ABSTRACT
Seaweed farming has now expanded across several continents from South East Asia to South America, Northern Europe, Canada and East Africa, contributing to global food security, supporting rural livelihoods, alleviating poverty and improving the health of oceans. Latin America (LA) covers a vast geographical area, which includes four different oceanic domains (Temperate Northern Pacific, Tropical Eastern Pacific, Temperate South America and Tropical Atlantic) and encompasses many types of coastal ecosystems with a wide range of seaweed species. LA has major potential for the development of seaweed farming activities; however, almost all the production is based on the harvesting of natural beds. This review describes the development of and prospects for the aquaculture seaweed industry in LA. The status of the seaweed aquaculture sector for green, brown and red seaweed and the main industry challenges are addressed. Regulation in the primary countries is also discussed. The expansion of the aquaculture industry in this region can be improved with new strains and farming methodologies, diversification of species, market expansion and an increase in domestic demand.
STATUS OF SEAWEED AQUACULTURE IN LATIN AMERICA
The seaweed aquaculture industry has dramatically increased production over the last 50 years and currently is responsible for 96% of global seaweed demand (Duarte et al. Citation2017). In 2016, global cultivation of aquatic plants, mainly seaweeds, reached a production volume of 30.2 million tonnes, of which 40.7% was from the tropical seaweeds Kappaphycus and Eucheuma for the extraction of carrageenan (FAO Citation2018a). Species of Gracilaria are also among the most cultivated species, mainly for agar production, while production of Pyropia (also as Porphyra), Undaria pinnatifida (Harvey) Suringar and Saccharina has been mainly for human consumption (FAO Citation2018a).
During the last 10 years, seaweed aquaculture has expanded rapidly due to increasing demand for edible seaweed, nutraceuticals, pharmaceuticals, antimicrobials and other compounds with biotechnological uses (Cottier-Cook et al. Citation2016; Shannon & Abu-Ghannam Citation2019). Seaweed is also gaining special attention as a potential source of new therapeutic compounds and other chemicals with novel industrial applications (Lorbeer et al. Citation2013). Moreover, seaweed aquaculture contributes to climate change adaptation by damping wave energy and protecting shores, and by elevating pH and supplying oxygen to the water, thereby locally reducing the effects of ocean acidification and deoxygenation (Duarte et al. Citation2017; Fernández et al. Citation2019). Consequently, the demand for seaweed products by western markets is expected to increase quickly in the future, due to an interest in alternative sources of protein, food supplements and sustainable textural compounds, that can meet future food demands (Kim et al. Citation2017). Accordingly, the seaweed aquaculture industry will have a pivotal role in supplying seaweed-derived products worldwide.
According to the FAO (Citation2018b), global seaweed production was approximately 30.8 million tonnes in 2016, of which 98% came from aquaculture and 2% from harvesting of natural beds. Latin America (LA) contributed only 1.2% of this production (373 thousand tonnes). Contrary to what occurs worldwide, only 4% of LA production came from aquaculture with 96% from natural beds. Chile contributes 88% of the total seaweed harvest in Latin America (329 thousand tonnes), while the remainder is divided between Peru (4.1%) and Mexico (3.7%). With respect to seaweed cultivation, Chile is the primary producer, being responsible for 95% of production (14,846 tonnes). This is followed by Brazil (4.68%), Mexico (1.15%), Ecuador (0.03%) and Peru (0.01%). Even if the FAO database is not precise, these numbers reflect the main pattern of industrial seaweed production in LA.
The seaweed aquaculture industry in LA is still in its infancy, and its development requires several biological, political and technological strategies. There is an urgent need for the establishment of regulations and best practices for seaweed farming. In addition, a trained workforce is required to provide the human resources for communities involved in this activity, and to facilitate the development of the industry (Rebours et al. Citation2014).
LA has a land surface area of 21 million km (Wurmann Citation2017) and a coastline of 59,591 km2. This ranges from 30°N to 55°S and includes four oceanic domains: Temperate Northern Pacific, Tropical Eastern Pacific, Temperate South America, and Tropical Atlantic. This vast geographical area encompasses many types of coastal ecosystems and includes diverse seaweed species with potential for the development of seaweed farming activity.
This review describes the development of and prospects for a seaweed aquaculture industry in LA. To this end, the status of the seaweed aquaculture sector for green, brown and red seaweeds is described, together with the main regulations and challenges facing the industry.
DEVELOPMENTS AND CHALLENGES OF LATIN AMERICAN SEAWEED INDUSTRY
Cultivation of brown seaweeds
The current Latin American aquaculture sector of brown seaweeds is scarcely developed, with only Mexico and Peru appearing in the 2016 cultivation statistics by FAO (Citation2018a). However, figures for Mexico are vague, although temporary permits and aquaculture on an experimental basis have been conducted for some commercial kelps (i.e. Macrocystis and Eisenia). Similarly, the 1 tonne of Macrocystis reported for Peru is probably derived from experimental pilot projects (FAO Citation2018a).
Recent progress in the domestication of Eisenia arborea (Areschoug) M.D. Rothman, Mattio & J.J. Bolton in Mexico has led to its testing as an alternative to Macrocystis pyrifera (Linnaeus) C. Agardh for feeding red abalone (Haliotis rufescens Swainson, 1822) in Baja California, Mexico (Zertuche-González et al. Citation2014). Although M. pyrifera appeared to be a better diet for large animals, the growth rates obtained with E. arborea, and the fact that this species could be cultivated in warmer waters, make this seaweed a good substitute.
Despite all Chilean brown seaweed production being based on wild harvest, attempts to cultivate and scale-up kelp production have been made at least for 10 years. In fact, Chile actively cultivated M. pyrifera, achieving a production of 12 tonnes (ww) in 2010 with an associated value of US$12,000 (FAO Citation2018a). This species is sold fresh for abalone feed and dry for alginate extraction. According to Camus et al. (Citation2018) the development of commercial cultivation is stimulated by increasing demand based on new markets such as human consumption or biofuel/chemical applications. Buschmann et al. (Citation2017) summarised two systems which had been tested: a tank cultivation system to produce unattached, floating sporophytes after several months, and one using gametophytes and sporophytes settled on artificial substrata, e.g. strings or ropes, inside tanks. In both cases, after sporophytes reached sufficient size, they were transferred to long-lines in marine farms. In southern Chile, hatchery and pilot cultivation of Macrocystis were successful (Gutierrez et al. Citation2006), and pilot-scale cultivation of M. pyrifera (, ) was accomplished for the production of biofuels, organic fertilisers and new food products (Buschmann et al. Citation2008).
Figs 1–6. Examples of commercial and pilot-scale cultivation of seaweed in Latin America.
Figs 1–2. Commercial pilot cultivation of Macrocystis pyrifera in southern Chile with a closeup of M. pyrifera cultivated using the long-line technique (J. Infante).
Figs 3–4. Pond cultivation of Ulva spp. at the 300 m2 facilities of the Autonomous University of Baja California with a specimen of cultivated Ulva (K. Navarro).
Fig. 5. Bottom cultivation of Agarophyton chilensis in southern Chile (D. Robledo).
Fig. 6. Pilot cultivation of Sarcothalia crispata (J. Zamorano).

