ABSTRACT
We describe two new species in the genus Gobiomorphus, a radiation of fresh and brackish water gudgeons known from Australia and New Zealand. These species are a prominent component of New Zealand’s freshwater ichthyofauna and most are widely distributed throughout both the North and South Islands. Two of the inland species, G. breviceps and G. basalis, are composed of disjunct northern and southern populations that are distinguishable with molecular data. We examine individuals from across the ranges of both species, identify morphological differences between them, and describe two new species: Gobiomorphus dinae n. sp. (distinct from G. basalis) and Gobiomorphus mataraerore n. sp. (distinct from G. breviceps). Although the species are similar, they vary in dorsal spine count (G. dinae) and pectoral fin ray count (G. mataraerore). We provide mitochondrial COI sequences for each species pair to facilitate identifications by DNA barcoding. These species represent examples of divergence in allopatry, with diagnostic characters arising over the last 2−5 million years in the G. breviceps/G. mataraerore pair, and fewer than 2 million years in the G. basalis/G. dinae pair. We also designate a lectotype for G. basalis (the paralectotype is G. cotidianus) in order to clarify confusion surrounding the original syntypes.
Introduction
New Zealand is home to seven species of the gudgeon genus Gobiomorphus, distributed throughout the fresh and brackish waters of both the North and South Islands and commonly known as bullies. They are stout, robust fishes, usually 50−80 mm in length but reaching up to 175 mm in the giant bully [G. gobioides (Valenciennes 1837)], and they are generally benthic and cryptic. Four of the species are exclusively or facultatively amphidromous, a life history strategy in which the eggs are laid in freshwater and larvae drift downstream to the ocean to feed before migrating as juveniles (rather than adults) back into freshwater rivers and streams to complete their life cycle. In three of the species [G. basalis (Gray Citation1842), G. breviceps (Stokell Citation1939) and G. alpinus Stokell Citation1939], the entire life history takes place in freshwater. Gobiomorphus alpinus has a very restricted distribution, only occurring in a few small freshwater alpine lakes on the South Island (McDowall and Stevens Citation2007), but G. basalis and G. breviceps are widespread. Gobiomorphus basalis (Cran’s bully) inhabits the North Island exclusively, distributed on either side of the central Taupo Volcanic Zone where ongoing volcanic activity appears to have largely extirpated obligate freshwater fishes beginning roughly 2 Mya (McDowall Citation1996). Gobiomorphus breviceps (Upland bully) is distributed widely on the South Island on both sides of the island’s dominant mountain range, the Southern Alps, as well as the southern portion of the North Island.
Genetically distinguishable subpopulations that accord with geographic features have been identified within both G. basalis and G. breviceps. Distinct clades within G. basalis distributed to the north and south of the Taupo Volcanic Zone on the North Island were identified by Shelley et al. (Citation2020) using nuclear SNP markers. Similarly, northern and southern clades within G. breviceps were identified between populations to the north of the Southern Alps (including the North Island) and those to the south, based on mitochondrial DNA sequence by Smith et al. (Citation2005). They hypothesised, based on a molecular clock analysis, that the highly distinct northern and southern clades were separated by the uplift of the Southern Alps in the late Miocene and Pliocene (2.1–8.4 Mya). Shallower genetic divergence within the northern clade, between populations on the North and South islands, is thought to be due to the more recent formation of the Cook Strait (c. 1 Mya) following sea-level rise towards the end of the Pleistocene glacial cycles. In both species, the northern and southern clades are clearly diagnosable based on genetics and geography but are morphologically cryptic. However, given the correlation between the genetic and distributional data, it is possible to segregate populations of each species and re-examine them for diagnostic morphological characters. We evaluate individuals from across the ranges of both G. basalis and G. breviceps, provide morphological distinctions between the geographic clades, and describe two species as new.
Materials and methods
Our counts and observations are based on preserved specimens identified as G. basalis and G. breviceps deposited at Te Papa Tongarewa, the National Museum of New Zealand (NMNZ). We examined individuals from across the geographic range of both species (listed in Material Examined), with particular emphasis on the identification of any morphological characters that could be used to distinguish the northern and southern geographic groups. We confirmed identification on all specimens using the primary diagnostic characters and descriptions in McDowall (Citation1975). We counted spines and rays in the dorsal, anal and pectoral fins (left side only unless the left fin was damaged, in which case the right fin was counted). The final elements in the second dorsal and anal fins were split to the base and counted as a single element. We counted lateral scales along the midline and noted the extent of body scalation, particularly on the head and nape. For a subset of specimens, we confirmed the fin ray counts on radiographs and additionally counted vertebrae (caudal and precaudal).
The live colouration of Gobiomorphus individuals may be quite striking, particularly that of the redfin bully [G. huttoni (Ogilby 1894)] and bluegill bully [G. hubbsi (Stokell 1959)]. Colouration in G. breviceps and G. basalis is more subtle, largely consisting of brown mottling on the body and fins with lighter, cream-coloured margins at the base of the pelvic fin and the distal edge of the first dorsal fin. As live colouration fades markedly in preservative, we focus our observations on external structures (fin ray and scale patterns and counts) that may be easily observed in both fresh and preserved specimens. In particular, because we are distinguishing cryptic species within two well-known taxa, we sought characters useful for checking identifications on specimens already preserved and housed in collections. Gobiomorphus was established by Gill (Citation1863) but not comprehensively treated until the works of Stokell (Citation1941) and especially McDowall (Citation1975); those studies provide a detailed diagnosis of the genus.
