ABSTRACT
Identification of planktonic flagellates is challenging, and multiple species are often lumped together, limiting our understanding of their diversity and ecology. Our aim was to investigate changes in the phytoplankton community composition over the annual cycle in Roskilde Fjord, Denmark, focusing on the identification of organisms smaller than 20 µm. Weekly microscope and pigment data indicated a low contribution of diatoms and dinoflagellates and the importance of cryptophytes, euglenophytes and green algae, with high contributions of alloxanthin and chlorophyll b. Community composition using Serial Dilution Cultures (SDC) confirmed the high contribution of cryptophytes, prasinophytes and euglenophytes in the phytoplankton community. In contrast, diatoms only played a minor role. Seasonal changes were observed within cryptophytes, with Teleaulax dominance during cold months shifting to Hemiselmis in summer. Small prasinophytes and pico-eukaryotes were always present but had a low relative contribution to the total phytoplankton biomass. Cultured organisms enabled the identification of Hemiselmis cf. cryptochromatica, recorded for the first time in European waters, and an undescribed Pyramimonas species. We describe the new species as Pyramimonas octopora sp. nov., placed within Pyramimonas subg. Vestigiferae, based on morphological and molecular (SSU rDNA) data. The distinctive features of the new species are the eight irregularly rounded perforations arranged in a square box with rounded corners around the centre of the base plate of the box scales, and the complex crown scales, not observed in previously described Pyramimonas species.
INTRODUCTION
Phytoplankton comprises different taxonomic groups, with organisms spanning over a broad size range, from less than one to a few hundred micrometres. The diversity of phytoplankton communities is related to the efficiency of resource use and ecosystem stability (Ptacnik et al. Citation2008). In many marine and estuarine ecosystems, small-sized phytoplankters are given less attention in comparison to larger phytoplankton such as diatoms and dinoflagellates, which often dominate the biomass. However, small phytoplankton is a pervasive part of the community and can dominate phytoplankton at specific periods of the year, especially when chlorophyll a concentrations are low (Smetacek Citation1981; Levasseur et al. Citation1984; Haraguchi et al. Citation2015). Thus, even though high biomass events are rarely associated with small-sized organisms (<20 µm), this size fraction is considered an important component of the planktonic communities due to their capability to cope with low nutrient conditions (Edwards et al. Citation2012), their high division potential (Banse Citation1982) and because they fit the optimal prey size for different predators (Hansen et al. Citation1994, Citation1997).
Despite the large diversity found among flagellates, they are often pooled together into coarse groups in which size is the only distinctive feature (Throndsen Citation1993; Zingone et al. Citation2015). This leads to loss of information on diversity, ecology and functionality of a significant portion of the phytoplankton community (Piwosz Citation2019). One of the main issues limiting a deeper characterization of small-sized phytoplankton is that many of them do not preserve their distinctive features with the fixatives commonly used in routine light microscopy analysis (Hasle Citation1978; Throndsen Citation1978a). Even among organisms that still preserve features such as shape and flagella, other key information like motility and colour is nevertheless lost, which in some cases can hamper proper identification (Throndsen Citation1993) and even distinction between autotrophic and heterotrophic organisms. Studies investigating the taxonomic composition of small flagellates usually increase the knowledge of local diversity, demonstrating that flagellate diversity is often overlooked (Thomsen Citation1992; Thomsen & Buck Citation1998; Bergesch et al. Citation2008; Percopo et al. Citation2011). In addition, species abundance and contribution change over seasons and years (Thomsen Citation1992; Cerino & Zingone Citation2006), demonstrating that the small flagellate assemblages are dynamic, with their composition responding to changes in the environment. Therefore, they are more than only a ‘background’ community.
At the mesohaline temperate estuary Roskilde Fjord (RF), Denmark, long-term data have shown that small organisms make up a large proportion of the phytoplankton community (Haraguchi Citation2018). Hence, the present study aims to investigate the phytoplankton community composition during the 2016 annual cycle in RF, focusing on the identification of small flagellates (<20 µm). Additionally, we include the description of a new species, Pyramimonas octopora sp. nov.
MATERIAL AND METHODS
Area description
Roskilde Fjord (RF) is a mesohaline, shallow (average depth is 4 m) and well-mixed estuary located in Denmark (Fig. S1). It has two large basins connected by a narrow channel, with the outer (northern) basin connected to the adjacent Kattegat by a narrow opening. Due to RF morphology, low river discharge and tidal influence, the freshwater residence time in the inner basin can be up to 8 months (Kamp-Nielsen Citation1992). The catchment area in the region is dominated by agriculture, resulting in high nutrient inputs that stimulate primary production (Staehr et al. Citation2017).