Another highly exploited brown seaweed on Chilean coasts is Lessonia. Attempts to cultivate Lessonia were also made, with the aim of avoiding depletion of natural beds. In northern Chile, experimental farms of Lessonia were established (Edding et al. Citation1990; Tala et al. Citation2004), and culture conditions for microscopic stages of L. nigrescens Bory were developed (Ávila et al. Citation1985). Since 2011, L. nigrescens has been separated into two cryptic species, L. berteroana Montagne and L. spicata (Suhr) Santelices (González et al. Citation2012; Tellier et al. Citation2011). Westermeier et al. (Citation2017) examined the population dynamics of L. trabeculata Villouta & Santelices in natural beds and compared this to results from cultivation. These authors concluded that wild populations recover slowly because of low growth rates and high herbivory. They noted that this was also true in sea cultivation, making commercial cultivation unprofitable, but that cultivation could be considered for repopulation projects.
Macrocystis pyrifera has also been suggested for use in integrated multi-trophic systems (IMTA) to reduce the nutrient load caused by salmon farming in Chile (Buschmann et al. Citation2001; Chopin et al. Citation2001; Fernández et al. Citation2019). Varela et al. (Citation2018) studied the influence of temperature, light and nutrients to find optimal conditions for growth close to salmon farms. This was done to evaluate not only the productivity of the seaweed in IMTA, but also its potential for bioremediation. As expected, productivity closely matched the seasonality of salmon production. Since salmon achieve commercial size in the summer, this is the period that produces more effluent which can be used by the seaweed to produce more biomass. However, the main challenge was to improve seaweed nitrate intake capacity by photosynthesis and consequent biomass production, considering the cultivation area available for IMTA.
It is clear that brown seaweed aquaculture in LA still has a long way to go before production is comparable to Asian competitors and even efforts in the USA (Kim et al. 2019). Despite the available knowledge for cultivation of Macrocystis and Lessonia, scaling-up production is a challenge. The high costs related to hatcheries are too expensive when harvesting can still be done from natural beds. Moving forward, production systems of brown seaweeds need to be optimised to make their cultivation profitable.
Cultivation of green seaweeds
In southern Brazil, several experimental farms for green seaweeds were established. Nonetheless, no artisanal or commercial cultivation is currently operational in the region (Pellizzari & Reis Citation2011). In Parana State, natural populations of the Monostroma complex were traditionally exploited and sold to restaurants in Sao Paulo State. This activity ceased due mainly to the reduction of natural populations, the lack of a defined market, and insufficient technological development for cultivation. However, according to Pellizzari & Reis (Citation2011) cultivation of Monostroma could still be justified, considering the increasing global demand for monostromatic algae by the cosmetics industry and Brazil’s third-place position in consumption of cosmetics.
In Brazil, there are two species of monostromatic Chlorophyta, Gayralia oxysperma (Kützing) K.L. Vinogradova ex Scagel et al. and Monostroma sp., with some economic potential (Pellizzari & Reis Citation2011). Pellizzari et al. (Citation2007) evaluated the feasibility of cultivating Gayralia sp. at Paranagua Bay, in nets placed in mangrove fringes. The species achieved a production of 58 g m−2 (dw) in the spring. Pellizzari et al. (Citation2008) selected one strain of Gayralia from a natural population for cultivation based on its physiological adaptations. According to them, strains collected in outer estuarine areas had larger thalli, higher growth rates and wider tolerance to environmental variation.
In the State of Rio Grande do Sul, Ulva clathrata (Roth) C. Agardh and U. ramulosa Smith were studied for their potential application as bio-extractor organisms in integrated aquaculture with shrimp (Pellizzari & Reis Citation2011). Of these species, U. ramulosa was a more effective biofilter for nitrogenous compounds. The bio-extractive efficiency of Ulva clathrata for inorganic compounds was demonstrated by Copertino et al. (Citation2009) in outdoor tanks receiving effluent water from a shrimp pond. In this work, a reduction in chlorophyll-a was observed in the water, suggesting that the decrease in nitrogenous compounds caused by U. clathrata reduced phytoplankton growth. In Santa Catarina State, Pallaoro et al. (Citation2016) verified that replacement of commercial feed of Litopenaeus vannamei (Boone 1931) with up to 50% U. lactuca Linnaeus did not affect their growth performance. Thus, Ulva in IMTA could not be only a biofilter, but also feed.
Research on novel properties and applications for Brazilian green monostromatic seaweeds has been conducted to support the development of commercial cultivation. This research included the identification of antiviral and antibiotic activities (Cassolato et al. Citation2008, Fernandes et al. Citation2014). Pellizzari & Reis (Citation2011) stated that the cultivation of Monostroma in southern Brazil was economically viable due to its high growth rate, simple cultivation techniques, and vast areas potentially available for cultivation. Nonetheless, several cultural and political changes are required to stimulate the development of this industry.
Ulva clathrata was cultivated by Aonori Aquafarms Inc. that initiated production with the brown shrimp Farfantepenaeus californiensis (Holmes 1900) in México. Results showed that the co-culture method can result in commercial yields, with 30 shrimp per m2 as the best stocking density based on shrimp performance (Peña-Rodríguez et al. Citation2017). Moreover, the chemical composition of U. clathrata indicates the potential for use as human and animal food (Peña-Rodríguez et al. Citation2011). Recently, the Autonomous University of Baja California (IIO-UABC) began production of U. lactuca and U. fasciata S.F. Gray in pilot commercial ponds (, ) that generated 180 g ww m−2 d−1 (Navarro Citation2016). This project has the participation of Productos Marinos de las Californias, whose parent company is in the United States. The collaboration included the production of the seaweed, its processing to convert it into raw material, and the development of recipes and products, while the parent company took charge of commercialization.
Cultivation of red seaweeds
The industrial cultivation of red seaweeds in LA has been mainly centred on Gracilaria chilensis C.J. Bird, McLachlan & E.C. Oliveira (now Agarophyton chilensis (C.J. Bird, McLachlan et E.C. Oliveira) Gurgel, J.N. Norris et Fredericq; Gurgel et al. Citation2018) in Chile (FAO Citation2018a; , ). The successful Chilean story has increased the interest of other LA countries in developing industrial seaweed aquaculture operations (Hayashi et al. Citation2013). Several countries in LA conducted trials based mainly on the exotic species Kappaphycus alvarezii (Doty) Doty ex P.C. Silva and Eucheuma striatus (F. Schmitz) Doty ex P.C. Silva, but none have progressed to commercial farms, with the exception of Brazil (Hayashi et al. Citation2017). The status and projections of red seaweed aquaculture in LA are described based on their regional applications.