To further facilitate the identification of specimens, we extracted DNA sequence from the mitochondrial COI gene from genomic sequences constructed for New Zealand Gobiomorphus in a previous study (Thacker et al. Citation2021). We assembled complete mitochondrial genomes from off-target reads using a procedure similar to that detailed in Raposo do Amaral et al. (Citation2015). We aligned whole genomic contigs against the mitochondrial genome of Eleotris acanthopoma (GenBank accession number AP004455) and transferred gene annotations using the Map to Reference assembly tool in Geneious Prime 2020.0.5 (www.geneious.com), then extracted the COI gene sequences and accessioned them at GenBank under numbers MZ891637-MZ891640.
Systematics
Gobiomorphus dinae new species
Dinah’s bully
Gobiomorphus basalis (in part) McDowall (Citation1975); McDowall (Citation1990); McDowall (Citation2000).
Holotype male, 75.5 mm, NMNZ P.061527 (ex. NMNZ P.056695), Mouth of tributary of Moawhango River, 2–3 miles north of Moawhango, North Island, New Zealand (33° 33.7457′ S 175° 51.6488′ E), collected by C. S. Woods, 16 March 1962.
Allotype female, 63.2 mm, NMNZ P.061528 (ex. NMNZ P.056695), same locality as holotype.
Paratypes female, 51.3 mm, CMC F1273, tributary of Hutt River (41.0799° S 175.1689° E), collected by C. S. Woods, A. C. MacFarlane et al., 2 November 1963; male, 68.6 mm, CMC F1275, tributary of Hutt River (41.0799° S 175.1689° E), collected by C. S. Woods, A. C. MacFarlane et al., 1 November 1963.
Diagnosis Gobiomorphus dinae is distinguished from G. basalis (Cran’s bully) in that it has one fewer dorsal spine (G. basalis have VIII; G. dinae have VII), usually more pectoral rays (18–19 instead of 16–18), and by its geographic range. It is distinguishable from G. breviceps and G. mataraerore in having more pectoral fin rays (18–19 instead of 14–16), and from G. alpinus in having more dorsal spines (VII as opposed to VI). Gobiomorphus dinae differs from G. hubbsi, G. huttoni, and G. gobioides in lacking open sensory pores on the head. It may be difficult to distinguish Gobiomorphus dinae from the common bully, G. cotidianus. Gobiomorphus cotidianus is widespread on both the North and South Islands and occurs throughout its range in both landlocked and amphidromous forms (Michel et al. Citation2008). The amphidromous form may be distinguished from G. dinae in that amphidromous G. cotidianus have open sensory pores on the head, at minimum a pair of lateral pores adjacent to the rear margins of the eyes and sometimes also a pair of median interorbital pores. Gobiomorphus dinae lacks these pores. The landlocked form of G. cotidianus does not have open sensory pores on the head and generally has fewer dorsal scales on the nape than the amphidromous form, such that the nape scalation pattern may be equivalent to that seen in G. dinae. Gobiomorphus dinae usually has one fewer anal ray than G. cotidianus (usually I, 8–9 vs. usually I, 10), and generally has a blunter head and more vertically inclined mouth; the head of G. cotidianus is flatter and more wedge-shaped in lateral view, and the mouth is correspondingly less acutely inclined. Mitochondrial COI (barcode) sequence for G. dinae is available under GenBank accession number MZ891637, and for G. basalis under MZ891638.
Description A summary table of counts and characters for all Gobiomorphus species is given in . Counts of the holotype are indicated with an asterisk. A Gobiomorphus with first dorsal spines VII*; second dorsal fin elements I, 9–10* (rarely 8); anal fin elements I, 8–10 (9*); pectoral fin rays 18–19*. Vertebrae 12* − 13 + 17* − 18 = 29* − 30. Pelvic fins ventral and separated, each with one spine and five branched rays. Caudal fin rounded, with 15 principal rays. Lateral scales 34–38*; cycloid scales present on lateral surfaces of body and on belly, sometimes small and embedded on belly and variably present on isthmus. Scales present on nape (anterior to origin of first dorsal fin) to variable extents, sometimes extending to halfway point between dorsal fin origin and level of eyes; no scales on cheek; rarely a few scales at top posterior corner of opercle. Head large and broad, with blunt snout, jaws reaching to or near the anterior margin of eyes. Head pores absent. Teeth minute, pointed, in several irregular rows on upper and lower jaws, none enlarged. Sexually dimorphic, with mature males attaining a larger size and possessing blunter, more rounded heads and larger fins than females. Genital papillae of males broad and rounded, tapering to fine point with small opening; genital papillae of females also broad but with wider opening and terminating in two projections, giving the papilla the overall shape of a W. Males also exhibit distinct colouration to females as discussed below.