Sampling
Surface water (2 litres) was sampled approximately weekly from March 2016 to January 2017 at a fixed shallow (c. 2-m depth) station (55°41ʹN, 12°5ʹE; Fig. S1). Samples were brought back to the laboratory for processing of nutrients and phytoplankton within 10–20 min after sampling. Temperature (°C) and salinity (‰) were measured immediately upon arrival in the laboratory with an YSI Professional Plus multi-parameter hand-held metre (YSI Inc., Yellow Springs, Ohio, USA).
Nutrients
Samples for inorganic nutrients (NO2–, NO3–, NH4+ and PO43–) were collected from GF/F-filtered water and stored frozen in 30-ml acid-washed plastic bottles until analysis. The samples were analysed on a San ++ Continuous Flow Analyser (Skalar Analytical B.V, Breda, Netherlands) as previously described (Grasshof Citation1976; Kaas & Markager Citation1998). Detection limits were 0.04, 0.1, 0.3 and 0.06 µM for NO2–, NO3–, NH4+ and PO43–, respectively. Dissolved inorganic nitrogen (DIN) concentrations were calculated as the sum of the concentrations of NO2–, NO3 – and NH4+. Dissolved inorganic phosphorus (DIP) is expressed as PO43– concentration.
Pigments
For the determination of pigment concentrations 300–500 ml of sample were collected on GF/F filters and stored at –80°C until analysis. Prior to analysis, pigments were extracted overnight at –20°C, in 10 ml acetone. Pigment extracts were analysed by an HPLC method (Wright et al. Citation1991) performed in a Shimazu LC 10A (Shimazu Corporation, Kyoto, Japan) system with a Supelcosil C18 column (250 × 4.6 mm, 5 µm). Pigments were identified by their retention times as compared to pure pigment standards (DHI Water & Environment, Denmark) at an absorption spectrum at 449 nm, following Wright et al. (Citation1991), as modified by Schlüter et al. (Citation2000). Chlorophyll a (Chl a) was used as a proxy of total phytoplankton biomass, whereas the following accessory pigments were assumed indicative of phytoplankton groups: alloxanthin (cryptophytes); chlorophyll b (green algae; euglenophytes), neoxanthin and violaxanthin (green algae); diadinoxanthin (‘golden-brown algae’, e.g. diatoms, dinoflagellates, haptophytes), fucoxanthin (diatoms); and peridinin (dinoflagellates) (Jeffrey et al. Citation1997).
Pigment data was analysed by the Bayesian compositional estimator (BCE) developed by Van Den Meersche et al. (Citation2008). The method uses a series of input ratios selected based on knowledge of the water body studied. We used the pigment ratios of Henriksen et al. (Citation2002) but replaced the diatom ratio by the ratio of Eutreptiella gymnastica obtained from Higgins et al. (Citation2011). This was done because of the minimal role of diatoms in RF (low concentration of fucoxanthin and microscopical confirmation of scarcity) and the frequent occurrence of Eutreptiella spp. For each sample, the pigment ratios were multiplied by the Chl a concentration to obtain Chl a proportion for each group. Group-specific carbon biomass was estimated assuming a C to Chl a ratio of 30, which is suitable for the area (Jakobsen & Markager Citation2016).
Community composition and cell enumeration
Serial dilution cultures (SDC; Throndsen Citation1978b, Citation1993) were conducted once per month on 10 occasions during the study. Five replicates of five 1:10 dilution steps (1 ml to 0.1 µl of the original sample) were incubated in 20-ml glass tubes containing L-1 medium (Guillard & Hargraves Citation1993) with a salinity of 12, similar to values found in the inner basin of RF. Tubes were kept at 10°C in saturated PAR light at an irradiance of 100–120 µmol m–2 s–1 (12:12 h light:dark regime), until analysis with a Nikon TI-U inverted light microscope. For each serial dilution, tubes were analysed after 2 and 4 weeks from sampling. Each tube was gently homogenized before observation and 5–10 drops were observed with a Nikon TI-U inverted light microscope equipped with a camera. Each droplet was screened under 100× and 400× magnification, using differential interference contrast, to assess the organisms in the whole subsample. For a more detailed view on organisms, especially the smaller ones, 1000× magnification was employed. When dinoflagellates were present, cells were stained with calcofluor and analysed using epifluorescence (Andersen & Throndsen Citation2003). All organisms, including the unidentified ones, were registered with pictures, drawings and descriptions, ensuring a unified catalogue for all dilution steps and replicates. Cell abundances of different taxonomical units were estimated from the most probable number (MPN) tables, considering the number of tubes showing growth (Andersen & Throndsen Citation2003). To account for cell size variations among different taxa, cell abundances were transformed into biovolumes by normalizing cell numbers by taxa volumes. Taxa volumes were calculated using a geometrical approach (Sun & Liu Citation2003), with the average cell dimensions (as the average of measurements of 10–30 live individual cells of each taxon, depending on the abundance in the samples). Volumes were converted to carbon using the general protist formula from Menden-Deuer & Lessard (Citation2000). Some organisms were isolated, and cultures established, allowing further investigation and species identification by electron microscopy (EM) and/or molecular phylogenetic analyses.