AGAROPHYTES
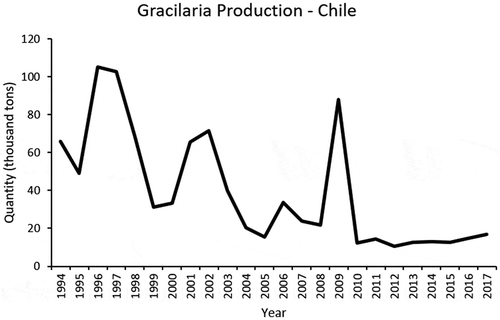
Chile has increased Gracilaria (pelillo in Spanish) production in the last five years, from 10.5 thousand tonnes (ww) in 2012 to 16.7 thousand tonnes in 2017. However, since 1994, production has fluctuated, with a maximum of 105 thousand tonnes in 1996 () (Sernapesca Citation1994–2017). These fluctuations seem to be related to thallus aging. This is because all production is based on vegetative propagation, and the fact that during harvesting, the new tissue of Gracilaria is always cut off and old tissue remains for the new production cycle. These problems were partially solved by seeding carpospores on ropes, but this increases cultivation time (Buschmann et al. Citation2008). Other problems seemed to be related to the presence of pests and epiphytes, which seem to be influenced by the effects of climate change and oscillation of seawater temperature. The green seaweed Rhizoclonium, forced the Chilean government to declare one production region in Río Maullín, Los Lagos, a plague area in 2015 (Ministerio de Economia, Fomento y Turismo Citation2015). The unpredictable price fluctuations and Gracilaria spp. cultivation in Asia are responsible for the apparent lack of interest in developing novel cultivation techniques (Hayashi et al. Citation2013). In addition, the availability of farming areas for Gracilaria in the southern Chile has been significantly reduced due to the establishment of salmon and mollusc aquaculture installations (Buschmann et al. Citation2008).
Fig. 7. Gracilaria (Agarophyton) production from 1994 to 2017 in quantity (1000 tonnes of wet weight) from commercial farms (SERNAPESCA 1994–2017).
Species of Gelidium (chasca in Spanish) have also been used in the agar industry in Chile. In this case, all production is based on harvesting from natural populations in the Central region (FAO Citation2018b). The landings of these species reached 1,644 wet tonnes in 1991 but declined such that in 2017 only 228 wet tonnes were harvested (Sernapesca Citation1991, Citation2017). The cultivation potential of some species of Gelidium was investigated using free-floating and net systems with some successful results (Buschmann et al. Citation2008). Otaíza et al. (Citation2018) proposed a cultivation method based on thallus fragmentation and the formation of secondary attachment structures (SAS) in Gelidium lingulatum Kützing. However, all cultivation described in literature has been experimental. According to Callaway (Citation2015), the global supply of bacteriological agar has been affected by the overharvesting of Gelidium stocks and the unsteady supply from some producing countries. This may provide an opportunity for new cultivation or repopulation programs for Gelidium in LA.
Brazil has exploited species of Gracilaria since 1960, reaching a maximum of 2000 tonnes (dw) annually between 1973 and 1974 for export to the Japanese market. Subsequently, a sharp drop was observed, and exports declined to 250 tonnes (dw) with negligible amounts currently exported (Marinho-Soriano Citation2017). Initial efforts to cultivate Gracilaria took place in Rio Grande do Norte State, under the auspices of university and government agencies (Hayashi et al. Citation2013). Subsequently, the artisanal cultivation of Gracilaria birdiae E.M. Plastino & E.C. Oliveira has become an income source for local producers in the northeastern States of Paraiba, Rio Grande do Norte and Ceara. Nonetheless, since this cultivation was based on the use of seedlings from natural beds, uncontrolled harvesting reduced their availability, and risked overexploitation (Hayashi et al. Citation2013). The farming of G. birdiae in estuaries using effluent from shrimp ponds was proposed (Bezerra & Marinho-Soriano Citation2010; Marinho-Soriano et al. Citation2006, Citation2009a). The latter strategy has also been studied for other Gracilaria species (Marinho-Soriano et al. Citation2002, Citation2009b). In Sao Paulo State, other colour variants of G. birdiae proved adequate for commercial farming in Ubatuba Bay, with growth rates up to 4.4% day−1 and agar yield and quality close to that obtained from wild specimens in this region (Ursi et al. Citation2013).
During the 1990s, the company Soriano S.A. established a Gracilaria sp. farm in Patagonia (Argentina). Harsh oceanographic conditions and heavy epiphytism did not allow cultivation of this species in the region, and new cultivation strategies are needed (Hayashi et al. Citation2013). Martín et al. (Citation2011) evaluated fluctuations in biomass and reproductive effort of natural populations of Gracilaria gracilis (Stackhouse) M.Steentoft, L.M. Irvine & W.F. Farnham in Bahia Bustamante (the same place as Soriano S.A.). They observed that both vegetative propagation and sexual spore production were involved in bed sustainability, and that reproductive thalli occurred throughout the year. Moreover, the best time to obtain spores from cystocarpic thalli was in the summer and early autumn. These results should support new cultivation trials for Gracilaria in the region, or provide the basis for sustainable exploitation of natural beds.
In Mexico, the cultivation of G. vermiculophylla and Gracilaria cornea J. Agardh were not commercially viable. Low profitability led to declining interest in Gracilaria cultivation projects (Robledo et al. Citation2013).
In Peru, cultivation of Gracilariopsis lemaneiformis (BorydeSaint-Vincent) E.Y.Dawson, Acleto & Foldvik was done in the mid to late 1990s with governmental support. However, these trials were not successful mainly because of high temperature, grazing activity, and epiphytism where the farms were established (Hayashi et al. Citation2013). Since then, no other work has been reported in the literature.
CARRAGEENOPHYTES
Species of Kappaphycus and Eucheuma have been the main carrageenophytes cultivated in Latin America and globally (e.g. Hurtado et al. 2019). From the 20 countries which compose Latin America, seven (Brazil, Colombia, Cuba, Ecuador, Mexico, Panama and Venezuela) attempted to introduce and establish commercial cultivation (Hayashi et al. Citation2017). However, none of them has been especially successful.
Brazil probably is the country closest to implementing commercial cultivation. The first experimental introduction of K. alvarezii took place in 1995, on the north coast of São Paulo State (De Paula et al. Citation1999). The only large-scale commercial cultivation was established in the state of Rio de Janeiro from 1998 to 2011 (Castelar et al. Citation2009). However, activity ended in 2012, presumably due to lack of profitability. Currently, 10 seaweed farms operate in Ilha Grande and Sepetiba Bay, with a small factory to process carrageenan (Hayashi et al. Citation2017).
In addition to commercial farming, small-scale cultivation of K. alvarezii has been carried out in the south and southeast regions of Brazil (Pellizzari & Reis Citation2011). In 2008, this species was introduced in Santa Catarina State. This state is responsible for 95% of mollusc production, so the co-cultivation of seaweed with mussels and oysters seems to be a viable option (Hayashi et al. Citation2017). A socioeconomic analysis of seaweed monoculture versus co-cultivation with mussels and oysters concluded that co-cultivation with oysters was at least twice as profitable (Santos et al. Citation2018a). As a consequence of these results, a new floatation model was developed to support both seaweeds and oysters in the same structure (Santos et al. Citation2018b) as well as mechanical harvesting for both organisms (Hayashi et al. Citation2017).
In Mexico, the industrial exploitation of seaweeds for the phycocolloid industry has existed for half a century; however, only recently has such activity increased (Robledo et al. Citation2013). Despite the lack of commercial seaweed farming, experimental cultivations have been carried out for Eucheuma uncinatum Setchell & Gardner, Chondracanthus canaliculatus (Harvey) Guiry, and Eucheuma isiforme (C. Agardh) J. Agardh (Hayashi et al. Citation2013). Nonetheless, pilot-scale cultivation of the exotic Chondrus crispus Stackhouse (Zertuche-González et al. Citation2001) and K. alvarezii (Muñoz et al. Citation2004) gave the best results. The cultivation of K. alvarezii as an alternative mariculture activity in the Yucatan Peninsula appears viable. This was supported by growth achieved in these tropical and subtropical waters during the dry, and part of the wet, season, when the main fisheries sector does not operate. The potential of seaweed cultivation as an alternative activity to artisanal fishing was demonstrated by socio-economic research in 2002–2003 focusing on community-based coastal resource management in protected areas (Robledo & Townsend Citation2006). However, no commercial farms were established. A contributing factor is that, in contrast with other countries of LA such as Chile and Brazil, there is no official effort to expand seaweed farming in Mexico (Robledo et al. Citation2013).