Table 1. Morphological characters for Gobiomorphus species, derived from McDowall (Citation1975), McDowall and Stevens (Citation2007) and our own observations.
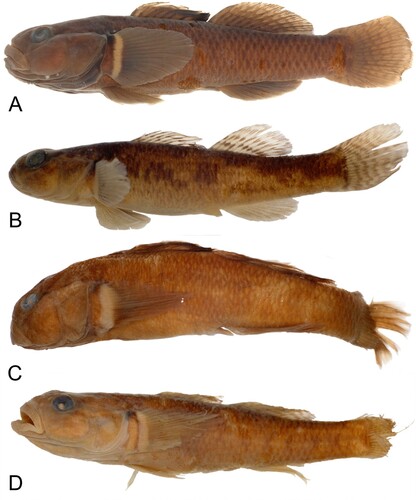
Colouration when fresh Colouration overall light brown to brownish/olive, with irregular pale to dark blotches laterally. Head dark with irregular blotching on cheeks and opercula. First dorsal fin dusky-grey, occasionally with orange speckling, outer fringe brightly coloured in males, being bright pinkish-orange to red. Second dorsal fin clear to dusky, with light to dark grey-olive spots that are sometimes arranged into bands. Second dorsal dusky, sometimes with faint pink-orange colouration at margin, speckled with light to dark grey-olive spots sometimes arranged into bands. Caudal fin dusky with speckles or bands as in second dorsal. Anal fin uniform yellowish-orange. Pelvic fins clear to dusky, pectoral fins clear to dusky with broad cream-coloured margin at base, dark spot at dorsal edge. Mature males much darker coloured than juveniles and females, with lighter band at distal edge of first dorsal fin. Live colouration for G. dinae and G. basalis is shown in .
Figure 1. Live colouration for Gobiomorphus basalis and G. dinae. A. G. basalis male (Kirikiri stream, Coromandel). B. G. basalis female (Kirikiri stream, Coromandel). C. G. dinae male (Turitea Stream, Manawatu). D. G. dinae female (Turitea Stream, Manawatu). Photos of G. basalis by Rod Morris, photos of G. dinae by Stella McQueen.

Colouration when preserved Colouration overall light brown, with irregular pale to dark blotches faintly present laterally. Head uniformly tan. Dorsal, anal and caudal fins pale with rows of faint darker patches; pectoral fins light brown, broad cream-coloured margin is prominent. Photo of preserved specimens is given in .
Figure 2. Gobiomorphus basalis A. male, 60.3 mm (NMNZ P.058870). B. female, 54.8 mm. (NMNZ P.056486). Gobiomorphus dinae C. male holotype, 75.5 mm (NMNZ P.061527). D. female allotype 63.2 mm (NMNZ P.061528).
Ecology Very little ecological data is available for G. dinae or the closely related G. basalis. Published information is predominantly observations from McDowall (Citation1990; Citation2000) that does not distinguish between the species. However, G. basalis and G. dinae are expected to exhibit similar ecological traits, so we present the combined ecological information here.
A benthic fish that is mostly found in slow to moderately flowing stream sections and at low to high altitudes (0–720 m). Predominantly occupies interstitial spaces among cobble and boulders. The insectivorous diet consists of a variety of aquatic invertebrates such as chironomids, mayflies, caddisflies, small crustaceans and snails (McDowall Citation2000).
Matures around age one and may live up to four years (McDowall Citation2000). Spawning occurs in Spring and Summer. Males establish spawning sites beneath large rocks and assume breeding colours to attract a female (McDowall Citation2000). A female deposits several hundred to a thousand oval-shaped eggs ∼2.3 mm in diameter when water hard which the male protects (McDowall Citation1990). McDowall (Citation2000) suggested that there was an unspecified difference in egg size between populations near the city of Auckland (G. basalis) and those near the city of Wellington (G. dinae). Several females may deposit eggs at a single spawning site and individual females may spawn multiple times throughout the spawning period. Eggs hatch after several weeks and larvae are benthic (McDowall Citation2000).
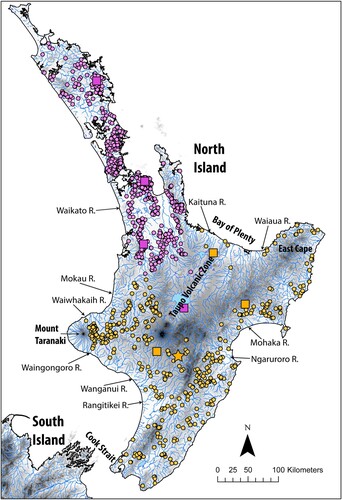
Distribution Widespread across the southern North Island, south of the Taupo Volcanic Zone highlands in the centre of the island. Distribution is effectively continuous across the majority of its range between the Waingongoro River, south of Mount Taranaki, and the Waiaua River at the northwest corner of East Cape. However, it is absent from the headwaters of long southerly and easterly flowing rivers such as the Wanganui, Whangaehu, Rangitikei, Ngaruroro and Mohaka rivers that drain from the Taupo Volcanic Zone. On the west coast it is absent from drainages on the western side of Mount Taranaki between the Waingongoro River and the Waiwhakaih River. It is also absent from west coast drainages between the Waiwhakaiho River and the Mokau River where an isolated population occurs in the upper reaches (as in G. mataraerore) marking the northern extent of the species on the west coast. Beyond its core distribution on the east coast, G. basalis and G. dinae are extremely rare in streams within the Taupo Volcanic Zone, in particular those flowing eastwards into the Bay of Plenty. While we were unable to examine specimens from each of the disjunct record locations, we were able to determine that G. dinae occurs in the easterly flowing Kaituna River catchment, while G. basalis occurs in the headwaters of the northerly flowing Waikato River. These observations align with expectations of species distributions based on geography. As such, we infer that isolated populations in rivers that flow east into the Bay of Plenty are likely G. dinae, although it needs independent verification. It is difficult to determine whether these isolated populations are relictual (McDowall Citation1996) or have resulted from historical translocations (e.g. Ross et al. Citation2018). Maps of the distributions of G. basalis and G. dinae are given in .
