?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Implementation of open sea seaweed aquaculture has been pursued in Europe for high biomass production to be used for different purposes, including the biorefinery pipeline. Offshore cultivation has been proposed to avoid conflict with other uses of coastal areas such as fishing and recreational activities, and preferably associated with other compatible activities such as wind energy production or fish aquaculture to optimise costs and operation. Saccharina latissima is a European kelp species that finds its southern distribution limit in northern Portugal and is considered a good candidate for commercial cultivation. The present work studied growth performance of this species in exposed conditions, at 5, 10 and 15 m depth from February until September. Photosynthetic performance was also assessed at those depths in summer. Saccharina latissima grew in offshore exposed conditions at the southern distribution limit of the species, with growth rates of 3.3%–4.5% day−1 between January and May, while withstanding high wave heights. Results indicate that for food applications the growing season in these conditions may be extended until May. If used for applications with less stringent demands for appearance – for example, feed or biofuels – harvest can be delayed further. Optimisation of technology and understanding the underlying biology of S. latissima is needed for cultivation in these conditions. This will include development of low-maintenance/high-resistance support structures and harvest techniques, in addition to strategies for early deployment to improve cultivation reliability and to extend the growth period.
INTRODUCTION
In Asian countries, seaweeds have been consumed as vegetables for many centuries (Murata & Nakazoe Citation2001), and an important kelp aquaculture industry has existed for decades, occupying vast marine areas (Liu et al. Citation2015; Hwang et al. Citation2019). In Western countries, interest in developing open sea seaweed aquaculture has emerged more recently. Production of biomass for biofuels was an initial driver. Cultivation of high-energy biomass at sea presents advantages over terrestrial crops, especially the lack of a requirement for fresh water or fertile land, in addition to higher yields (Gao & McKinley Citation1994). Later, due to low technology readiness for low-cost deployment and harvesting of the biomass, the biorefinery concept arose, and drivers have shifted to production of higher value biomass, for application in functional food and feed products, nutraceuticals, and extraction of bioactive biomolecules (Baghel et al. Citation2015; van Hal et al. Citation2014; Shannon & Abu-Ghannam Citation2019).
Because the establishment of large areas for seaweed cultivation may conflict with other established uses of coastal areas, such as fishing and recreational activities, offshore cultivation has been proposed, preferably associated with compatible activities like wind energy production or fish aquaculture farms (Jansen et al. Citation2016; Troell et al. Citation2009).
In Europe, development of open water seaweed cultivation has focused on kelp species. This was based on methods first developed for Saccharina japonica (Areschoug) C.E.Lane, C.Mayes, Druehl & G.W.Saunders in China in the 1940s, which include seedling rearing in a hatchery, with subsequent grow out at sea in floating structures (Food and Agriculture Organization 2004–2018). One of the kelp species more commonly used for sea cultivation has been Saccharina latissima (Linnaeus) C.E.Lane, C.Mayes, Druehl & G.W.Saunders, a European kelp species characterised by fast growth, high biomass yield, wide geographic distribution, biofiltration capacity, and commercialisation potential (Handa et al. Citation2013; Peteiro et al. Citation2016; Reid et al. Citation2013; Sanderson et al. Citation2012), making it a good candidate for cultivation. In addition, its chemical composition gives it great potential for applications such as food, food and feed supplements, bioactive molecules, and feedstock for biofuels (Bartsch et al. Citation2008; Forbord et al. Citation2012; Marinho et al. Citation2015a; Peteiro & Freire Citation2013).
Offshore cultivation of S. latissima has been tested at various locations across Europe, including sheltered areas, such as the Galician rias (Freitas et al. 2015; Peteiro & Freire Citation2013) and Norwegian fjords (Handa et al. Citation2013), as well as in exposed coastal environments (Buck & Buchholz Citation2005; Wang et al. Citation2014).
The northern Portuguese coast is highly energetic, with significant wave heights potentially exceeding 10 m in winter (Portuguese Instituto Hidrográfico, Citation2015), and is also the southern distribution limit of S. latissima in Europe. This is a cold water species reported to grow best at water temperatures ranging from 10 to 15 °C (e.g. Bolton & Luning Citation1982) and in moderately exposed environments (Peteiro & Freire Citation2013). However, previous studies have suggested that populations near their distribution limits may be adapted to local conditions, allowing them to cope with temperature conditions higher than the reported optimum for the species (Gerard & Dubois Citation1988).
The goal of the present work was to study the viability of cultivation of S. latissima in a high-energy environment, at its southern distribution limit, during one growth season. Differences in growth with depth during spring and summer were assessed. Growth performance was based on thallus length and weight increase, and photosynthetic activity was assessed in situ during summer by pulse amplitude–modulated (PAM) chlorophyll a fluorometry.