Since the Peruvian Government does not regulate the wild harvest of red seaweeds, cultivation of Chondracanthus chamissoi was attempted in Peru in the early 2000s as an alternative to harvesting from natural beds and to minimise overharvesting. Higher values of gel strength and viscosity were found relative to harvests from natural beds and could enhance exports of the species (Hayashi et al. Citation2013). However, since C. chamissoi is used for human consumption as a gourmet ingredient in the traditional Peruvian dish ceviche, investment in cultivation for this purpose may be more viable than for the carrageenan industry.
Chile also carried out experimental cultivation to reduce harvest pressure on natural beds. This has been mainly with Gigartina skottsbergii Setchell & N.L. Garner, Sarcothalia crispata, Callophyllis variegata (Bory) Kützing and Chondracanthus chamissoi (C. Agardh) Kützing. Despite improvements in cultivation techniques (Ávila et al. Citation2011; Bulboa et al. Citation2010; Correa et al. Citation1999; Fonck et al. Citation2008), there are challenges to be resolved before commercial cultivation is realised. These include high mortality, poor sporeling attachment, heavy epiphytism and slow growth (Bulboa et al. Citation2007; Romo et al. Citation2001, Citation2006). Effort has concentrated on strategies for vegetative propagation. Bulboa et al. (Citation2013) proposed a new technique for cultivating Chondracanthus chamissoi in tanks based on secondary attachment discs (SAD). The SADs adapted to outdoor tank conditions, thus demonstrating technical feasibility. Following this work, Sáez & Macchiavello (Citation2018) and Macchiavello et al. (Citation2018) tested the growth of tank-grown SAD in the sea, with 40% of SADs persisting in the sea with growth rates of 4% day−1 (Sáez & Macchiavello Citation2018). SADs colonised substrates quickly and formed reproductive structures. Macchiavello et al. (Citation2018) evaluated the growth of SADs for one year and found greater formation of SADs and biomass in winter and spring. However, they also observed a direct correlation between biomass and epiphytes.
Hernández-González et al. (Citation2007) demonstrated that frond fragments and rhizoids of Gigartina skottsbergii, can generate new plants in the sea with similar growth rates to those observed in nursery settings. Sarcothalia crispata (Bory) Leister was also the focus of public and private projects (). Zamorano (Citation2016) described recent progress and proposed a cultivation system for S. crispata that included the following: hatchery stage to produce sporelings; differential growth of gametophytic and sporophytic plants; site selection based on local regimes of water movement to reduce herbivory, fouling and epiphytes; and the economics of the proposed cultivation strategy for implementation in southern Chile.
OTHER SPECIES AND APPLICATIONS
Significant diversification of seaweed cultivation and exploitation has occurred in LA over the last decade. Cultivation of red seaweeds has been promoted in the region due to the increasing demand for raw materials by the phycocolloid sector, feed for abalone farming, seaweed flour production for animal and human nutrition, and the development of new food items. One example is the aforementioned Chondracanthus chamissoi, used for the preparation of the dish ceviche (Hayashi et al. Citation2013). The potential of C. chamissoi in Peru led to its cultivation from 2012 to 2014, when Peruvian production from aquaculture was 131, 44, and 2 tonnes, respectively (FAO Citation2018b). Unfortunately, according to the FAO (Citation2018c), production of C. chamissoi ceased in 2015.
In the last decade, Chile has exported Callophyllis variegata (Bory) Kützing to Japan; nonetheless, this economic activity is currently being jeopardised due to the reduction of the natural beds in southern Chile, the lack of sustainable harvesting programs, and the absence adequate cultivation strategies (Hayashi et al. Citation2013). The edible seaweed Pyropia columbina (Montagne) W.A. Nelson (as Porphyra columbina Montagne) has a long history of commercialisation by traditional coastal populations in Chile. Mariculture trials on this species and other Pyropia spp. (as Porphyra) have been performed, with some success (Buschmann et al. Citation2008). Nonetheless, the limited market for this seaweed has halted the investment needed to establish industrial cultivation. More recently, other species of Pyropia (Porphyra) with higher market potential have been investigated in Chilean waters, generating new prospects for the aquaculture of Porphyra (Buschmann et al. Citation2008).
REGULATION OF SEAWEED CULTIVATION IN LA
Among the 20 countries which compose Latin America, only seven have national plans or policies for aquaculture development: Chile (SUBPESCA Citation2003), Peru (PRODUCE Citation2010), Brazil (MPA Citation2015), Colombia (AUNAP Citation2014), Uruguay (DINARA Citation2008), Argentina (UCAR Citation2015), Costa Rica (MIDEPLAN Citation2014), and El Salvador (MAG Citation2016). Of these, only Chile, Peru and Brazil mention programs for seaweed cultivation. All policies were supported in some way by FAO, and have guidelines for the organisation and promotion of sustainable aquaculture.
Brazil and Peru have in common areas of coastline dedicated to aquaculture, including the cultivation of seaweed. Their governments give concessions for use of these predetermined areas through a bidding process, for 20 and 30 years, respectively (PERU Citation2004; Santos Citation2014). Brazil divided aquaculture areas into onerous (from 2 to 10 ha) and non-onerous areas (up to 2 ha), so smaller producers can use the concession free of charge. Peru charges annually for the concession. Santa Catarina State, in southern Brazil, was the first and only state with aquaculture areas delimited by Local Plans for Marine Aquaculture Development (in Portuguese, Plano Local de Desenvolvimento da Maricultura – PLDM), and currently almost all producers have their own concessions, formalising the activity (Suplicy et al. Citation2017).
In Chile, there are no exclusive areas for aquaculture. Concessions for cultivation areas are given by the Subsecretary of Fisheries and Aquaculture (Subsecretaria de Pesca y Acuicultura) from the Ministry of Economy, Development and Tourism. This occurs after analysis of the aquaculture project and a previous assessment analysis of the region from the government’s Environmental Impact Assessment System. Municipal authorisation must also be obtained before beginning the activity (CHILE Citation1989; Zuniga-Jara & Soria-Barreto Citation2018).
Brazil is probably the only country which has specific regulations for seaweed cultivation. The Normative Instruction from the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA) number 185 (ICMBIO Citation2008) restricts commercial cultivation of Kappaphycus alvarezii between the north coast of Sao Paulo State and south coast of Rio de Janeiro. The issue is that these states do not have their PLDM’s ready, so all producers are working informally. Santa Catarina is the only state where producers work formally in aquaculture areas; however, because K. alvarezii is an introduced species, commercial cultivation of this species is not allowed. This is even after more than 10 years of studies, reports and negotiations with the government (Reis et al. Citation2017).
In 2016, Chile innovated with a new law specifically intended to benefit artisanal fishermen or small enterprises that create specific plans for seaweed cultivation and the repopulation of natural beds (Chile Citation2016). This kind of policy is essential to promote the activity and to protect natural resources from overharvesting.