Figure 3. Distribution maps for G. basalis (pink) and G. dinae (orange). Dots represent known localities from museum records; squares indicate localities of lots examined for this study. The locality of the holotype of G. dinae (Mouth of tributary of Moawhango River) is indicated with an orange star. The holotype locality of G. basalis is the Waihou River, Northland, New Zealand, but the precise coordinates are unknown.
Remarks Gobiomorphus basalis (Cran’s bully) was described by Gray (Citation1842: as Eleotris basalis), from the Waihou River, Coromandel, New Zealand. This locality is within the northern portion of the range, which extends from the Waihou River north throughout the northern portion of the North Island and extending to the Waitetuna River in the west. Thus, the northern populations retain the name G. basalis.
Between 1842, when the species was described, and the revision by McDowall (Citation1975), nearly all of the literature records referring to G. basalis actually refer to the more common and widespread G. cotidianus, including a generic revision and later field guide by Stokell (Citation1941, Citation1955) and a guide to the Natural History of Canterbury (Burnet et al. Citation1969). McDowall (Citation1975) determined in his revision that the name G. basalis was being used to refer to a different, undescribed species, which he described as G. cotidianus. He provided clear diagnoses of both species and reviewed the types and the relevant literature but did not clarify the type status other than to say that the confusion was due to ‘misidentification of the syntypes of G. basalis’ (McDowall Citation1975, p. 23).
Examination of the syntypes of G. basalis (NHMUK 1842.3.5.6–7) revealed that they represent two different species: one is G. basalis, and the second is G. cotidianus. We here designate the eight-spined syntype (NHMUK 1842.3.7.6) as the lectotype for G. basalis; the other former syntype (NHMUK 1842.3.7.7) becomes a paralectotype and is here identified as G. cotidianus. The former syntypes are very similar in overall colouration (uniformly tan) and scale coverage (body and belly covered with imbricate, cycloid scales, extending on the nape to between the eyes). The counts for the lectotype of G. basalis are dorsal VIII I,9; anal I,9; pectoral 18 and vertebrae 13 + 17 = 30. The counts for the paralectotype, corresponding to G. cotidianus, are dorsal VII, anal I,9; pectoral 18, and vertebrae 13 + 16 = 29. Neither of the former syntypes has open pores on the head. The fishes differ in dorsal spine count and in overall shape; the G. basalis lectotype has a broader body, shorter head and more vertically inclined mouth. The paralectotype identified as G. cotidianus is uniformly slender, with a larger, less vertically inclined mouth. These differences are slight but noticeable.
Etymology The specific epithet dinae honours Dinah Arndt, in recognition of her unstinting support of freshwater fish research and fieldwork across both Australia and New Zealand.
Gobiomorphus mataraerore new species
Kaharore bully
Gobiomorphus breviceps (in part) McDowall (Citation1975); McDowall (Citation1990); McDowall (Citation2000).
Holotype male, 49.3 mm, NMNZ P.061525 (ex. NMNZ P.004441), South Karori Stream, North Island, Wellington, (41° 20.00′ S 174° 41.00′ E), collected by C. S. Woods, 09 May 1962.
Allotype female, 48.2 mm, NMNZ P.061526 (ex. NMNZ P.036970), Mangarae Stream, Tangarakau, North Island, New Zealand (39° 07.0451′ S 174° 53.1377′ E), collected by Dean Caskey, 09 December 1998.
Paratypes female, 87.1 mm, CMC F1071, tributary of Wairau River (41.61° S 173.22° E), collected by C. S. Woods, A. C. MacFarlane et al., 19 October 1952; male, 85.7 mm, CMC F1125, Lake Grassmere (41.73075° S 174.1673° E), collected by C. S. Woods, A. C. MacFarlane et al., date unknown.
Diagnosis Gobiomorphus mataraerore is distinguished from G. breviceps (Upland bully) in having one fewer pectoral ray (G. breviceps has 15–16; G. mataraerore has 14), usually fewer lateral scales (37–44 in G. mataraerore as opposed to 40–53 in G. breviceps), and by its geographic range. It is additionally distinguished from all other species in Gobiomorphus in having 14 pectoral rays (rather than 15–20 in the other species). Gobiomorphus mataraerore also differs from G. hubbsi, G. huttoni, G. gobioides and amphidromous G. cotidianus in lacking open sensory pores on the head. Mitochondrial COI (barcode) sequence for G. mataraerore is available under GenBank accession number MZ891639, and for G. breviceps under MZ891640.