MATERIAL AND METHODS
Study site
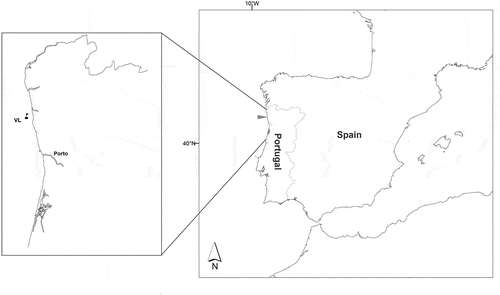
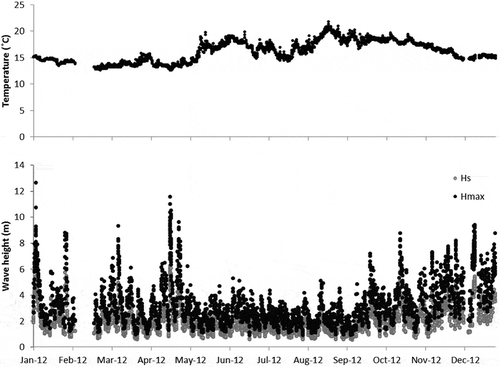
The deployment site was located approximately 6 km (3.24 nautical miles) off the coast, in the north of Portugal, at 41°28′N, 8°50′W (). Site depth was around 40 m. This area is subject to coastal upwelling with maxima from July to September due to the increase in the intensity and steadiness of northerly winds in June, July and August (Fiuza et al. Citation1982; Lemos & Pires Citation2004). During the study period, from January to September 2012, SST ranged between 12.6 and 21.8 °C, significant wave height ranged between 0.5 and 6.2 m, and maximum wave height ranged from 0.9 and 12.6 m (; data from the Portuguese Instituto Hidrográfico, Leixões buoy, located c. 10 nautical miles southwest from deployment site; Portuguese Instituto Hidrográfico, Citation2015). Nutrient data was available for this period, collected by a different project (PMD et al. 2016). Nitrates ranged between 4.1 and 7.0 µM in February and between 1.2 and 7.0 µM in June, and phosphates ranged between 1.2 and 2 µM in February and between 0.5 and 1.1 µM in June (PMD et al., 2016).
Fig. 2. Temperature (top) and wave height (bottom) were measured approximately every 3 hours in a hydrographical buoy located 10 nautical miles southwest from deployment site. Data from the Portuguese Hydrographic Institute (Citation2015). Hs, significant wave height; Hmax, maximum wave height.
Deployment of S. latissima seedlings
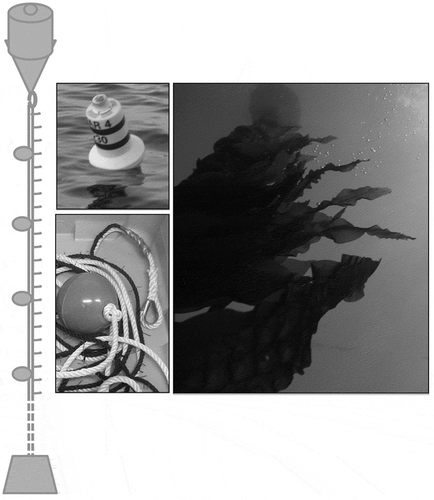
Seedlings of Saccharina latissima attached to strings originated from the Spanish Institute of Oceanography, where they were produced from gametophytes under controlled temperature and light intensity, as described in Peteiro & Freire (Citation2009). Seeded string with 0.5- to 1.0-cm seedlings was cut into 6-cm fragments which were then inserted into 16-mm ropes, at 10- to 20-cm intervals, which resulted in around 200 segments per 30-m length of rope. These were attached to vertical longlines (VLs), which were kept in place by means of a surface buoy and a bottom weight, balanced with several intermediate buoys (). Two VLs were deployed on 13 January 2012, with the help of a fishing vessel, at 41°28.17′N, 8°50.98′W (VL1) and 41°28.27′N, 8°50.97′W (VL2).
Monitoring
Four monitoring visits were conducted, on 18 February, 14 May, 10 July and 07 September. At each visit, vertical profiles of dissolved oxygen, photosynthetically active radiation (PAR), salinity, temperature, and water density were obtained using a CTD (CTD90M, Sea & Sun Technology, Trappenkamp, Germany), from 0.4 m to approximately 40 m deep.
During the February visit, photographs were taken at different depths, but no seaweed collection was undertaken, due to the small size of sporophytes. In May and July, around 10 individuals were collected by divers, at each of three depths: 5, 10 and 15 m.
After collection, sporophytes were transported to the laboratory in ice boxes. In the laboratory they were measured (stipe, blade and total length) and weighed. For each sampling time and depth, 10 sporophytes were split into blades and stipes, washed with filtered seawater to remove epibionts, pooled together (stipes and blades separately) and freeze-dried.
After the May monitoring, VL1 was lost, its buoy being found on 02 July at a beach around 130 km south. Thus, on subsequent monitoring trips, only VL2 was sampled.
Photosynthetic activity as in vivo chlorophyll a fluorescence
To characterise photosynthetic response of sporophytes at different depths, PAM fluorometry of Photosystem II was used during the July monitoring visit when three replicate rapid light curves (RLCs) were obtained at 5, 10 and 15 m depth from S. latissima sporophytes with a DIVING-PAM fluorometer (Walz, Heinz Walz GmbH, Effeltrich, Germany) using the PAM halogen light source. All fluorescence measurements were conducted using the Universal Sample Holder (DIVING-USH, Walz) to prevent the influence of external light. Each RLC was started as soon as the sample holder was put in place. Therefore, algae were in the dark less than 5 s before the first fluorescence measurement took place. The sample holder was positioned at the base of each blade to obtain the RLCs and a 10-s interval was allowed between each light level. The following light levels were used: 0, 1, 19, 62, 132, 223, 345, 666 and 1001 µmol photons m−2 s−1. Blade absorptance was measured separately for algal blades collected at the three reported depths (5, 10 and 15 m) using the DIVING-USH and the DIVING-PAM PAR light sensor in contrast to the actinic light delivered by the PAM (Heinz Walz GmbH Citation1998).
C/N content analyses
Nitrogen and carbon content were determined in blade and stipe tissues by isotope ratio mass spectrometry using an organic elemental analyser (Flash 2000, Thermo Fisher Scientific, Bremen, Germany).
Data analysis
Relative growth rate (RGR) was determined as
RGR (% day−1) = (Ln (FL) − Ln (IL))/T * 100, where FL is the final length, IL is the initial length and T is the number of days in culture (e.g. Kraan et al. Citation2000).