CONCLUSIONS AND FUTURE PROSPECTS
Latin America has an important role in the global seaweed industry. Unfortunately, most seaweed production in LA is still based on harvesting natural beds. Consequently, it is urgent to include the most critical aspects in the regulation of seaweed farming. Policies supporting growth of small-scale seaweed aquaculture and repopulation of sites where overexploitation of seaweed has occurred are urgently needed in many regions. Chile enacted one policy which stimulated seaweed cultivation and repopulation. Several researchers are working to diversify the seaweed industry currently based on only Agarophyton chilensis. In the near future, commercial cultivation of other species will likely begin. Given the increasing demand for Chondracanthus chamissoi in Peruvian cuisine and government regulation of wild harvests, the development of coastal farms would be a natural next step.
Despite Brazil having a formal plan for aquaculture development that includes seaweed, political instability has affected seaweed cultivation and industry development. Between 2005 and 2014, the Brazilian Ministry of Fisheries and Aquaculture had seven new ministers, until its cancellation in 2015 (Suplicy et al. Citation2017). Subsequently, the ministry was included within the Ministry of Agriculture, Livestock and Food Supply and Ministry of Industry, Foreign Trade and Services. More recently it fell under the Special Secretary of Aquaculture and Fisheries. This discontinuity of decision makers, managers and technical staff delayed all plans for aquaculture development and seaweed cultivation.
In Mexico and Argentina, policy has been either absent or scarce. Special attention is needed in all countries at many different levels to develop sustainable and profitable seaweed production. Policies to promote domestication of native species and improve already established techniques of introduced species are needed.
In conclusion, it is clear that LA has great potential for seaweed aquaculture, and that the benefits associated with this sector clearly outweigh any negative aspects. Seaweed farming has the potential to improve the livelihoods of many coastal communities by becoming an alternative source of income. In addition, the activity can provide several ecosystem services which may benefit other industries and release existing harvesting pressures that have led to the overexploitation of natural seaweed beds. To achieve sustainable development of the sector in LA, a wide-ranging effort is needed to address market and aquaculture system development, strain improvement, marine spatial planning and policy-making.
ACKNOWLEDGEMENTS
We would like to thank Mr. Gunter Villena (Peru), Gonzalo Soriano (Argentina), and Dr. Cristian Bulboa and Dr. Raul Ugarte (Chile) for the support and up-to-date information in the Regulation in LA.
REFERENCES
- Ávila M., Hoffmann A. & Santelices B. 1985. Interacciones de temperatura, densidad de flujo fotónico y fotoperíodo sobre el desarrollo de etapas microscópicas de Lessonia nigrescens (Phaeophyta, Laminariales). Revista Chilena de Historia Natural 58: 71–82.
- Ávila M., Piel M.I., Caceres J.H. & Alveal K. 2011. Cultivation of the red alga Chondracanthus chamissoi: sexual reproduction and seedling production in culture under controlled conditions. Journal of Applied Phycology 23: 529–536. DOI: 10.1007/s10811-010-9628-1.
- AUNAP. 2014. Plan Nacional para el Desarrollo de la Acuicultura Sostenible en Colombia. Autoridad Nacional de Acuicultura y Pesca. http://aunap.gov.co/wp-content/uploads/2016/04/Plan-Nacional-para-el-Desarrollo-de-la-Acuicultura-Sostenible-Colombia.pdf; searched on 10 December 2018.
- Bezerra A.F. & Marinho-Soriano E. 2010. Cultivation of the red seaweed Gracilaria birdiae (Gracilariales, Rhodophyta) in tropical waters of northeast Brazil. Biomass and Bioenergy 34: 1813–1817. DOI: 10.1016/j.biombioe.2010.07.016.
- Bulboa C., Macchiavello J., Véliz K. & Oliveira E.C. 2010. Germination rate and sporeling development of Chondracanthus chamissoi (Rhodophyta, Gigartinales) varies along a latitudinal gradient on the coast of Chile. Aquatic Botany 92: 137–141. DOI: 10.1016/j.aquabot.2009.10.016.
- Bulboa C., Véliz K., Sáez F., Sepúlveda C., Vega L. & Macchiavello J. 2013. A new method for cultivation of the carragenophyte and edible red seaweed Chondracanthus chamissoi based on secondary attachment disc: development in outdoor tanks. Aquaculture 410–411: 86–94. DOI: 10.1016/j.aquaculture.2013.06.018.
- Bulboa C.R., Macchiavello J.E., Veliz K., Macaya E. & Oliveira E.C. 2007. In vitro recruitment of Ulva sp. and Enteromorpha sp. on gametophytic and tetrasporophytic thalli of four populations of Chondracanthus chamissoi from Chile. Journal of Applied Phycology 19: 247–254. DOI: 10.1007/s10811-006-9130-y.
- Buschmann A.H., Camus C., Infante J., Neori A., Álvaro I., Hernández-González M.C., Pereda S.V., Gómez- Pinchetti J.L., Golberg A., Tadmor-Shalev N., et al. 2017. Seaweed production: overview of the global state of exploitation, farming and emerging research activity. European Journal of Phycology 52: 391–406. DOI: 10.1080/09670262.2017.1365175.
- Buschmann A.H., Hernández-González M.D.C. & Daniel V. 2008. Seaweed future cultivation in Chile: perspectives and challenges. International Journal of Environment and Pollution 33: 432–456. DOI: 10.1504/IJEP.2008.020571.
- Buschmann A.H., Troell M. & Kautsky N. 2001. Integrated algal farming: a review. Cahiers de Biologie Marine 42: 83–90.
- Callaway E. 2015. Lab staple agar runs low: dwindling seaweed harvest imperils reagent essential for culturing microbes. Nature 528: 171–172.
- Camus C., Infante J. & Buschmann A. 2018. Overview of 3 years precommercial seafarming of Macrocystis pyrifera along the Chilean coast. Reviews in Aquaculture 10: 543–559. DOI: 10.1111/raq.12185.
- Cassolato J.E.F., Noseda M.D., Pujol C.A. Pellizari F.M., Damonte E.B. & Duarte M.E.R. 2008. Chemical structure and antiviral activity of the sulfated heterorhamnan isolated from the green seaweed Gayralia oxysperma. Carbohydrate Research 343: 3085–3095.
- Castelar B., Reis R.P. & Kirk R. 2009. Invasive potential of Kappaphycus alvarezii off the south coast of Rio de Janeiro state, Brazil: a contribution to environmentally secure cultivation in the tropics. Botanica Marina 52: 283–289.
- CHILE. 1989. Ley General De Pesca Y Acuicultura. Ministerio de Economía, Fomento y Reconstrucción. https://www.leychile.cl/N?i=30265&f=1991-09-06&p=; searched on 10 December 2018.
- CHILE. 2016. Ley 20925. Crea Bonificación para el Repoblamiento y Cultivo de Algas. Ministerio de Economía, Fomento y Reconstrucción. https://www.leychile.cl/N?i=1091690&f=2016-06-17&p=; searched on 10 December 2018.
- Chopin T., Buschmann A.H., Halling C., Troell M., Kautsky N., Kraemer G.P., Zertuche-González J.A., Yarish C. & Neefus C. 2001. Integrating seaweeds into marine aquaculture systems: a key toward sustainability. Journal of Phycology 37: 975–986. DOI: 10.1046/j.1529-8817.2001.01137.x.
