone Haliotis discus hannai and sea cucumber Stichopus japonicus. Aquaculture 216: 87–93. 10.1016/S0044-8486(02)00203-X
- King N, Lake R 2013. Bivalve shellfish harvesting and consumption in New Zealand, 2011: data for exposure assessment. New Zealand Journal of Marine and Freshwater Research 47: 62–72. 10.1080/00288330.2012.744319
- Lavitra T, Rasolofonirina R, Eeckhaut I 2010. The effect of sediment quality and stocking density on survival and growth of the sea cucumber Holothuria scabra reared in nursery ponds and sea pens. Western Indian Ocean Journal of Marine Science 9: 153–164.
- MacTavish T, Stenton-Dozey J, Vopel K, Savage C 2012. Deposit-feeding sea cucumbers enhance mineralization and nutrient cycling in organically-enriched coastal sediments. PloS ONE 7: 1–11. 10.1371/journal.pone.0050031
- Maxwell K, Gardner J, Heath P 2009. The effect of diet on the energy budget of the brown sea cucumber, Stichopus mollis (Hutton). Journal of the World Aquaculture Society 40: 157–170. 10.1111/j.1749-7345.2009.00239.x
- Mercier A, Ycaza RH, Ezpinoza R, Arriaga VM, Hamel J-F 2012. Hatchery experience and useful lessons from Isostichopus fuscus in Ecuador and Mexico. In: Hair CA, Pickering TD, Mills DJ eds. Asia–Pacific tropical sea cucumber aquaculture. Proceedings of an international symposium held in Noumea, New Caledonia. Canberra, ACIAR Proceedings No. 136. Pp. 79–90.
- Nell JA 2001. The history of oyster farming in Australia. Marine Fisheries Review 63: 14–25.
- Nelson EJ, MacDonald BA, Robinson SMS 2012. A review of the northern sea cucumber Cucumaria frondosa (Gunnerus, 1767) as a potential aquaculture species. Reviews in Fisheries Science 20: 212–219. 10.1080/10641262.2012.719043
- Paltzat DL, Pearce CM, Barnes PA, McKinley RS 2008. Growth and production of California sea cucumbers (Parastichopus californicus Stimpson) co-cultured with suspended Pacific oysters (Crassostrea gigas Thunberg). Aquaculture 275: 124–137. 10.1016/j.aquaculture.2007.12.014
- Pawson DL 1970. The marine fauna of New Zealand: sea cucumbers (Echinodermata: Holothuroidea). New Zealand Department of Scientific and Industrial Research Bulletin 201: 1–70.
- Purcell SW, Agudo NS 2013. Optimisation of mesh enclosures for nursery rearing of juvenile sea cucumbers. PLoS ONE 8: e64103. 10.1371/journal.pone.0064103
- Purcell SW, Samyn Y, Conand C 2012. Commercially important sea cucumbers of the world. FAO species catalogue for fishery purposes No 6. Rome, Food and Agriculture Organization of the United Nations. 150 p.
- Purcell SW, Simutoga M 2008. Spatio-temporal and size-dependent variation in the success of releasing cultured sea cucumbers in the wild. Reviews in Fisheries Science 16: 204–214. 10.1080/10641260701686895
- Raj LK 1998. Photo-identification of Stichopus mollis. SPC Beche-de Mer Information Bulletin 10: 29–31.
- Ren Y, Dong S, Chuanxin Q, Wang F, Tian X, Gao Q 2012. Ecological effects of co-culturing sea cucumber Apostichopus japonicus (Selenka) with scallop Chlamys farreri in earthen ponds. Chinese Journal of Oceanology and Limnology 30: 71–79. 10.1007/s00343-012-1038-6
- Renbo W, Yuan C 2004. Breeding and culture of the sea cucumber, Apostichopus japonicus. Liao. In: Lovatelli A, Conand C, Purcell S, Uthicke S, Hamel J-F, Mercier A eds. Advances in sea cucumber aquaculture and management. FAO Fisheries technical paper No. 463. Rome, Food and Agriculture Organization of the United Nations. Pp. 277–286.
- Ricker WE 1979. Growth rates and models. In: Hoar WS, Randall DJ, Brett JR eds. Fish physiology. New York, Academic Press. Pp. 677–743.
- Robinson G, Pascal B 2012. Sea cucumber farming experiences in south-western Madagascar. In: Hair CA, Pickering TD, Mills DJ eds. Asia–Pacific tropical sea cucumber aquaculture. Proceedings of an international symposium held in Noumea, New Caledonia. Canberra, ACIAR Proceedings No. 136. Pp. 142–145.