The light extinction coefficient (k) in the water column was calculated from PAR vertical profiles. Considering the Lambert-Beer law, the decline in PAR with depth may be described by the following equation:
where PARsurf and PARZ are the PAR light intensity at the surface and at depth Z (µmol photons m−2 s−1), respectively; k is the light extinction coefficient (m−1); and Z is depth (m).
By applying logarithms to equation 1, a linear relationship between PAR and depth may be obtained:
Therefore, k is the slope of a linear regression between the natural logarithms of PAR and Z.
Equation 2may be rearranged as
Fluorescence yields obtained with the DIVING-PAM were used to compute oxygen production using the following equation, according to other authors (e.g. Figueroa et al. Citation2003; Hancke et al. Citation2008; Hurd et al. Citation2014):
where ФPSII is the quantum yield of charge separations in Photosystem II (PSII; mol e- mol photon−1); E is absorbed radiance (µmol photons m−2 s−1) obtained from the product of incident PAR and thallus absorptance; CF is a correction factor to account for the fraction of light absorbed by PSII. The product is the electron transport rate (µmol e- m−2 s−1) and Γ is the stoichiometric ratio of oxygen evolved per electron generated at PSII − 0.25 O2 (e-)−1 (Falkowski & Raven Citation1997) .
One of the assumptions of this method is the value of the parameter CF. The commonly used value for CF is 0.5 (Morris & Kromkamp Citation2003), and this assumes that light is equally distributed among Photosystem I (PSI) and PSII. If this assumption is not met, it does not influence the shape of the RLCs but merely its asymptote. Moreover, it does not affect the comparison of the RLCs obtained at different depths. There is an overall consensus that PAM readings may be a good indicator of photosynthetic oxygen production in seaweeds as long as effective quantum yields are above 0.1 (Hurd et al. [Citation2014] and references therein). In this study, values < 0.1 were obtained only at maximum irradiances in some of the replicates. However, using equation 3 to convert linearly fluorescence measurements to oxygen production does not preclude the comparisons of the RLCs at various depths, and the shape of the curves based on either electron transport rate (ETR) or oxygen values is the same, even if the ‘real’ oxygen production is biased due to some deviation at high light from the linear behaviour predicted by equation 3. Given the above arguments and because these measurements are used to compare the photosynthetic response of organisms located at different depths, and the measurement conditions were exactly the same, this approach was considered adequate for the purposes of the present work, even if some bias may exist in calculated oxygen productions. As stated above, the result of equation 3, expressed in µmol O2 m−2 s−1, was converted to mg O2 g−1 h−1 after weighing dry circles of S. latissima blades, with an area similar to the circles illuminated by the DIVING-PAM USH, and finding the relationship between area and dry weight.
Eilers & Peeters’ (Citation1988) production–light function (equation 4) was fitted to RLCs obtained at each depth using nonlinear regression with Statistica software v6.1 (Citation2004).
where a, b and c are parameters that may be related to the usual photosynthesis–light curve characteristics by the following formulas:
where PARopt stands for optimum light intensity (µmol photons m−2 s−1); Pmax is maximal production rate [mg O2 g (DW)−1 h−1]; and a is the initial slope of the photosynthesis–light curve [mg O2 g (DW)−1 h−1/(µmol photons m−2 s−1)].
Statistical analyses
Length and fresh weight of sporophytes sampled at each depth in the May survey were used to test the null hypothesis about the absence of significant buoy and depth effects using a nested analysis of variance (ANOVA). For the July survey, when data were available for one buoy only, a one-way ANOVA was used to test the null hypothesis for the absence of significant depth effects in this case. Triplicate estimates of each RLC parameter (PARopt, Pmax and α) obtained at each depth (5, 10 and 15 m; ‘Photosynthetic activity as in vivo chlorophyll a fluorescence’) were used to test the null hypothesis for the absence of significant depth effect using one-way ANOVA. Differences in C and N contents between stipes and blades and times of collection were analysed by a two-way ANOVA using the values obtained at different depths as replicates. The Student-Newman-Keuls (SNK) test was used for post hoc comparisons. All analyses were performed with Statistica software v6.1. Differences at P < 0.05 were considered statistically significant.
RESULTS
Environmental variables
Regarding water density, the water column was stable in all sampling surveys with density increasing as a function of depth (not shown). However, pycnocline depth varied in the different surveys: in February, around 15 m; in May, from the surface down to 10 m; in July, from the surface down to 20 m depth, and in September there was a very shallow pycnocline between the surface and c. 5 m depth and another, less pronounced, extending from there to > 40 m.
Sea surface temperature (SST) was lowest in February (12 °C) and highest in May (19 °C) followed by July (15 °C) and September (16 °C; ). A strong thermocline was observed in May, with a sharp temperature decrease between the surface and 10 m depth.
Fig. 4. Vertical profiles of (a) temperature and (b) salinity obtained during the four sampling surveys.
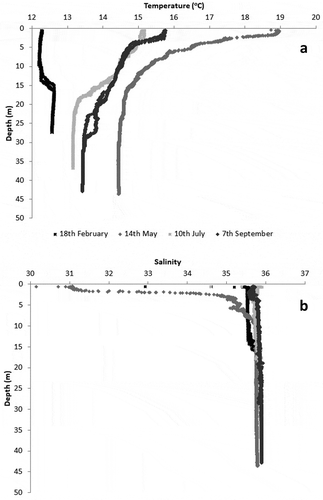
A strong halocline was observed in May, with a thin surface layer of relatively low-salinity water, which was assumed to be related to recent rainfall ().