- Copertino M., Da S., Tormena T. & Ulrich S. 2009. Biofiltering efficiency, uptake and assimilation rates of Ulva clathrata (Roth) J. Agardh (Chlorophyceae) cultivated in shrimp aquaculture waste water. Journal of Applied Phycology 21: 31–45. DOI: 10.1007/s10811-008-9357-x.
- Correa J.A., Beltrán J., Buschmann A.H. & Renato W. 1999. Healing and regeneration responses in Gigartina skottsbergii (Rhodophyta, Gigartinales): optimization of vegetative propagation for cultivation. Journal of Applied Phycology 11: 315–327. DOI: 10.1023/A:1008106527820.
- Cottier-Cook E.J., Nagabhatla N., Yacine B., Campbell M.L., Thierry C., Dai W., Fang J., He P., Hewitt C.L., Kim G.H., et al. 2016. Policy brief. Safeguarding the future of the global seaweed aquaculture industry. United Nations University Institute of Water, Environment and Health (UNU-INWEH) and Scottish Association for Marine Science (SAMS), South Hamilton, UK. 12 pp.
- De Paula E.J., Pereira R.T.L. & Ohno M. 1999. Strain selection in Kappaphycus alvarezii var. alvarezii (Solieriaceae, Rhodophyta) using tetraspore progeny. Journal of Applied Phycology 11: 111–121. DOI: 10.1023/A:1008085614360.
- DINARA. 2008. Política Nacional para el Desarrollo de la Acuicultura Sostenible en la República Oriental del Uruguay. Ministerio de Ganadería, Agricultura y Pesca Dirección Nacional de Recursos Acuáticos. http://www.mgap.gub.uy/sites/default/files/multimedia/politica_acuicola_sostenible.pdf; searched on 10 December 2018.
- Duarte C.M., Wu J., Xiao X., Bruhn A. & Krause-Jensen D. 2017. Can seaweed farming play a role in climate change mitigation and adaptation? Frontiers in Marine Science 4: 1–10. DOI: 10.3389/fmars.2017.00100.
- Edding M., Venegas M., Orrego P. & Fonck E. 1990. Culture and growth of Lessonia trabeculata (Phaeophyta, Laminariales) juvenile sporophytes in La Herradura de Guayacan Bay, northern Chile. Hydrobiologia 204/205: 361–366. DOI: 10.1007/BF00040257.
- FAO. 2018a. The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals. Rome, Italy. 210 pp.
- FAO. 2018b. The global status of seaweed production, trade and utilization. Globefish Research Programme Volume 124. Rome, Italy. 120 pp.
- FAO. 2018c. FishStat. Global aquaculture production 1950–2016. http://www.fao.org/fishery/statistics/global-aquaculture-production/query/en; searched on 04 March 2018.
- Fernandes D.R.P., Oliveira V.P. & Valentin Y. 2014. Seaweed biotechnology in Brazil: six decades of studies on natural products and their antibiotic and other biological activities. Journal of Applied Phycology 26: 1923–1937. DOI: 10.1007/s10811-014-0287-5.
- Fernández P.A., Leal P.P. & Henríquez L.A. 2019. Co-culture in marine farms: macroalgae can act as chemical refuge for shell-forming molluscs under an ocean acidification scenario. Phycologia 58: 542–551. DOI: 10.1080/00318884.2019.1628576.
- Fonck E., Martínez R., Vásquez J. & Bulboa C. 2008. Factors that affect the re-attachment of Chondracanthus chamissoi (Rhodophyta, Gigartinales) thalli. Journal of Applied Phycology 20: 311–314. DOI: 10.1007/s10811-007-9251-y.
- González A., Beltrán J., Hiriart-Bertrand L., Flores V., De Reviers B., Correa J.A. & Santelices B. 2012. Identification of cryptic species in the Lessonia nigrescens complex (Phaeophyceae, Laminariales). Journal of Phycology 48: 1153–1165. DOI: 10.1111/jpy.2012.48.issue-5.
- Gurgel C.F.D., Norris J.N., Schmidt W.E., Le H.N. & Fredericq S. 2018. Systematics of the Gracilariales (Rhodophyta) including new subfamilies, tribes, subgenera, and two new genera, Agarophyton gen. nov. and Crassa gen. nov. Phytotaxa 374: 1–23. DOI: 10.11646/phytotaxa.374.1.1.
- Gutierrez A., Correa T., Muñoz V., Santibañez A., Marcos R., Cárceres C. & Buschmann A. 2006. Farming of the giant kelp Macrocystis pyrifera in southern Chile for development of novel food products. Journal of Applied Phycology 18: 259–267. DOI: 10.1007/s10811-006-9025-y.
- Hayashi L., Reis R.P., Dos Santos A.A., Castelar B., Robledo D., De Vega G.B., Msuya F.E., Eswaran K., Yasir S.M., Ali M.K.M.K., et al. 2017. The cultivation of Kappaphycus and Eucheuma in tropical and sub-tropical waters. In: Tropical seaweed farming. trends, problems and opportunities (Ed. by A.Q. Hurtado, A.T. Critchley & I.C. Neish), pp. 55–90. Springer, Cham, Switzerland.
- Hayashi L., Bulboa C., Kradolfer P., Soriano G. & Robledo D. 2013. Cultivation of red seaweeds: a Latin American perspective. Journal of Applied Phycology 26: 719–727. DOI: 10.1007/s10811-013-0143-z.
- Hernández-González M.C., Buschmann A.H., Cifuentes M., Correa J.A. & Westermeier R. 2007. Vegetative propagation of the carrageenophytic red alga Gigartina skottsbergii Setchell et Gardner: indoor and field experiments. Aquaculture 262: 120–128. DOI: 10.1016/j.aquaculture.2006.04.048.
- Hurtado A.Q., Neish I.C. & Critchley A.T. 2019. Phyconomy: the extensive cultivation of seaweeds, their sustainability and economic value, with particular reference to important lessons to be learned and transferred from the practice of eucheumatoid farming. Phycologia 58: 472–483. DOI: 10.1080/00318884.2019.1625632.
- ICMBIO. 2008. Instrução Normativa IBAMA N° 185, de 22 de julho de 2008. http://www.icmbio.gov.br/cepsul/images/stories/legislacao/Instrucao_normativa/2008/in_ibama_185_2008_permitircultivokappaphycus_alvarezii_rs_sc_revoga_in_ibama_165_2007.pdf; searched on 10 December 2018.
- Kim J.K., Stekoll M. & Yarish C. 2019. Opportunities, challenges and future directions of open-water seaweed aquaculture in the United States. Phycologia 58: 446–461. DOI: 10.1080/00318884.2019.1625611.
- Kim J.K., Yarish C., Hwang E.K., Park M. & Kim Y. 2017. Seaweed aquaculture: cultivation technologies, challenges and its ecosystem services. Algae 32: 1–13. DOI: 10.4490/algae.2017.32.3.3.
- Lorbeer A.J., Tham R. & Zhang W. 2013. Potential products from the highly diverse and endemic macroalgae of southern Australia and pathways for their sustainable production. Journal of Applied Phycology 25: 717–732. DOI: 10.1007/s10811-013-0003-x.