- Sewell MA 1990. Aspects of the ecology of Stichopus mollis (Echinodermata: Holothuroidea) in north-eastern New Zealand. New Zealand Journal of Marine and Freshwater Research 24: 97–103. 10.1080/00288330.1990.9516405
- Slater MJ, Carton AG 2007. Survivorship and growth of the sea cucumber Australostichopus (Stichopus) mollis (Hutton 1872) in polyculture trials with green-lipped mussel farms. Aquaculture 272: 389–398. 10.1016/j.aquaculture.2007.07.230
- Slater MJ, Carton AG 2009. Effect of sea cucumber (Australostichopus mollis) grazing on coastal sediments impacted by mussel farm deposition. Marine Pollution Bulletin 58: 1123–1129. 10.1016/j.marpolbul.2009.04.008
- Slater MJ, Carton AG 2010. Sea cucumber habitat differentiation and site retention as determined by intraspecific stable isotope variation. Aquaculture Research 41: 695–702.
- Slater MJ, Jeffs AG 2010. Do benthic sediment characteristics explain the distribution of juveniles of the deposit-feeding sea cucumber Australostichopus mollis? Journal of Sea Research 64: 241–249. 10.1016/j.seares.2010.03.005
- Slater MJ, Jeffs AG, Carton AG 2009. The use of the waste from green-lipped mussels as a food source for juvenile sea cucumber, Australostichopus mollis. Aquaculture 292: 219–224. 10.1016/j.aquaculture.2009.04.027
- Stenton-Dozey J 2007. Finding hidden treasure in aquaculture waste. Water Atmosphere 15: 9–11.
- Toral-Granda V, Lovatelli A, Vasconcellos M Editors. 2008. Sea cucumbers. A global review of fisheries and trade. Fisheries and aquaculture technical paper No 516. Rome, FAO. 317 p.
- Xiyin L, Guanhui Z, Quiang Z, Liang W, Benxue G 2004. Studies on hatchery techniques of the sea cucumber, Apostichopus japonicus. In: Lovatelli A, Conand C, Purcell S, Uthicke S, Hamel J-F Mercier A eds. Advances in sea cucumber aquaculture and management. FAO Fisheries technical paper No. 463. Rome, Food and Agriculture Organization of the United Nations. Pp. 287–296.
- Yokoyama H 2013. Growth and food source of the sea cucumber Apostichopus japonicus cultured below fish cages – potential for integrated multi-trophic aquaculture. Aquaculture 372–375: 28–38. 10.1016/j.aquaculture.2012.10.022
- Zamora LN, Jeffs AG 2011. Feeding, selection, digestion and absorption of the organic matter from mussel waste by juveniles of the deposit-feeding sea cucumber, Australostichopus mollis. Aquaculture 317: 223–228. 10.1016/j.aquaculture.2011.04.011
- Zamora LN, Jeffs AG 2012a. The ability of the deposit-feeding sea cucumber Australostichopus mollis to use natural variation in the biodeposits beneath mussel farms. Aquaculture 326–329: 116–122. 10.1016/j.aquaculture.2011.11.015
- Zamora LN, Jeffs AG 2012b. Feeding, metabolism and growth in response to temperature in juveniles of the Australasian sea cucumber, Australostichopus mollis. Aquaculture 358–359: 92–97. 10.1016/j.aquaculture.2012.06.024
- Zamora LN, Jeffs AG 2013. A review of the research on the Australasian sea cucumber Australostichopus mollis (Echinodermata: Holothuroidea) (Hutton 1872) with emphasis on aquaculture. Journal of Shellfish Research 32: 613–627. 10.2983/035.032.0301
- Zamora LN, Jeffs AG 2014a. Macronutrient selection, absorption and energy budget of juveniles of the Australasian sea cucumber, Australostichopus mollis, feeding on mussel biodeposits at different temperatures Aquaculture Nutrition. doi:10.1111/anu.12144
- Zamora LN, Jeffs AG 2014b. Evaluation of transportation methods of juveniles of the Australasian sea cucumber, Australostichopus mollis. Aquaculture Research. doi:10.1111/are.12400
- Zhou Y, Yang H, Liu S, Yuan X, Mao Y, Liu Y et al. 2006. Feeding and growth on bivalve biodeposits by the deposit feeder Stichopus japonicus Selenka (Echinodermata: Holothuroidea) co-cultured in lantern nets. Aquaculture 256: 510–520. 10.1016/j.aquaculture.2006.02.005