Electron microscopy
Whole mounts of different flagellate cultures were examined with a JEM-1010 transmission electron microscope (Jeol Ltd., Tokyo, Japan). Briefly, drops of cultures were placed on formvar/carbon-coated grids; then cells were fixed with 1% OsO4 vapour. Grids were left to dry at c. 50°C and gently rinsed with distilled water to remove salts. Finally, grids were stained with 4% uranyl acetate for 5 min and rinsed very quickly in distilled water.
For thin sectioning of Pyramimonas octopora, two fixation methods were used. In the first method, the material was fixed 1:1 with 1% OsO4 in 0.2 M cacodylate buffer for 40 min, dehydrated in an ethanol series followed by propylene oxide, and embedded in Epon 812. During the second fixation method, cells were fixed 1:1 with 1.5% glutaraldehyde in 0.2 M cacodylate buffer and 0.18-M sucrose for 80 min, rinsed in the buffer with decreasing sucrose content over 2.5 h, and post fixed in 1% OsO4 in distilled water overnight. It was then dehydrated and embedded as above. Thin sections were collected on single-hole slot grids and stained in uranyl acetate and lead citrate. They were observed with a JEM-1010 transmission electron microscope.
Cryptophytes were examined with a JSM-6335F scanning electron microscope (Jeol Ltd., Tokyo, Japan) for investigation of cell periplast features. Cultures (800 µl) were fixed for 30 min in a mixture of 960 µl OsO4 (4%), 960 µl L-1 medium (12 salinity) and 480 µl HgCl2. Samples were concentrated onto 1- or 5-µm pore-sized filters, depending on the cell size. Filters were rinsed with Milli-Q water, dehydrated through an alcohol series and critical-point dried (Bal-Tec CPD 030, Balzers, Liechtenstein).
Molecular phylogeny
Cells of established cultures were concentrated by centrifugation and the pellets kept frozen (–20°C) until extraction. DNA was extracted using the CTAB method following Lundholm et al. (Citation2002). For most species, the SSU rDNA region was amplified using the primers ND1 and ND6, and for sequencing additionally the primers ND2, ND3, ND9 and ND7 (Ekelund et al. Citation2004). For cryptophytes, SSU and LSU rDNA regions were amplified with a semi-nested PCR following Hoef-Emden (Citation2008). For SSU, the first PCR was done with the primers CrN1F and ITS055R, followed by a second step with the primers CrN1F and BR. The LSU region was amplified first by the primers CrN1F and LSU1433R, followed by a second step using the primers CrN3F and LSU1433R.
PCR products were purified using a QIAquick PCR purification kit (Qiagen, Germany) and analysed on an automated sequencer (ABI3730XL, Applied Biosystems). Alignments for each major taxonomic group were established based on taxonomically relevant taxa from GenBank and similar sequences found using the BLAST algorithm available in GenBank (Altschul et al. Citation1997). For several taxa, basic sequence comparisons confirmed the morphological taxonomic identity and no further phylogenetic analyses were performed. Sequence alignments were done in BioEdit (Hall Citation1999). For Pyramimonas, the SSU rDNA alignment included 48 taxa and 1683 nucleotide positions, with four taxa belonging to the genera Mamiella, Micromonas and Mantoniella as outgroup. Distance, parsimony, and likelihood analyses were performed using PAUP* version 4.0b.8 (Swofford Citation2003). Maximum parsimony (MP) analyses were done with 1,000 heuristic searches and distance analyses used NJ with the GTR (general time-reversible) model. The optimal model for the maximum likelihood (ML) analyses was a general time-reversible model with the proportion of invariable sites and gamma distribution (GTR + I + G), according to the Akaike information criterion with a 99% level of significance in Modeltest version 3.7 (Posada & Crandall Citation1998). Heuristic searches performed ML analyses with 10 random-addition replicates and the tree bisection-reconnection (TBR) branch-swapping algorithm. In NJ, MP and ML analyses, 1,000 bootstrap replicates were performed. Bayesian analyses were performed using MrBayes 3.1.2 (Ronquist & Huelsenbeck Citation2003), using four chains run for 1,200,000 generations. The temperature was set to 0.2, the sample frequency was 100, and the number of burn-in generations was 3,000.