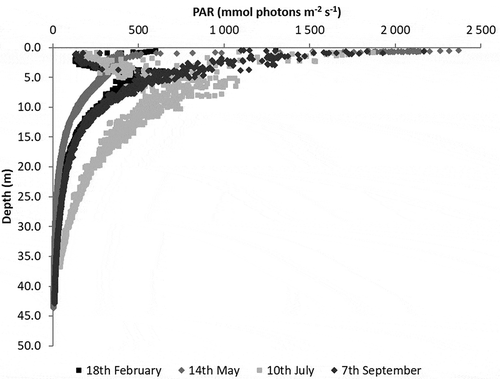
PAR strongly decreased with depth (). A strange near-surface pattern was observed during February and July surveys, presumably related to the boat shadow; whereas, the expected pattern was observed in May and September, when an exponential decrease with depth occurred. Surface PAR reached almost 1000 µmol m−2 s−1 in February and values in excess of 2000 µmol m−2 s−1 in the remaining surveys. The light extinction coefficient (k) varied between 0.08 m−1 in July and 0.12 m−1 in February and May.
Growth
Photographs taken during sampling surveys show that seaweed growth occurred mainly from February to May and that growth decreased with depth, as was expected (). Growth at 20 m was negligible and the algae seemed unable to survive below 25 m. At 20 and 25 m, almost only epibionts grew on the longline. In May, most sporophytes had some epibionts, including hydrozoans and bryozoans, at all depths. In July, not only blades (c. 70% coverage) but also stipes had epibionts. Several crabs (Polybius henslowi) were observed, apparently feeding on the hydrozoans, and shoals of small fish were observed near the surface close by. On 07 September, the last monitoring date, the sporophytes were highly eroded (data not shown); their blades were covered with epibionts, and mussels grew on the stipes.
Fig. 6. Photographs taken at 5, 10, 15, and 20 m depth during February, May and July sampling surveys.

Total length of sporophytes sampled in May and July had decreased significantly (P < 0.05; Tables S1 and S2) with depth, as expected. In May, a maximum length of 170 cm was obtained at 5 m, 99 cm at 10 m, and 75 cm at 15 m. No significant differences were found between samples collected from the two buoys. Unfortunately, this comparison was possible only for the May survey, due to the loss of buoy 1. In the period from May to July, average total length increased from 99.7 to 113.4 cm at 5 m and from 56.9 to 93.1 cm at 10 m; whereas, at 15 m no increase in length was observed (). Similar patterns were found for individual fresh weight, with values significantly (P < 0.05; Tables S3 and S4) decreasing with depth and presenting high variability, averaging 45.6, 19.4, and 10.5 g for 5, 10 and 15 m, respectively, in May () for buoy 1. Buoy 2 presented higher average weight at 5 m (65.9 g) but lower values at 10 and 15 m (16.3 and 5.3 g, respectively), although values are not significantly different from those for buoy 1. In July, both length and fresh weight decreased significantly (P < 0.05; Tables S5–S8) with depth. Average biomass on buoy 2 in July increased to 120, 80.2, and 7.7 g at 5, 10 and 15 m, respectively, although these values were certainly inflated by epibionts.
Fig. 7. Average (a) thallus length and (b) fresh weight of sampled individuals (n = 10) at 5, 10, and 15 m during May and July sampling surveys. Letters indicate groups differentiated by SNK tests, when these were significant at P < 0.05.
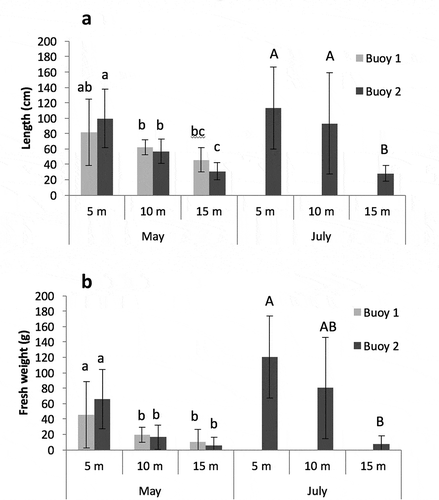
RGR was much higher between January and May (3.3%–4.5% day−1) than between May and July (0.1%–0.7% day−1). Variation in RGR with depth differred in the two periods; whereas during the first period RGR decreased with depth, in the second the highest RGR was found at 10 m.
Pulse amplitude–modulated fluorimetry of PSII
shows the oxygen-based photosynthesis–light curves obtained from the PAM RLCs and the corresponding fit to the Eilers & Peeters function (equation 4; cf. ‘Material and Methods’). Also shown are the photosynthesis–light parameters obtained at each depth together with maximum electron transport rate (ETRmax) and r2 values for each curve fit. PARopt, α and Pmax were in the ranges 314.6–482.0 μmol photons m−2 s−1, 0.12–0.18 mg O2 g(DW)−1 h−1/(µmol photons m−2 s−1) and 17.8–28.6 mg O2 g(DW)−1 h−1, respectively. ETRmax varied from 31.4 to 51.1 µmol e- m−2 s−1. Maximum quantum yields (not shown) were slightly above 0.7. Significant depth effects on RLC parameters were obtained only for α, between results obtained at 10 and 15 m depth, with the former being significantly larger than the latter (P < 0.05; Tables S9–S14).
Fig. 8. Rapid light curves (RLCs) obtained in situ by divers with a DIVING-PAM at 5, 10 and 15 m during the July survey. The abscissa represents incident PAR irradiance. Also shown are RLC parameters PARopt (µmol photons m−2 s−1), Pmax (mg O2 g(DW)−1 h−1) and α [mg O2 g(DW)−1 h−1/(µmol photons m−2 s−1)]; ERTmax (µmol e- m−2 s−1); and the coefficient of determination (r2).