Description A summary table of counts and characters for all Gobiomorphus species is given in . Counts of the holotype are indicated with an asterisk. A Gobiomorphus with first dorsal spines VI*–VII; second dorsal fin elements I, 9*–10; anal fin elements I, 8*–10; pectoral fin rays 15*–16. Vertebrae 12–13 + 17 = 29–30. Pelvic fins ventral and separated, each with one spine and five branched rays. Caudal fin rounded, with 15 principal rays. Lateral scales 37–44 (41*), in larger specimens with papillae on distal edge of alternate median scales; cycloid scales present on lateral surfaces of body, absent on belly, variably present on dorsal surface anteriad to origin of first dorsal fin, no scales on cheek, opercle, or nape. Head large and broad, with blunt snout, jaws reaching to or near the anterior margin of eyes. Head pores absent. Teeth minute, pointed, in several irregular rows on upper and lower jaws, none enlarged. Sexually dimorphic, with mature males attaining larger size, possessing blunter, more rounded heads, larger fins than females. Genital papillae of males broad, rounded, tapering to fine point with small opening; genital papillae of females broad, with wider opening and terminating in ragged edge with several projections.
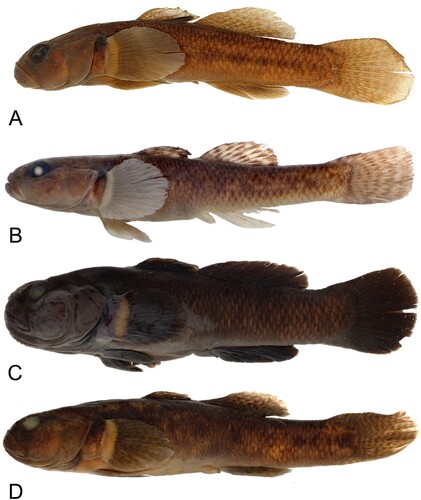
Colouration when fresh Colouration overall greyish to light brown, with irregular large golden-brown blotches on entire body, often arranged in a row along sides. Amongst larger blotches smaller orange-yellow spots. Entire head covered in small, polygonal, orange spots of varying density, shapes. First dorsal fin dusky-grey with orange spots, outer fringe brightly coloured in males, typically orange, sometimes orange-pink, yellow-green or cream. Second dorsal, caudal fins clear to pale orange with dusky grey markings. Anal, pelvic fins clear to dusky-grey. Pectoral fin base with dark spot at dorsal edge; fin clear with broad cream/yellow coloured margin at base, some orange speckling most intense at base. Mature males exhibiting more vibrant colouration than females and juvenile males, particularly during breeding season. Live colouration for G. mataraerore and G. breviceps is shown in .
Figure 4. Live colouration for Gobiomorphus mataraerore and G. breviceps. A. G. mataraerore male (Turitea Stream, Manawatu). B. G. mataraerore female (Turitea Stream, Manawatu). C. G. breviceps male (Ohau River, MacKenzie Basin). D. G. breviceps male (back) and female (front; Stony Stream, Lammerlaw Range). Photos of G. mataraerore by Stella McQueen, photos of G. breviceps by Rod Morris.

Colouration when preserved Colouration overall olive brown, with irregular pale to dark blotches faintly present laterally. Head uniformly brown. Dorsal, caudal fins pale with rows of faint darker patches; anal fin pale with only faint spots; pectoral fins pale cream, with prominent broad cream-coloured margin. Photo of preserved specimens is given in .
Figure 5. Gobiomorphus mataraerore A. male holotype, 49.3 mm (NMNZ P.061525). B. female allotype, 48.2 mm (NMNZ P.061526). Gobiomorphus breviceps C. male, 90.1 mm (NMNZ P.058930). D. female, 83.9 mm (NMNZ P.058930).
Ecology Ecological information for G. mataraerore is limited and it is mostly not possible to distinguish information available for G. breviceps from G. mataraerore. However, they are expected to exhibit similar ecological traits, so we present ecological information for both species combined. A benthic fish that is common across most freshwater habitats including wetlands, lakes, ponds and small to large streams. Predominantly occupies interstitial spaces among cobble and boulders. In fluvial environments, juveniles prefer quiet stream sections, while adults occupy both riffles and quiet stretches wherever boulders provided cover (Cadwallader Citation1975; McDowall Citation2000). They occur, with no clear preference, at low to high altitudes (0−835 m). The predominantly insectivorous diet consists of a variety of aquatic invertebrates such as chironomids, mayflies, caddisflies, small crustaceans and snails. They may also eat their own eggs (McDowall Citation2000). Food is taken from all levels of the water column, although predominantly the benthos (Hopkins Citation1970; Cadwallader Citation1975).