Statistical analysis
Carbon biomass estimated from pigments and SDC were compared using a simple linear regression, using estimates from the pigments as the independent variable. The linear model and plots were done in R (R Core Team Citation2015).
RESULTS
Seasonal environment variability
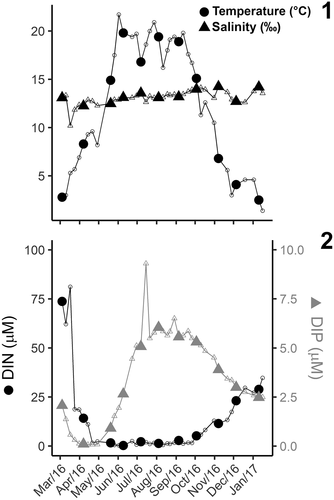
Salinity during the study period was relatively stable, ranging from 10 to 14, while temperature varied between 1.4°C in winter and 21.7°C in summer (). Variability in temperature and nutrients shows that the study captured distinct phases of the annual cycle in RF, with high DIN concentrations in winter compared to summer, while DIP concentrations were lower in winter and increased over summer (). DIN and DIP were quickly depleted between March and April () coinciding with the spring bloom, as indicated by the sharp increase in Chl a (). Periods with increased Chl a concentrations (>10 µg l–1) were associated with high biomass of cryptophytes (). All SDCs were established from communities with relative low phytoplankton biomass, indicated by Chl a < 5 µg l–1 (), and the total C biomass estimated from pigments was less than 141 µg l–1 ().
Figs 1, 2. Seasonal cycle of environmental parameters in Roskilde Fjord during the study period.
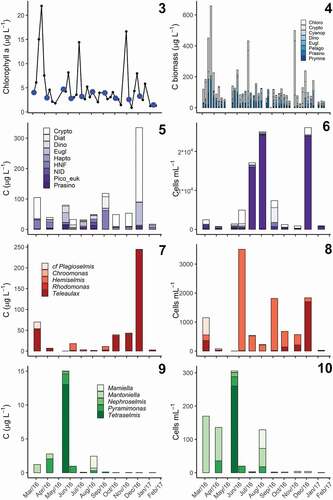
Figs 3–10. Seasonal variation in phytoplankton community composition in Roskilde Fjord.
Community composition
Despite relatively similar Chl a concentrations (1.46–4.70 µg l–1) among the samples used for flagellate analyses, both pigment composition and SDC indicated distinct communities over the annual cycle (). Cryptophytes were found in all the samples, and overall were the group with highest biomass contribution during the year, with C biomass ranging from 3.7 to 451.8 µg l–1 in early August and late March, respectively (). Although cryptophyte biomass values larger than 100 µg C l–1 were mostly observed in spring, isolated peaks were also observed in summer and autumn, but did not coincide with the SDC (). In summer, cryptophyte biomass was lower than 10 µg C l–1 and increased to 20–70 µg C l–1 over autumn (). Prasinophytes were the second most important group, with biomass in the range 0–174 µg C l–1. Two biomass peaks of prasinophytes, with biomass above 100 µg C l–1, were observed in late March and late May, followed by a period with biomass values of about 30–40 µg C l–1 in July (). After September, prasinophyte biomass was consistently low (). Euglenophytes (0–94.8 µg C l–1) and dinoflagellates (0–103.5 µg C l–1), had similar contributions to the overall biomass, although they were less important than cryptophytes and prasinophytes. While euglenophytes contributed with low to moderate biomass (c. 20–40 µg C l–1) over the year, dinoflagellate biomass had a marked occurrence in summer, with peaks in the range of 40–100 µg C l–1 (). Pigments also indicated the presence of cyanophytes, pelagophytes, chlorophytes, and prymnesiophytes, although their contributions were smaller than those of the groups described before ().
Total carbon biomass estimated from SDC/MPN was 17–335 µg l–1 and showed relatively small variation over the months in agreement with Chl a concentration (), except in December 2016, when the SDC/MPN estimated high abundances of cryptophytes and euglenophytes while Chl a concentrations were not notably different from the other samples (). Although the total biomass estimated from pigments ranged from 26.2 to 657.7 µg C l–1 over the whole period (), the range for the coinciding SDC observations was much smaller (43.9–141.1 µg C l–1), being comparable to most of the SDC estimates, except for December 2016. A linear regression comparing total biomass estimates from both methods yields a poor correlation (p = 0.461); however, it is strongly biased by the high biomass estimated by the SDC in December 2016. If the December sample is excluded, the linear model becomes significant (p = 0.022; R2 = 0.436; slope = 0.818), indicating that there is agreement between C biomass estimated by the two methods and that the SDC tends to yield lower values than the pigments.