![Fig. 8. Rapid light curves (RLCs) obtained in situ by divers with a DIVING-PAM at 5, 10 and 15 m during the July survey. The abscissa represents incident PAR irradiance. Also shown are RLC parameters PARopt (µmol photons m−2 s−1), Pmax (mg O2 g(DW)−1 h−1) and α [mg O2 g(DW)−1 h−1/(µmol photons m−2 s−1)]; ERTmax (µmol e- m−2 s−1); and the coefficient of determination (r2).](/cms/asset/41ab7109-e6e3-484e-872e-3e96ed011be1/uphy_a_1625610_f0008_b.gif)
Carbon and nitrogen content
The nitrogen content was significantly higher (P < 0.05; Tables S15, S16) in blades than in stipes (). In blades values remained rather constant, decreasing slightly from May to July; whereas, in stipes, N content increased significantly (P < 0.05; Tables S15, S16), from 1.6% ± 0.1% in May to 2.1% ± 0.0% in July. Carbon content was similar in stipes and blades, increasing significantly (P < 0.05; Tables S17, S18), to 24.9% ± 2.3% (stipes) and 23.3% ± 3.6% (blades) in July (). The carbon-to-nitrogen ratio was significantly higher (P < 0.05; Tables S19, S20) in stipes than in blades, remained similar between May and July in stipes, and increased (although not significantly) in blades, from 8.2% ± 0.6% to 10.6% ± 1.8% from May to July ().
Table 1. C and N content and molar C:N ratio for stipes and blades of sporophytes collected in May and July (mean ± s).a
DISCUSSION
Environmental variables
The study site was located in northern Portugal and corresponded with the lower latitude margin of the distribution of S. latissima in Europe. As a consequence, local populations are subjected to higher water temperatures than the average of its distribution range. Previous studies have shown that the optimum temperature range for this species is 10–15 °C (e.g. Bolton & Luning Citation1982). During the study period, SST was always above 12 °C, with values increasing in May and peaking in August, when temperature reached 20 °C. The decrease in temperature observed in July throughout the water column may have resulted from an upwelling event, common in this period (Fiuza et al. Citation1982; Lemos & Pires Citation2004). SST values from May onwards were higher than the reported optimum for the species, although the vertical temperature profile in May showed a sharp thermocline which may have attenuated the negative effects of high temperatures on sporophytes placed below 5 m, because temperature dropped to 16 and 14 °C, respectively.
Based on the light extinction coefficient obtained in the February and May surveys (c. 0.12 m−1), it follows that at 20 m, light intensity is c. 10% of that at the surface. This estimate may be obtained solving equation 2 for PARz/PARsurf. However, given the usual rough sea conditions in the area, the value obtained in these surveys for the light extinction coefficient likely underestimates the average; therefore, light intensity is probably lower than expected at 20 m. Photic depth has been defined as the depth where light intensity is 1% of its surface value (Valiela Citation1995). According to Spalding et al. (Citation2003), this limit may be as low 0.56% for brown algae, 0.12% for red algae and 0.01% for nongeniculate coralline algae. Following Runcie et al. (Citation2008), values may be even lower for crustose red algae (0.00023%). Considering the results presented here, it appears that for S. latissima, a lower photic depth should be considered corresponding roughly to the depth where PAR is 10% of the surface value, because growth was not observed deeper than 15 m.
Equation 7 may be rearranged as
Therefore, if PARz/PARsurf = 0.1 (10% of surface light) at 20 m, as mentioned before, the next equation may be used to calculate an approximate vertical limit for S. latissima production as a function of K:
The availability of data on the light extinction coefficient and surface light intensity for different regions may form a good basis to define, from equation 9, vertical depth ranges for S. latissima in different areas that may lead to significant growth.
Growth
The purpose of the present work was to establish whether S. latissima was able to grow at the southern distribution limit of the species, under rough sea conditions at an offshore location. Buck & Buchholz (Citation2004, 2005) tested several carrier constructions and different mooring systems in the North Sea for cultivation of S. latissima. They reported an average biomass yield of 4 kg m−1 after 6 months, with fronds able to withstand current velocities of 2 m s−1 and wave heights up to 6.4 m. In the present study, S. latissima withstood higher wave heights (maximum and significant wave heights were around 10 and 7 m, respectively). Peteiro & Freire (Citation2013) found that S. latissima had higher length and mass when cultivated in a moderately exposed environment than in a sheltered site, suggesting that high-energy environments per se are not detrimental to S. latissima growth. Those authors reported average thallus lengths of 166 and 136 cm for moderately exposed and sheltered sites, respectively, in a Galician bay. These values are not very different from another study in the same ria in Spain, reporting an average length of 162 cm (Freitas et al. 2015). Average length obtained in the present study was around 30% lower than those values. Reasons behind this difference may be the cultivation method – that is, closer to the surface than the 5 m we used – and a longline configuration that prevented self-shading. Growth rates obtained in the present study (3.5%–4.3% at 120 days) are comparable with values reported by Freitas et al. (2015) of 4.5% (after 102 days) and 2.7% (at 140 days). Wang et al. (Citation2014) reported a maximum growth rate of around 1 cm day−1 (around 0.9 cm day−1 here) in a cultivation site near salmon cages in Tristein, Norway.
High variability in length and fresh weight (FW) between individuals may be due to differences in developmental stage at deployment, because most of them were around 0.5–1.0 cm, but smaller seedlings were also present in the seeded string, and there may have been a shading effect from faster growing individuals on smaller ones. In the present study, during the first period, RGR decreased with depth; in the second, the highest RGR was found at 10 m. Between May and July, temperature at the surface may have been above the reported optimum, and light levels may have been excessive, leading to photoinhibition and higher growth of fronds deployed deeper where environmental variables are more fit for their growth.