- Macchiavello J., Sepúlveda C., Basaure H., Sáez F., Yañes D., Marín C. & Vega L. 2018. Suspended culture of Chondracanthus chamissoi (Rhodophyta: gigartinales) in Caleta Hornos (northern Chile) via vegetative propagation with secondary attachment discs. Journal of Applied Phycology 30: 1149–1155. DOI: 10.1007/s10811-017-1307-z.
- MAG. 2016. Plan Nacional de Desarrollo Sustentable de la Pesca y la Acuicultura de El Salvador. Ministerio de Agricultura y Ganadería. http://propesca.org/sites/default/files//on_blank/Plan%20Estrat%C3%A9gico%20Acuicultura%20en%20El%20Salvador%20-%20FUNDES.pdf; searched on 10 December 2018.
- Marinho-Soriano E. 2017. Historical context of commercial exploitation of seaweeds in Brazil. Journal of Applied Phycology 29: 665–671. DOI: 10.1007/s10811-016-0866-8.
- Marinho-Soriano E., Morales C. & Moreira W.S.C. 2002. Cultivation of Gracilaria (Rhodophyta) in shrimp pond effluents in Brazil. Aquaculture Research 33: 1081–1086. DOI: 10.1046/j.1365-2109.2002.00781.x.
- Marinho-Soriano E., Moreira W.S.C. & Carneiro M.A.A. 2006. Some aspects of the growth of Gracilaria birdiae (Gracilariales, Rhodophyta) in an estuary in northeast Brazil. Aquaculture International 14: 327–336. DOI: 10.1007/s10499-005-9032-z.
- Marinho-Soriano E., Nunes S.O., Carneiro M.A.A. & Pereira D.C.E. 2009a. Nutrients’ removal from aquaculture wastewater using the macroalgae Gracilaria birdiae. Biomass and Bioenergy 33: 327–331. DOI: 10.1016/j.biombioe.2008.07.002.
- Marinho-Soriano E., Panucci R.A., Carneiro M.A.A. & Pereira D.C. 2009b. Evaluation of Gracilaria caudata J. Agardh for bioremediation of nutrients from shrimp farming wastewater. Bioresource Technology 100: 6192–6198. DOI: 10.1016/j.biortech.2009.06.102.
- Martín L.A., De Zaixso A.L.B. & Leonardi P.I. 2011. Biomass variation and reproductive phenology of Gracilaria gracilis in a Patagonian natural bed (Chubut, Argentina). Journal of Applied Phycology 23: 643–654. DOI: 10.1007/s10811-010-9555-1.
- MIDEPLAN. 2014. Plan Nacional de Desarrollo 2015–2018 “Alberto Cañas Escalante”. Ministerio de Planificación Nacional y Política Económica. http://extwprlegs1.fao.org/docs/pdf/cos145028.pdf; searched on 10 December 2018.
- Ministério de Economia, Fomento y Turismo. 2015. Resolución 1105 EXENTA. Programa de Vigilancia de Rhizoclonium spp. en el área de plaga declarada por la Subsecretaría de Pesca y Acuicultura en el Río Maullín. https://www.leychile.cl/Navegar?idNorma=1085034; searched on 09 December 2018.
- MPA. 2015. Plano de Desenvolvimento da Aquicultura Brasileira 2015–2020. Ministério da Pesca e Aquicultura. http://seafoodbrasil.com.br/wp-content/uploads/2015/09/Plano_de_Desenvolvimento_da_Aquicultura-2015-2020.pdf; searched on 10 December 2018.
- Muñoz J., Freile-Pelegrín Y. & Robledo D. 2004. Mariculture of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) color strains in tropical waters of Yucatán, México. Aquaculture 239: 161–177. DOI: 10.1016/j.aquaculture.2004.05.043.
- Navarro K. 2016. Cultiva UABC macroalga para la elaboración de alimentos. http://newsnet.conacytprensa.mx/index.php/documentos/15207-cultiva-uabc-macroalga-para-la-elaboracio-n-de-alimentos; searched on 24 March 2018.
- Otaíza R.D., Rodríguez C.Y., Cáceres J.H. & Sanhueza A.G. 2018. Fragmentation of thalli and secondary attachment of fragments of the agarophyte Gelidium lingulatum (Rhodophyta, Gelidiales). Journal of Applied Phycology 30: 1921–1931. DOI: 10.1007/s10811-018-1391-8.
- Pallaoro M.F., Vieira F.N. & Hayashi L. 2016. Ulva lactuca (Chlorophyta, Ulvales) as co-feed for Pacific white shrimp. Journal of Applied Phycology 28: 3659–3665. DOI: 10.1007/s10811-016-0843-2.
- Pellizzari F., Oliveira E.C. & Yokoya N.S. 2008. Life-history, thallus ontogeny, and the effects of temperature, irradiance and salinity on growth of the edible green seaweed Gayralia spp. (Chlorophyta) from southern Brazil. Journal of Applied Phycology 20: 75–82. DOI: 10.1007/s10811-007-9183-6.
- Pellizzari F. & Reis R.P. 2011. Seaweed cultivation on the southern and southeastern Brazilian coast. Brazilian Journal of Pharmacognosy 21: 305–312. DOI: 10.1590/S0102-695X2011005000057.
- Pellizzari F.M., Absher T., Yokoya N.S. & Oliveira E.C. 2007. Cultivation of the edible green seaweed Gayralia (Chlorophyta) in southern Brazil. Journal of Applied Phycology 19: 63–69. DOI: 10.1007/s10811-006-9111-1.
- Peña-Rodríguez A., Magallón-Barajas F.J., Cruz-Suárez L.E., Elizondo-González R. & Moll B. 2017. Effects of stocking density on the performance of brown shrimp Farfantepenaeus californiensis co-cultured with the green seaweed Ulva clathrata. Aquaculture Research 48: 2803–2811. DOI: 10.1111/are.2017.48.issue-6.
- Peña-Rodríguez A., Mawhinney T.P., Ricque-Marie D. & Cruz-Suárez L.E. 2011. Chemical composition of cultivated seaweed Ulva clathrata (Roth) C. Agardh. Food Chemistry 129: 491–498. DOI: 10.1016/j.foodchem.2011.04.104.
- PERU. 2004. Ley 27460. Ley de promocion y desarrollo de la acuicultura y sus modificaciones. http://www2.produce.gob.pe/RepositorioAPS/1/jer/PROPESCA_OTRO/marco-legal/1.2.%20Ley%20Acuacultura%20l27460.pdf; searched on 10 December 2018.
- PRODUCE. 2010. Plan Nacional de Desarrollo Acuícola 2010–2021. Ministério de la Producción. https://www.produce.gob.pe/index.php/sector-acuicultura/plan-nacional-de-desarrollo-acuicola; searched on 10 December 2018.
- Rebours C., Marinho-Soriano E., Zertuche-González J.A.M., Hayashi L., Vásquez J.A., Kradolfer P., Soriano G., Ugarte R., Abreu M.H., Bay-Larsen I. et al. 2014. Seaweeds: an opportunity for wealth and sustainable livelihood for coastal communities. Journal of Applied Phycology 26: 1939–1951. DOI: 10.1007/s10811-014-0304-8.
- Reis R.P., Castelar B. & Santos A.A. 2017. Why is algaculture still incipient in Brazil? Journal of Applied Phycology 29: 673–682. DOI: 10.1007/s10811-016-0890-8.