In accordance with the pigment data, the biomass estimated from SDC showed that cryptophytes were present throughout the year and constituted the most important group (>50% on five occasions), followed by heterotrophic nanoflagellates (10%–50% on five occasions) and euglenophytes (10%–50% on four occasions; , S2). Although prasinophytes and pico-eukaryotes did not contribute much to the total C biomass, they were present in all observations (, S2), and the latter reached high cell abundances (>1.5 × 104 cells ml–1) on three occasions (). Photosynthetic dinoflagellates were abundant in the SDC in May and August 2016 (, S2), coinciding with pigment samples with a high contribution of dinoflagellates (). In those samples, Heterocapsa rotundata (Lohmann) Gert Hansen, plus an unidentified small species of the Suessiaceae and unidentified gymnodinioids were recorded. The SDC from September 2016 was the only case in which diatoms were abundant compared to autotrophic flagellates (, S2). In that sample, Skeletonema marinoi Sarno & Zingone, Chaetoceros cf. tenuissimus, Cylindrotheca closterium (Ehrenberg) Reimann & J.C. Lewin and unidentified centric taxa smaller than 10 µm were recorded. Haptophytes were also recorded in most of the observations (except in April, July and December 2016), but at low concentrations, as shown by the pigment data (). Chrysochromulina was the most common haptophyte, recorded in all haptophyte observations, and organisms belonging to Pavlovophyceae were also recorded on three occasions during summer (May, June and August 2016).
A closer look at the composition of the main autotrophic groups showed some seasonal variation. All the euglenophytes observed belonged to the genus Eutreptiella (). Although these could not be identified to species level, morphological variations were observed at different times of the year, with smaller cells (c. 20 µm) observed in the cold months (April, December and January) and larger cells (>30 µm) in warmer months (May and July). Cryptophytes and prasinophytes showed seasonality in the generic composition. Teleaulax was the main contributor to cryptophyte biomass in March, April and October 2016 until January 2017 (). From June to November 2016, high abundances of small Hemiselmis were observed (), and they dominated the cryptophyte biomass between June and September 2016, when the cryptophyte biomass was generally low (). Small-sized organisms, mainly prasinophytes and pico-eukaryotes, were more abundant in spring and summer, respectively (,). Mantoniella was recorded in all samples, but the highest prasinophyte biomass and abundance, in May, was dominated by Tetraselmis (). Species of Pyramimonas were observed mainly from April to June (), in reasonable agreement with the pigments ().
Figs 11–27. Examples of identified organisms in Roskilde Fjord. All micrographs are LM, except Fig. 20, which is SEM. Scale bars = 5 µm.
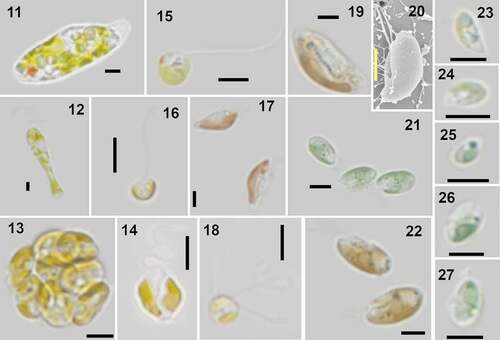
Establishment of cultures supported the identification of organisms that could not be identified by light microscopy. The SDC allowed for observation of diversity among small flagellates and showed this assembly’s composition to change over the annual cycle of 2016. Examples of some of the organisms are shown in . Although some organisms were recorded by the SDC (e.g. heterotrophic nanoflagellates, diatoms and dinoflagellates), they were not always identified. In total, 15 flagellate taxa were identified (), belonging to cryptophytes (7), prasinophytes (4), haptophytes (2) and pico-eukaryotes (2). Information on the strains, including methods used for identification and GenBank accession numbers, are summarized in . Of the identified species in RF, we highlight the occurrence of Hemiselmis cryptochromatica C.E. Lane & J.M. Archibald, recorded for the first time in European waters, and Pyramimonas octopora sp. nov. Some cultures could not be identified to species level due to problems with sequencing or lack of reference data: Eutreptiella (April, May, November and January), Tetraselmis (May and August), Hemiselmis (July, October and November) and pico-eukaryotes (April and May).
Table 1. Flagellates identified to species level, the month in which they were isolated, strain code, techniques used for identification, and GenBank accession number
Phylogenetic analyses of cryptophyte SSU rDNA (Fig. S3) revealed a clade comprising the genus Teleaulax, in which T. amphioxeia (W. Conrad) D.R.A. Hill strains from March and April clustered with T. amphioxeia strains from GenBank with high support, and the T. acuta from October clustered with T. acuta (Butcher) D.R.A. Hill from GenBank with high support. A separate Rhodomonas clade comprised R. salina (Wisłouch) Butcher from June clustering with R. salina as well as other Rhodomonas taxa from GenBank. Within a Chroomonas clade, C. vectensis N. Carter from January clustered together with another strain of the same species. In the Hemiselmis clade, H. virescens Droop from March clustered with high support with sequences of two H. cf. virescens from GenBank, while another strain from GenBank was found outside that branch but within the Hemiselmis clade. Hemiselmis cryptochromatica formed a well-supported distinct branch within the Hemiselmis clade, in which our June isolate clustered with a sequence obtained from the culture from which the type material was derived.