Kim et al. (Citation2015) cultivated S. latissima at a similar latitude, although under lower temperatures in the northeast Atlantic (Long Island Sound, USA). They reported average yields of 6 to 9 kg FW m−1 for a 5-month growth period, whereas Marinho et al. (2015b) reported a biomass yield over two growing seasons of around 3.0 kg FW m−1 in Denmark. Highest mean length and individual weight were 123 cm and 92 g FW, respectively, at the end of the second growing season in May. In our study, although no precise measures of yield were taken, a very rough estimation may be achieved by considering that the sporophytes collected at different depths corresponded to a segment of seeded string inserted every 20 cm in the vertical longlines. This is a conservative estimate and thus yield as kg m−1 would equal weight *5 [as is times 5] of total biomass collected at each depth. From these calculations, a yield of 2.5–3.5 kg m−1 at 5 m can be estimated. Compared to other studies, these are relatively low values. However, as stated before, the cultivation method was not optimised for maximum production but to assess growth viability in harsh sea conditions at the southern distribution limit of the species.
Carbon and nitrogen content
Nitrogen content values measured in this study are within the range reported in other studies for blades of sporophytes cultivated in the sea close to fish farms (Handa et al. Citation2013; Sanderson et al. Citation2012) and with mussel rafts (Freitas et al. 2015), which confirms that nutrient concentration in seawater in this area is suitable for seaweed cultivation. The increase in C:N observed from May to July in blades resulted from an increase in C, suggesting a higher accumulation of carbohydrates in summer, coincident with the period of slower growth, when reserve storage compounds increase (Black Citation1950; Schiener et al. Citation2015).
Pulse amplitude–modulated fluorometry of PSII
Maximum quantum yields obtained in the present study (> 0.7) compare well with maximum yields obtained in situ by Spurkland & Iken (Citation2012) for S. latissima. In , RLC parameter values obtained for different seaweeds are synthesised together with values obtained in this study. These values show that results presented here are within the ranges reported in the literature. The value of such a comparison is limited by several factors such as differences in time of the day, water temperature and nutrient concentrations when RLCs were obtained. It is well known that RLC parameters undergo changes in relation to daily solar cycle related to photoprotective mechanisms (e.g. Gevaert et al. Citation2003). It is also known that temperature influences these parameters (e.g. Morris & Kromkamp Citation2003). The same applies to nutrient concentrations: under nutrient limitation (e.g. nitrogen limitation), quantum yields tend to decrease (e.g. Bergmann et al. Citation2002). Another factor that contributes to differences in parameter values is whether relative or absolute ETR was quantified by different authors. In the former case, ETRmax was obtained as in the present work except without the multiplication by thallus absorptance, leading to slightly higher values.
Table 2. Comparison of ETR parameter values obtained in different studies.
Despite the limitations described above, results obtained in this work suggest that depression of photosynthesis may occur at PAR light intensities between c. 300 and 400 μmol photons m−2 s−1. These light intensities may be reached at different depths, depending on sunlight intensity and water transparency. For example, in the July survey, such light levels were measured between 12 and 18 m depth. However, in previous surveys (18 February and 14 May), such light levels were observed between 3 and 10 m and between 1 and 5 m, respectively.
Despite the relatively high α, considering the ranges presented in , S. latissima does not exhibit significant growth at light levels below 10% of the surface irradiance (cf. Environmental Variables subsection). This may be a result of overestimation of the light extinction coefficient or of high metabolic losses of this species. Whatever the reasons, results presented herein suggest that photosynthetic performance is good in the depth range of 0–15 m.
CONCLUSIONS
Saccharina latissima grew in offshore exposed conditions at the southern distribution limit for the species, presenting good growth rates while withstanding high wave heights. The growing season may be extended until May for food applications, with epibionts appearing subsequently. If used for other applications that are less demanding in terms of appearance, like feed or biofuels, harvest can be further delayed. Optimisation of technological and biological aspects is needed for cultivation of S. latissima in these conditions. Technological issues are related to development of low-maintenance/high-resistance support structures and harvest techniques. Early deployment, to extend the growth period, should be tested.
Supplemental Material
Download MS Word (37.9 KB)Acknowledgements
We thank the two anonymous reviewers for the helpful comments and suggestions which helped improve the article.
SUPPLEMENTAL DATA
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Baghel R.S., Trivedi N., Gupta V., Neori A., Reddy C.R.K., Lali A. & Jha B. 2015. Biorefining of marine macroalgal biomass for production of biofuel and commodity chemicals. Green Chemistry 17: 2436–2443. DOI:10.1039/C4GC02532F.
- Bartsch I., Wiencke C., Bischof K., Buchholz C.M., Buck B.H., Eggert A., Feuerpfeil P., Hanelt D., Jacobsen S., Karez. R. et al. 2008. The genus Laminaria sensu lato: recent insights and developments. European Journal of Phycology 43: 1–86.
- Bergmann T., Richardson T.L., Paerl H.W., Pinckney J.L. & Schofield O. 2002. Synergy of light and nutrients on the photosynthetic efficiency of phytoplankton populations from the Neuse River Estuary, North Carolina. Journal of Plankton Research 24: 923–933. DOI:10.1093/plankt/24.9.923.
- Black W.A.P. 1950. The seasonal variation in weight and chemical composition of the common British Laminariaceae. Journal of the Marine Biological Association of the United Kingdom 29: 45–72. DOI:10.1017/S0025315400056186.
- Bolton J.J. & Luning K. 1982. Optimal-growth and maximal survival temperatures of Atlantic Laminaria species (Phaeophyta) in culture. Marine Biology 66: 89–94. DOI:10.1007/BF00397259.
- Buck B.H. & Buchholz C.M. 2004. The offshore-ring: a new system design for the open ocean aquaculture of macroalgae. Journal of Applied Phycology 16: 355–368. DOI:10.1023/B:JAPH.0000047947.96231.ea.