- Robledo D., Gasca-Leyva E. & Fraga J. 2013. Social and economic dimensions of carrageenan seaweed farming in Mexico. In: Social and economic dimensions of carrageenan seaweed farming. FAO fisheries and aquaculture technical paper (Ed. by D. Valderrama, J. Cai, N. Hishamunda & R.N. Ridler), pp. 185–204. FAO, Rome, Italy.
- Robledo D.R. & Townsend W. 2006. Seaweeds and mangroves: improving environmental practices in coastal communities to secure sustainable livelihoods. In: Coastal resource management in the wider Caribbean. Resilience, adaptation and community diversity (Ed. by Y. Breton, D. Brown, B. Davy, M. Haughton & L. Ovares), pp. 172–191. International Development Research Centre, Canada; Ian Randle Publishers, Kingston, Jamaica.
- Romo H., Alveal K. & Werlinger C. 2001. Growth of the commercial carrageenophyte Sarcothalia crispata (Rhodophyta, Gigartinales) on suspended culture in central Chile. Journal of Applied Phycology 13: 229–234. DOI: 10.1023/A:1011173515578.
- Romo H., Avila M., Núñez M., Pérez R., Candia A. & Aroca G. 2006. Culture of Gigartina skottsbergii (Rhodophyta) in southern Chile. A pilot scale approach. Journal of Applied Phycology 18: 307–314. DOI: 10.1007/s10811-006-9026-x.
- Sáez F. & Macchiavello J. 2018. Secondary attachment discs: a new alternative for restoring populations of Chondracanthus chamissoi (Gigartinales, Rhodophyta). Latin America Journal of Aquatic Research 46: 140–146. DOI: 10.3856/vol46-issue1-fulltext-14.
- Santos A.A. 2014. Potencial de cultivo da macroalga Kappaphycus alvarezii em Santa Catarina. PhD Thesis. Federal University of Santa Catarina, Brazil. 151 pp.
- Santos A.A., Dorow R., Araujo L.A. & Hayashi L. 2018a. Socioeconomic analysis of the seaweed Kappaphycus alvarezii and mollusks (Crassostrea gigas and Perna perna) farming in Santa Catarina State, Southern Brazil. Custos E Agronegócio Online 14: 443–472.
- Santos A.A., Novaes A.L.T., Marchiori N.C. & Hayashi L. 2018b. Novel flotation model for the experimental culture of macroalgae Kappaphycus alvarezii in Florianópolis, Brazil. Agropecuária Catarinense 31: 45–48. DOI: 10.22491/RAC.2018.v31n2.4.
- SERNAPESCA. 1991. Anuarios estadísticos de pesca 1991. Servicio Nacional de Pesca y Acuicultura de Chile. http://www.sernapesca.cl/informes/estadisticas; searched on 09 December 2018.
- SERNAPESCA. 1994–2017. Anuarios estadísticos de pesca y acuicultura 1994–2017. Servicio Nacional de Pesca y Acuicultura de Chile. http://www.sernapesca.cl/informes/estadisticas; searched on 09 December 2018.
- SERNAPESCA. 2017. Anuarios estadísticos de pesca 2017. Servicio Nacional de Pesca y Acuicultura de Chile. http://www.sernapesca.cl/informes/estadisticas; searched on 09 December 2018.
- Shannon E. & Abu-Ghannam N. 2019. Seaweeds as nutraceuticals for health and nutrition. Phycologia 58: 563–577. DOI: 10.1080/00318884.2019.1640533.
- SUBPESCA. 2003. Politica Nacional de Acuicultura. Subsecretaría de Pesca y Acuicultura, Ministerio de Economía, Fomento y Turismo. http://www.subpesca.cl/portal/616/w3-article-60019.html; searched on 10 December 2018.
- Suplicy F.M., Vianna L.F.N., Rupp G.S., Novaes A.L.T., Garbossa L.H.P., Souza R.V., Guzenski J., Costa S.W., Silva F.M. & Santos A.A. 2017. Planning and management for sustainable coastal aquaculture development in Santa Catarina State, south Brazil. Reviews in Aquaculture 9: 107–124. DOI: 10.1111/raq.12107.
- Tala F., Edding M. & Vásquez J. 2004. Aspects of the reproductive phenology of Lessonia trabeculata (Laminariales : Phaeophyceae) from three populations in northern Chile. New Zealand Journal of Marine and Freshwater Research 38: 255–266. DOI: 10.1080/00288330.2004.9517235.
- Tellier F., Vega J.M.A., Broitman B., Vásquez J.A., Valero M. & Faugeron S. 2011. The importance of having two species instead of one in kelp management: the Lessonia nigrescens species complex. Cahiers de Biologie Marine 52: 455–465.
- UCAR. 2015. Programa de Desarrollo Pesquero y Acuícola Sustentable (PRODESPA). Unidad para el Cambio Rural (UCAR) del Ministerio de Agroindustria de la Nación. https://plataformacelac.org/programa/1049; searched on 10 December 2018.
- Ursi S., Costa V.L., Hayashi L., Pereira R.T.L., Paula E.J. & Plastino E.M. 2013. Intraspecific variation in Gracilaria birdiae (Gracilariales, Rhodophyta): growth and agar yield and quality of color strains under aquaculture. Botanica Marina 56: 241–248. DOI: 10.1515/bot-2012-0219.
- Varela D.A., Hernríquez L.A., Fernández P.A., Leal P., Hernández-González M.C., Figueroa F.L. & Buschmann A.H. 2018. Photosynthesis and nitrogen uptake of the giant kelp Macrocystis pyrifera (Ochrophyta) grown close to salmon farms. Marine Environmental Research 135: 92–102. DOI: 10.1016/j.marenvres.2018.02.002.
- Westermeier R., Murúa P., Patiño D.J. & Muller D.G. 2017. Population biology and long-term mariculture studies in the brown alga Lessonia trabeculata in Atacama, Chile. Journal of Applied Phycology 29: 2267–2275. DOI: 10.1007/s10811-016-1019-9.
- Wurmann G.C. 2017. Regional review on status and trends in aquaculture development in Latin America and the Caribbean – 2015. FAO fisheries and aquaculture circular no. 1135/3, Rome, Italy. 36 pp.
- Zamorano J. 2016. Pilot scale cultivation of Sarcothalia crispata in southern Chile. In: Book of abstracts. 22nd International Seaweed Symposium Proceedings. p. 87. Copenhagen, Denmark.
- Zertuche-González J.A., García-Lepe G., Pacheco-Ruíz I., Chee A., Gendrop V. & Guzmán J.M. 2001. Open water Chondrus crispus Stackhouse cultivation. Journal of Applied Phycology 13: 249–253. DOI: 10.1023/A:1011197119042.
- Zertuche-González J.A., Sánchez-Barredo M., Guzmán-Calderón J.M. & Altamirano-Gómez Z. 2014. Eisenia arborea J.E. Areschoug as abalone diet on an IMTA farm in Baja California, México. Journal of Applied Phycology 26: 957–960. DOI: 10.1007/s10811-013-0138-9.
- Zuniga-Jara S. & Soria-Barreto K. 2018. Prospects for the commercial cultivation of macroalgae in northern Chile: the case of Chondracanthus chamissoi and Lessonia trabeculata. Journal of Applied Phycology 30: 1135–1147. DOI: 10.1007/s10811-017-1298-9.