Formal description of new species
Pyramimonas octopora L. Haraguchi, Moestrup, H.H. Jakobsen & Lundholm sp. nov.
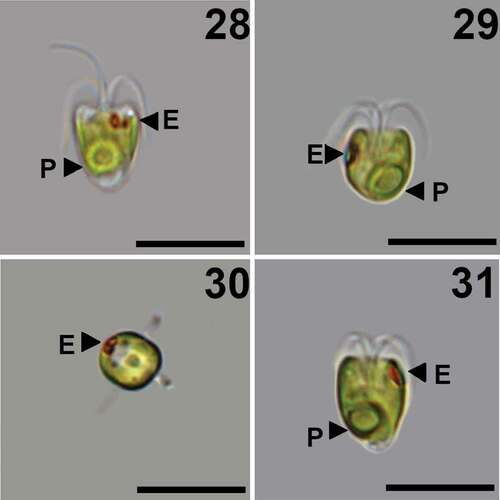
Figs 28–31. Pyramimonas octopora sp. nov., LM. Scale bars = 10 µm.
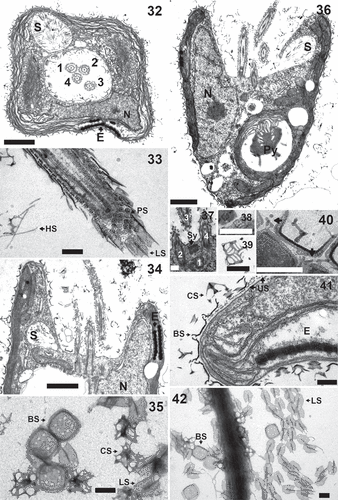
Figs 32–42. Pyramimonas octopora sp. nov., TEM. Figures 32–34, 36–41 are from thin sections; Figs 35, 42 are from whole mount preparations stained with uranyl acetate. All scale bars = 200 nm, except for Figs 32, 34 and 36 in which the scale bars = 1 µm.
Diagnosis: Elongated cells with average cell length 9.5 ± 1.2 µm and width 7.2 ± 1.1 µm. The eyespot comprises two rows of carotenoid droplets, near the apical end of the cell in two adjacent chloroplast lobes. The four flagella are slightly longer than the cell. Each flagellum is covered by a layer of small pentagonal scales, in addition to limuloid scales and hair scales. A layer of box scales covers the cell body, each box scale with eight round irregular perforations in the base plate, arranged in a rounded square around the centre of the base plate. Crown scales have a squared base with pointed edges. A cross-shaped structure is formed by struts from the middle of each side of the outer rim and meeting in the centre. In each quadrant defined by the cross-shaped structure, the outer parts of the rim are connected by two smaller structures arranged at about 45 degrees in relation to the cross, forming a complex pattern. From each corner, a tubular structure rises upward, all four meeting at the top, from where a central tubular column connects to the base plate centre.
Holotype: A fixed and plastic-embedded sample of culture Poct_06 is deposited at C, the herbarium at the Natural History Museum of Denmark (museum number C-A-99692) ( illustrates the holotype). Accession number MW451603 for the SSU sequence of the holotype strain.
Type Locality: Type material was collected at the inner portion of Roskilde Fjord, Denmark (55°41ʹN, 12°5ʹE) in June 2016. Salinity was 13.1 and temperature 19.8°C.
Etymology: Latin adjective octoporus, -a, -um, eight pores, referring to the box scale with eight round irregular perforations in the base plate.
Morphology
LIGHT MICROSCOPY
The cells have an average length of 9.5 ± 1.2 µm and a width of 7.2 ± 1.1 µm (n = 27, mean ± s). The apical portion of the cell is truncated, but some notches can occasionally be observed, corresponding to the four anterior lobes of the cell. Cell morphology varies from inverse pyramidal to U-shaped (). The single chloroplast is bright light green, and cup-shaped with four anterior lobes (). The pyrenoid is excentric and located in the antapical part of the cell (). Four thick flagella emerge from the flagellar pit, which is about ⅓ of the cell length (). The flagella are slightly longer than the cell. The two eyespots are located near the cell apex, often at the edge of a cell corner ().