Reported to reach maturity anywhere between 1 and 3.5 years of age and may live up to four years (Hamilton et al. Citation1997; McDowall Citation2000). Spawns in Spring and Summer (McDowall Citation1990). Mature male bullies establish territories and defend them against conspecifics. Males establish a territory, then entice females to lay their eggs at a nest site, commonly the underside of a boulder. Females reported to deposit several hundred to a thousand oval-shaped eggs, typically 2.3−2.6 mm in diameter when water hard, in a single layer which the male protects (Hamilton et al. Citation1997; McDowall Citation1990; McDowall Citation2000). Several females may deposit eggs at a single spawning site and individual females may spawn multiple times throughout the spawning period. Different clutches are laid adjacent to each other (McDowall Citation2000). The guarding males generally stay with the eggs until they hatch, typically 3–5 weeks later (McDowall Citation1990; Hamilton et al. Citation1997).
Distribution Widespread but intermittently distributed across the southern North Island and northern South Island. Within the North Island, distributed south of the Taupo Volcanic Zone highlands in the centre of the island, primarily found in river systems that drain into the South Taranaki Bight and Cook Strait between the Waingongoro and Ruamahanga rivers. The species is absent from drainages on the western side of Mount Taranaki between the Waingongoro River and Waitara River, where there are records in the upper reaches of the Manganui River. it is also absent from west coast drainages between the Waitara River and the Mokau River where an isolated population occurs in the upper reaches marking the northern extent of the species. Disjunct records exist in the Ngaruroro and Tukituki Rivers that flow off the east coast into southern Hawkes Bay that mark the northern extent of the species on the eastern side of the island. Maps of the distribution of G. breviceps and G. mataraerore are given in .
Figure 6. Distribution maps for G. breviceps (yellow) and G. mataraerore (blue). Dots represent known localities from museum records; squares indicate localities of lots examined for this study. The locality of the holotype of G. mataraerore (Mangarae Stream, Tangarakau) is indicated with a blue star. The holotype locality of G. breviceps is the Kowai River, Canterbury, New Zealand, but the precise coordinates are unknown.
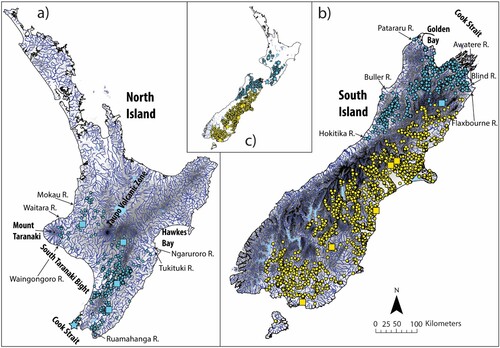
On the South Island the species is present north of the Southern Alps, between the Hokitika River in the southwest and at least the Awatere River in the northeast. The exact northeast boundary between the northern and southern species is not established here due to a lack of samples from this region. However, we assume that populations in the Blind River and Flaxbourne River immediately east of the Awatere River also belong to G. mataraerore as there are no particularly strong elevational divides separating them. However, these records need independent verification. Neither G. mataraerore nor G. breviceps are present in west coast catchments south of the Hokitika River. Largely continuous distribution within range, except absent from catchments draining the northwest coast between Buller River and Patararu River in the far north. Also, a notably patchy distribution in rivers draining into Golden Bay in the northeast.
Remarks Gobiomorphus breviceps, the Upland bully, was first described by Stokell (Citation1939) as Philypnodon breviceps. The holotype specimen is at the museum of Canterbury, Christchurch (CMC F77) and was collected from the Kowai River, tributary of the Waimakariri River, near Christchurch on New Zealand’s South Island. Examination of this holotype as well as two paratypes (NHMUK 1940.2.12.1-2) confirms the identification as G. breviceps. The type locality is from the southern part of the range, so the southern species retain the original name and we describe the northern species here.
Etymology The specific name mataraerore is derived from the Maori words ‘mata’, meaning face (referring to the distinctive facial expression of Gobiomorphus fishes), and ‘rae’ meaning forehead (referring to the elongate forehead), and ‘rore’ in honour of the type locality that lies within the region traditionally referred to as Kaharore (a traditional bird snare). Noun in apposition.
Discussion
The descriptions of G. mataraerore and G. dinae bring the total number of Gobiomorphus species in New Zealand to nine, and in particular add to the known diversity of exclusively freshwater (landlocked) fish species. These two species pairs represent classic cases of divergence in allopatry. For G. basalis and G. dinae, the populations are split by the Taupo Volcanic Zone, a volcanic region that bisects the North Island and has been active from 2 Mya up until the present day (Wilson et al. Citation1995; McDowall Citation1996; Shelley et al. Citation2020), with lava flows subsuming and extirpating fishes confined to freshwater habitats and isolating populations on either side. A similar distributional pattern is seen in the mudfishes Neochanna apoda Günther, 1867 and N. diversus Stokell, 1949 (Gleeson et al. Citation1999). The species G. breviceps and G. mataraerore are present on either side of the Southern Alps, a mountain chain that crosses the South Island from southwest to northeast and was uplifted over the past 5 Mya. Several freshwater fish species, including other Neochanna species and several Galaxias, also show divergence across this vicariant boundary (Michel et al. Citation2008; Wallis and Trewick Citation2009; Craw et al. Citation2016). Exclusively freshwater species such as these are much more liable to experience population segregation due to vicariance than migratory ones. The amphidromous New Zealand Gobiomorphus species (G. hubbsi, G. huttoni, G. gobioides and G. cotidianus) are all widespread and, due to their migratory life history, much more likely to experience population mixing and gene flow over time, as well as being more able to repopulate drainages from which they have been extirpated (Unmack Citation2001; Thacker et al. Citation2007, Citation2008).