- Buck B.H. & Buchholz C.M. 2005. Response of offshore cultivated Laminaria saccharina to hydrodynamic forcing in the North Sea. Aquaculture 250: 674–691. DOI:10.1016/j.aquaculture.2005.04.062.
- Duarte P., Ramos M., Calado G. & Jesus B. 2013. Laminaria hyperborea photosynthesis-irradiance relationship measured by oxygen production and pulse-amplitude-modulated chlorophyll fluorometry. Aquatic Biology 19: 29–44. DOI:10.3354/ab00515.
- Eilers P.H.C. & Peeters J.C.H. 1988. A model for the relationship between light-intensity and the rate of photosynthesis in phytoplankton. Ecological Modelling 42: 199–215. DOI:10.1016/0304-3800(88)90057-9.
- Falkowski P.G. & Raven J.A. 1997. Aquatic photosynthesis. Blackwell Science, Oxford, UK, 375 pp.
- Figueroa F.L., Conde-Alvarez R. & Gómez I. 2003. Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynthesis Research 75: 259–275. DOI:10.1023/A:1023936313544.
- Fiuza A.F.D., Demacedo M.E. & Guerreiro M.R., 1982. Climatological space and time-variation of the Portuguese coastal upwelling. Oceanologica Acta 5: 31–40.
- Food and Agriculture Organization. 2004-2018. Cultured aquatic species information programme. Laminaria japonica (Ed. by J. Chen), FAO Fisheries and Aquaculture Department (online). Rome. Updated 1 January 2004. http://www. fao.org/fishery/culturedspecies/Laminaria_japonica/en; searched on 07 April 2018.
- Forbord S., Skjermo J., Arff J., Handa A., Reitan K.I., Bjerregaard R. & Luning K. 2012. Development of Saccharina latissima (Phaeophyceae) kelp hatcheries with year-round production of zoospores and juvenile sporophytes on culture ropes for kelp aquaculture. Journal of Applied Phycology 24: 393–399. DOI:10.1007/s10811-011-9784-y.
- Freitas J.R.C., Salinas Morrondo J.M. & Cremades Ugarte J. 2015. Saccharina latissima (Laminariales, Ochrophyta) farming in an industrial IMTA system in Galicia (Spain). Journal of Applied Phycology 28: 377–385. DOI:10.1007/s10811-015-0526-4.
- Gao K. & McKinley K.R. 1994. Use of macroalgae for marine biomass production and CO2 remediation: a review. Journal of Applied Phycology 6: 45–60. DOI:10.1007/BF02185904.
- Gerard V.A. & Dubois K.R. 1988. Temperature ecotypes near the southern boundary of the kelp Laminaria Saccharina. Marine Biology 97: 575–580. DOI:10.1007/BF00391054.
- Gevaert F., Creach A., Davoult D., Migne A., Levavasseur G., Arzel P., Holl A.C. & Lemoine Y. 2003. Laminaria saccharina photosynthesis measured in situ: photoinhibition and xanthophyll cycle during a tidal cycle. Marine Ecology Progress Series 247: 43–50. DOI:10.3354/meps247043.
- Hancke T.B., Hancke K., Johnsen G. & Sakshaug E. 2008. Rate of O2 production derived from pulse-amplitude-modulated fluorescence: testing three biooptical approaches against measured O2-production rate. Journal of Phycology 44: 803–813. DOI:10.1111/j.1529-8817.2008.00509.x.
- Handa A., Forbord S., Wang X.X., Broch O.J., Dahle S.W., Storseth T.R., Reitan K.I., Olsen Y. & Skjermo J. 2013. Seasonal- and depth-dependent growth of cultivated kelp (Saccharina latissima) in close proximity to salmon (Salmo salar) aquaculture in Norway. Aquaculture 414: 191–201. DOI:10.1016/j.aquaculture.2013.08.006.
- Heinz Walz GmbH. 1998. Underwater fluorometer DIVING-PAM: submersible photosynthesis yield analyser Handbook of operation. Heinz Walz GmbH, Effeltrich, Germany.
- Hidrográfico Português Instituto. 2015. Portuguese navy. Accessed on 3 November http://www.hidrografico.pt.
- Hurd C.L., Harrison P.J., Bischof K. & Lobban C.S. 2014. Seaweed ecology and physiology. Cambridge University Press, Cambridge, UK. 551 pp.
- Hwang E.K., Yotsukura N., Pang S.J., Su L. & Shan T.F. 2019. Seaweed breeding programs and progress in eastern Asian countries. Phycologia 58: 484–495. DOI: 10.1080/00318884.2019.1639436.
- Jansen H.M., Van Den Burg S., Bolman B., Jak R.G., Kamermans P., Poelman M. & Stuiver M. 2016. The feasibility of offshore aquaculture and its potential for multi-use in the North Sea. Aquaculture International 24: 735–756. DOI:10.1007/s10499-016-9987-y.
- Kim J.K., Kraemer G.P. & Yarish C. 2015. Use of sugar kelp aquaculture in Long Island Sound and the Bronx River estuary for nutrient extraction. Marine Ecology Progress Series 531: 155–166. DOI:10.3354/meps11331.
- Kraan S., Tramullas A.V. & Guiry M.D. 2000. The edible brown seaweed Alaria esculenta (Phaeophyceae, Laminariales): hybridization, growth and genetic comparisons of six Irish populations. Journal of Applied Phycology 12: 577–583. DOI:10.1023/A:1026519030398.
- Lemos R.T. & Pires H.O. 2004. The upwelling regime off the west Portuguese coast, 1941–2000. International Journal of Climatology 24: 511–524. DOI:10.1002/(ISSN)1097-0088.
- Liu F., Pang S.J. & Gao S.Q. 2015. Growth performance of unialgal gametophytes of the brown alga Saccharina japonica in mass culture conditions. Journal of Applied Phycology 28: 1145–1152. DOI:10.1007/s10811-015-0675-5.