TRANSMISSION ELECTRON MICROSCOPY
This species presents morphological characteristics common to species of Pyramimonas subg. Vestigiferae McFadden, D.R.A. Hill & Wetherbee. These include two bi-layered eyespots (), an excentric pyrenoid with invading thylakoids (), a square synistosome (), and square underlayer scales ornamented with perforations ().
The flagella are covered by an underlayer of pentagonal scales about 40 nm wide (usually 38–42 nm) overlain by nine rows of limuloid scales. Each limuloid scale is 260–300 nm long and 170–190 nm wide, with a longitudinal spine directed anteriorly (). At least two ribs diverged from the centre of the scale towards the margins, one located close to the spine base and the other more posteriorly (). Three perforations of irregular size are found in the anterior part of the scale, one very close to the base of the longitudinal spine and the other two near the anterior end (). Hair scales are present () but were not examined in detail.
Figs 43–45. Diagrammatic representation of the most characteristic scales of Pyramimonas octopora sp. nov. Drawings by Nikolaj Toftemark Nielsen.
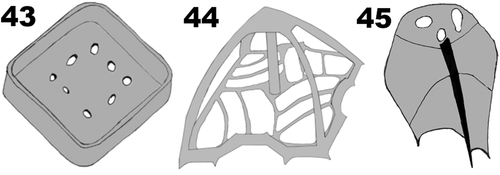
The cell body is covered with a layer of square box scales (), in which the only ornamentation are eight perforations on the base, arranged in a square with round corners around the centre. The perforations are irregular in shape and size and occasionally very small. Each box scale is about 200 nm wide and about 80 nm high (). The crown scales, situated on top of the box scales (), have a square base with pointed edges, with a cross structure from the middle portion of each side of the outer rim, meeting at the centre. In each quadrant defined by the cross, the two parts of the rim were connected by two smaller structures arranged at an angle of c. 45 degrees in relation to the central cross, forming an intricate pattern on the base of the crown scale (). From each corner, a tubular structure rises upwards, the four structures meeting at the top, from where a central tubular column connects to the centre of the base (). A layer of small square scales with perforations () is present only in the flagellar pit (). Footprint scales can be found between the box scales and measure approximately 60 nm in length when measured in thin sections, one end being slightly thicker than the rest ().
Cell motion
In culture, cells usually swim forward in a straight line, rotating along the cell axis. To turn to a new direction, the cells stop, turn and then resume swimming. Cells can also be found with their flagella attached to a surface and moving in circles.
Phylogenetic analyses
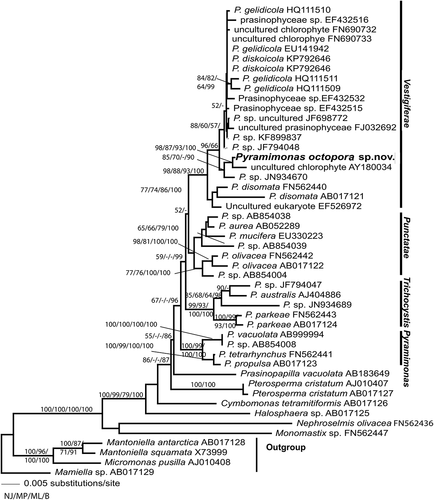
Phylogenetic trees were based on alignments of 1683 positions of the SSU rDNA (). The genus Pyramimonas made up a monophyletic clade, weakly supported (67/-/- as NJ/MP/ML), to which ‘Prasinopapilla vacuolata’ was the closest sister taxon (). The designation ‘Prasinopapilla vacuolata’ was not validly published (no description) and no further information on the organism was found, except what was mentioned in GenBank. Pyramimonas octopora clustered within the Vestigiferae clade, which was monophyletic and highly supported (98/88/83). The clade Vestigiferae appeared as sister to the clade Punctatae, with no statistical support. Within the Vestigiferae clade, three main clades were observed: the basal clade included P. disomata Butcher ex McFadden, D.R.A. Hill & Wetherbee and received high bootstrap values (98/88/93 as NJ/MP/ML), whereas the two other clades were supported by the moderate bootstrap values 96/66/- (NJ/MP/ML). The second clade comprised P. octopora, an unidentified Pyramimonas (strain RCC1987 from the Beaufort Sea; JN934670) and an uncultured chlorophyte (clone CCW80 from rRNA library-based sample from Cape Cod, US; AY180034). The third clade comprised the cold-water species P. diskoicola Harðardóttir, Lundholm, Moestrup & T.G. Nielsen and P. gelidicola McFadden, Moestrup & Wetherbee, and a few unidentified organisms ().
DISCUSSION
Our results on flagellate diversity and composition in a well-studied and monitored environment demonstrate that part of the phytoplankton diversity has been overlooked by traditional monitoring. The findings highlight the importance of small phytoplankton biodiversity surveys.