We examined individuals from across the ranges of all four species, including samples from near the species boundaries. Gobiomorphus basalis and G. dinae are rare in the Taupo Volcanic Zone, but we included two lots from the area, one from each species. In both cases, the counts and observations of the individuals in the Taupo Volcanic Zone matched those of their conspecifics and were not ambiguous or intermediate. Similarly, for G. mataraerore, we examined one population from the northern side of the species boundary, and the counts coincided with the remainder of the individuals observed from farther north rather than G. breviceps.
Phylogenetically, G. breviceps and G. mataraerore are sister species, but G. basalis and G. dinae are not. Instead, G. dinae is sister to a clade containing, sequentially, G. basalis followed by the sister pair of G. cotidianus and the highly endemic South Island species G. alpinus (Shelley et al. Citation2020; Thacker et al. Citation2021). In that case, the phylogenetic pattern suggests that G. cotidianus arose in the north, reacquired the ancestral capability for amphidromy, and dispersed into its current wide range, eventually seeding the populations of landlocked G. alpinus. The vicariant barrier separating G. breviceps and G. mataraerore is older than that separating G. basalis and G. dinae, but in both cases, easily observable and consistent morphological differences have evolved. Both are serial structures (fin rays), and so any potential selective advantage deriving from one more or fewer is likely to be slight. More probably, these differences simply result from drift and lineage sorting, accomplished relatively rapidly due to the strong allopatric barriers. In these cases, the clades identified in molecular phylogeographic analyses correspond not only with geographic range but also with consistently observable morphological characters, greatly facilitating identification for taxa even if geographic information is lacking.
Conclusion
We describe two new species, Gobiomorphus dinae and G. mataraerore, each representing geographically isolated subpopulations of existing species. Gobiomorphus dinae is separated from G. basalis by the Taupo Volcanic Zone on the North Island, and G. mataraerore is separated from G. breviceps by the Southern Alps. Both species pairs are similar but distinguishable by fin ray counts. We additionally resolve confusion surrounding the syntypes of G. basalis: the two syntypes are different species, and we designate one as the lectotype of G. basalis and the other as a paralectotype identified as G. cotidianus.
Material examined
For each lot, the first number in parentheses is the total number of individuals, followed by the number of individuals with counts tabulated for that lot (generally the largest). NHMUK: Natural History Museum, London. CMC: Canterbury Museum, Christchurch. NMNZ: National Museum of New Zealand.
Gobiomorphus basalis NHMUK 1842.3.7.6 (1), NMNZ P.056486 (19:7), NMNZ P.056513 (5:5), NMNZ P.056861 (31:5), NMNZ P.058870 (14:6)
Gobiomorphus dinae NMNZ P.056295 (30:6), NMNZ P.056695 (28:6), NMNZ P.056766 (69:6), NMNZ P.057316 (23:5), NMNZ P.058915 (20:6)
Gobiomorphus breviceps NHMUK 1940.2.12.1-2 (2), CMC F77 (1), NMNZ P.004363 (22:5), NMNZ P.057508 (20:5), NMNZ P.58850 (10:6), NMNZ P.058930 (20:6), NMNZ P.060592 (43:3)
Gobiomorphus mataraerore NMNZ P.004441 (17:4), NMNZ P.004451 (27:5), NMNZ P.004459 (28:5); NMNZ P.004466 (47:7), NMNZ P.036970 (8:5), NMNZ P.057869 (38:1)
Acknowledgements
We would like to acknowledge the help of Lee Rauhina-August (NIWA) for helping co-ordinate the naming of Gobiomorphus mataraerore and Kura Moeahu of Te Ātiawa Taranaki Whānui for gifting the name. We thank Todd Clardy and Bill Ludt (Section of Ichthyology, Natural History Museum of Los Angeles County) for preparing the radiographs, and James MacLaine (Natural History Museum, London) and Paul Scofield (Canterbury Museum, New Zealand) for their generous assistance in locating and imaging type specimens. Special thanks to Paul Scofield for locating and designating paratypes for both new species between lockdowns due to the COVID-19 pandemic. We also thank Stella McQueen and Rod Morris for generously providing us with live photographs of Gobiomorphus species.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Burnet AM, Cranfield HJ, Benzie V. 1969. The freshwater fishes. In: Knox GA, editor. The natural history of Canterbury. Auckland: Reed Books; p. 620.
- Cadwallader PL. 1975. Feeding relationships of galaxiids, bullies, eels and trout in a New Zealand river. Marine and Freshwater Research. 26:299–316.
- Craw D, Upton P, Burridge CP, Wallis GP, Waters JM. 2016. Rapid biological speciation driven by tectonic evolution in New Zealand. Nature Geoscience. 9:140.