- Marinho G., Holdt S. & Angelidaki I. 2015a. Seasonal variations in the amino acid profile and protein nutritional value of Saccharina latissima cultivated in a commercial IMTA system. Journal of Applied Phycology 25: 1991–2000. DOI:10.1007/s10811-015-0546-0.
- Marinho G., Holdt S., Birkeland M. & Angelidaki I. 2015b. Commercial cultivation and bioremediation potential of sugar kelp, Saccharina latissima, in Danish waters. Journal of Applied Phycology 27: 1963–1973. DOI:10.1007/s10811-014-0519-8.
- Morris E.P. & Kromkamp J.C. 2003. Influence of temperature on the relationship between oxygen- and fluorescence-based estimates of photosynthetic parameters in a marine benthic diatom (Cylindrotheca closterium). European Journal of Phycology 38: 133–142. DOI:10.1080/0967026031000085832.
- Murata M. & Nakazoe J. 2001. Production and use of marine algae in Japan. Jarq-Japan Agricultural Research Quarterly 35: 281–290. DOI:10.6090/jarq.35.281.
- Nitschke U., Connan S. & Stengel D.B. 2012. Chlorophyll a fluorescence responses of temperate Phaeophyceae under submersion and emersion regimes: a comparison of rapid and steady-state light curves. Photosynthesis Research 114: 29–42. DOI:10.1007/s11120-012-9776-z.
- Peteiro C. & Freire O. 2009. Effect of outplanting time on commercial cultivation of kelp Laminaria saccharina at the southern limit in the Atlantic coast, NW Spain. Chinese Journal of Oceanology and Limnology 27: 54–60. DOI:10.1007/s00343-009-0054-7.
- Peteiro C. & Freire O. 2013. Biomass yield and morphological features of the seaweed Saccharina latissima cultivated at two different sites in a coastal bay in the Atlantic coast of Spain. Journal of Applied Phycology 25: 205–213. DOI:10.1007/s10811-012-9854-9.
- Peteiro C., Sanche N. & Martinez B., 2016. Mariculture of the Asian kelp Undaria pinnatifida and the native kelp Saccharina latissima along the Atlantic coast of Southern Europe: an overview. Algal Research-Biomass Biofuels and Bioproducts 15: 9–23.
- Reid G.K., Chopin T., Robinson S.M.C., Azevedo P., Quinton M. & Belyea E. 2013. Weight ratios of the kelps, Alaria esculenta and Saccharina latissima, required to sequester dissolved inorganic nutrients and supply oxygen for Atlantic salmon, Salmo salar, in integrated multi-trophic aquaculture systems. Aquaculture 408: 34–46. DOI:10.1016/j.aquaculture.2013.05.004.
- Runcie J.W., Gurgel C.F.D. & McDermid K.J. 2008. In situ photosynthetic rates of tropical marine macroalgae at their lower depth limit. European Journal of Phycology 43: 377–388. DOI:10.1080/09670260801979303.
- Sanderson J.C., Dring M.J., Davidson K. & Kelly M.S. 2012. Culture, yield and bioremediation potential of Palmaria palmata (Linnaeus) Weber & Mohr and Saccharina latissima (Linnaeus) C.E. Lane, C. Mayes, Druehl & G.W. Saunders adjacent to fish farm cages in northwest Scotland. Aquaculture 354: 128–135. DOI:10.1016/j.aquaculture.2012.03.019.
- Saroussi S. & Beer S. 2007. Acclimations of macroalgae as reflected in photosynthetic parameters derived from PAM fluorometry, and possible implications for abundance patterns. Marine Ecology-An Evolutionary Perspective 28: 377–383. DOI:10.1111/mae.2007.28.issue-3.
- Schiener P., Black K.D., Stanley M.S. & Green D.H. 2015. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. Journal of Applied Phycology 27: 363–373. DOI:10.1007/s10811-014-0327-1.
- Shannon E. & Abu-Ghannam N. 2019. Seaweeds as nutraceuticals for health and nutrition. Phycologia 58: 563–577. DOI:10.1080/00318884.2019.1640533.
- Spalding H., Foster M.S. & Heine J.N. 2003. Composition, distribution, and abundance of deep-water (>30m) macroalgae in central California. Journal of Phycology 39: 273–284. DOI:10.1046/j.1529-8817.2003.02010.x.
- Spurkland T. & Iken K. 2012. Seasonal growth of Saccharina latissima. Phycological Research 60: 261–275. DOI:10.1111/j.1440-1835.2012.00657.x.
- Statsoft. 2004. Statistica software version 6.1. http://www.statsoft.com/Products/STATISTICA-Features
- Troell M., Joyce A., Chopin T., Neori A., Buschmann A.H. & Fang J.G. 2009. Ecological engineering in aquaculture - potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 297: 1–9. DOI:10.1016/j.aquaculture.2009.09.010.
- Valiela I. 1995. Marine ecological processes. Springer, New York, New York, USA.
- van Hal J.W., Huijgen W.J.J. & Lopez-Contreras A.M. 2014. Opportunities and challenges for seaweed in the biobased economy. Trends in Biotechnology 32: 231–233. DOI:10.1016/j.tibtech.2014.02.007.
- Wang X.X., Broch O.J., Forbord S., Handa A., Skjermo J., Reitan K.I., Vadstein O. & Olsen Y. 2014. Assimilation of inorganic nutrients from salmon (Salmo salar) farming by the macroalgae (Saccharina latissima) in an exposed coastal environment: implications for integrated multi-trophic aquaculture. Journal of Applied Phycology 26: 1869–1878. DOI:10.1007/s10811-013-0230-1.