- Gill TN. 1863. On the gobioids of the eastern coast of the United States. Proceedings of the Academy of Natural Sciences of Philadelphia. 15:267–271.
- Gleeson DM, Howitt RLJ, Ling N. 1999. Genetic variation, population structure and cryptic species within the black mudfish, Neochanna diversus, an endemic galaxiid from New Zealand. Molecular Ecology. 8:47–57.
- Gray JE. 1842. Three hitherto unrecorded species of fresh-water fish, brought from New Zealand and presented to the British Museum by Dr. Dieffenbach. Zoological Miscellany. 1:73.
- Hamilton WJ, Stott MK, Poulin R. 1997. Nest site characteristics and male reproductive success in the upland bully, Gobiomorphus breviceps (Eleotridae). Ecology of Freshwater Fish. 6:150–154.
- Hopkins CL. 1970. Some aspects of the bionomics of fish in a brown trout nursery stream. Fishery Research Bulletin of New Zealand. 4:1–38.
- McDowall RM. 1975. A revision of the New Zealand species of Gobiomorphus (Pisces: Eleotridae). Records of the National Museum of New Zealand. 1:1–32.
- McDowall RM. 1990. New Zealand freshwater fishes: a natural history and guide. Auckland: Heinemann-Reed; p. 553
- McDowall RM. 1996. Volcanism and freshwater fish biogeography in the northeastern North Island of New Zealand. Journal of Biogeography. 23:139–148.
- McDowall RM. 2000. The Reed field guide to New Zealand freshwater fishes. Auckland: Reed Books; p. 224.
- McDowall RM, Stevens MI. 2007. Taxonomic status of the Tarndale bully Gobiomorphus alpinus (Teleostei: Eleotridae), revisited–again. Journal of the Royal Society of New Zealand. 37:15–29.
- Michel C, Hicks BJ, Stölting KN, Clarke AC, Stevens MI, Tana R, Meyer A, van den Heuvel MR. 2008. Distinct migratory and non-migratory ecotypes of an endemic New Zealand eleotrid (Gobiomorphus cotidianus) – implications for incipient speciation in island freshwater fish species. BMC Evolutionary Biology. 8:49.
- Raposo do Amaral F, Neves LG, Resende MFR, Mobili F, Miyaki CY, Pellegrino KCM, Biondo C. 2015. Ultraconserved elements sequencing as a low-cost source of complete mitochondrial genomes and microsatellite markers in non-model amniotes. PLoS ONE. 10(9):e0138446.
- Ross PM, Knox MA, Smith S, Smith H, Williams J, Hogg ID. 2018. Historical translocations by Māori may explain the distribution and genetic structure of a threatened surf clam in Aotearoa (New Zealand). Scientific Reports. 8:1–8.
- Shelley JJ, David BO, Thacker CE, Hicks AS, Jarvis MG, Unmack PJ. 2020. Phylogeography of the Cran’s bully Gobiomorphus basalis (Gobiiformes: Eleotridae) and an analysis of species boundaries within the New Zealand radiation of Gobiomorphus. Biological Journal of the Linnean Society. 130:365–381.
- Smith PJ, McVeagh SM, Allibone R. 2005. Extensive genetic differentiation in Gobiomorphus breviceps from New Zealand. Journal of Fish Biology. 67:627–639.
- Stokell G. 1939. A new freshwater fish of the genus Philypnodon. Transactions and Proceedings of the Royal Society of New Zealand. 69:129–133.
- Stokell G. 1941. A revision of the genus Gobiomorphus. Transactions and Proceedings of the Royal Society of New Zealand. 70:265–276.
- Stokell G. 1955. Freshwater fishes of New Zealand. Christchurch: Simpson and Williams; p. 145
- Thacker CE, Shelley JJ, McCraney WT, Unmack PJ, McGee MD. 2021. Delayed adaptive radiation among New Zealand stream fishes: joint estimation of divergence time and trait evolution in a newly delineated island species flock. Systematic Biology. syab014. doi:10.1093/sysbio/syab014.
- Thacker CE, Unmack PJ, Matsui L, Rifenbark N. 2007. Comparative phylogeography of five sympatric Hypseleotris species (Teleostei: Eleotridae) in south-eastern Australia reveals a complex pattern of drainage basin exchanges with little congruence across species. Journal of Biogeography. 34:1518–1533.
- Thacker CE, Unmack PJ, Matsui L, Duong P, Huang E. 2008. Phylogeography of Philypnodon species (Teleostei: Eleotridae) across south-eastern Australia: testing patterns of connectivity across drainage divides and among coastal rivers. Biological Journal of the Linnean Society. 95:175–192.
- Unmack PJ. 2001. Biogeography of Australian freshwater fishes. Journal of Biogeography. 28:1053–1089.
- Wallis GP, Trewick SA. 2009. New Zealand phylogeography: evolution on a small continent. Molecular Ecology. 18:3548–3580.
- Wilson CJN, Houghton BF, Kampt PJJ, McWilliams MO. 1995. Volcanic and structural evolution of Taupo Volcanic Zone, New Zealand: a review. Journal of Volcanology and Geothermal Research. 68:1–28.