Methodological considerations
In this study, phytoplankton community composition was assessed by two very distinct methods: pigments and SDC/MPN. Overall, there was a fair agreement between the methods, which showed similar trends for phytoplankton biomass and composition, except for one out of 11 samples. The methods have very different strengths and limitations, and therefore discrepancies were expected. On the other hand, the number of samples was too small to make a robust comparison between methods and more studies are required to make a proper comparison between the two methods. The main limitations of the methods are described below, indicating potential sources of bias for each method.
Marker pigments are well suited for analysing a large number of samples but suffer limitations for inferring the taxonomic composition because many pigment markers, such as diadinoxanthin and chlorophyll b, are shared by different algal groups (Jeffrey et al. Citation1997). When HPLC data use specific procedures to project the community composition, as the Bayesian estimator used in this study, the results can be used to estimate the biomass contribution of different phytoplankton groups. However, as the pigment ratios vary with the environment, those procedures require good knowledge of the local populations and their pigments (Higgins et al. Citation2011), as was the case for RF where pigment ratios exist from previous studies (Henriksen et al. Citation2002). Another problem might come from pigments derived from non-phytoplanktonic groups, as mixotrophic ciliates, which are common in RF and can be abundant at specific times of the year (Haraguchi et al. Citation2018).
The SDC allows for a much deeper taxonomic classification, but it is laborious and time-consuming, limiting its application (Throndsen Citation1978b; Cerino & Zingone Citation2006). In addition, estimates from SDC are somewhat biased as they favour species that grow well under culture conditions (Cerino & Zingone Citation2006), and cell abundances estimated by the SDC/MPN method often underestimate the number of cells. Small grazers might be present in the tubes, as the case of this study, and their presence might impact the occurrence of certain taxa. Additionally, as species with different growth rates are present in phytoplankton, the method might indicate the false absence of an organism that grows too fast or too slow even though it was present in the sample (Cullen & MacIntyre Citation2016). Likely because of these issues, the SDC/MPN application to assess the whole community has been very limited but it has been indicated as a potential method to assess cell viability in ballast waters (Cullen & MacIntyre Citation2016). The semi-quantitative nature of the method is also a disadvantage; however, it has been shown that precision and reproducibility for cell viability can also be obtained for the MPN method when validation protocols are used (MacIntyre et al. Citation2019).
Our SDC/MPN results were able to reproduce some patterns previously observed in RF, such as the dominance of small phytoplankton (Haraguchi et al. Citation2018) and the summer reduction of the cryptophyte Teleaulax (Altenburger et al. Citation2020). Given the many constraints associated with the method, which limits the quantification capability, it was a surprise to see that it reproduced total biomass trends from the pigments. In addition, the possibility to establish cultures allowed for a more detailed investigation of a few organisms, an effort that was already relevant for the knowledge on the local biodiversity. However, it is important to highlight that part of the phytoplankton could not be properly assessed in this study, and the efforts were too limited to link biodiversity and ecology.
Metagenomics features as one of the methods to assess biodiversity that are well established (Johnson & Martiny Citation2015). Massana et al. (Citation2015) in a survey in different coastal environments in Europe showed that the diversity of larger protists is relatively well known, whereas small flagellate diversity remains much less explored. A comparison between SDC and next-generation sequencing for diatom resting spores demonstrated a good agreement between records obtained with both methods (Piredda et al. Citation2017). Piredda et al. (Citation2017) also emphasized the importance of curated reference sequences, highlighting a potential synergy between SDC and metagenomics.
Cryptophyte diversity: ecological implications and challenges
Cryptophytes were the main taxonomic group among the nanoflagellates in RF according to pigment data and were found by SDC during all months. However, quantitative studies focusing on species composition and dynamics of cryptophytes in marine systems are scarce (Laza-Martínez et al. Citation2012). In the Kattegat basin, adjacent to RF, cryptophytes have been recorded at all sampling occasions, the most common species found by Hill et al. (Citation1992) being Plagioselmis prolonga Butcher ex Novarino, I.A.N. Lucas & S. Morrall, Hemiselmis virescens and Teleaulax acuta. Teleaulax amphioxeia is the most important cryptophyte in RF in terms of biomass (Haraguchi et al. Citation2018; Altenburger et al. Citation2020), and the species was recently described as having a dimorphic life cycle, in which the form known as T. amphioxeia is the diploid stage and the former Plagioselmis prolonga is the haploid stage (Altenburger et al. Citation2020). In our study, T. acuta and T. amphioxeia (the diploid form) were observed to occur together, especially during the winter months. The haploid form of T. amphioxeia (the former Plagioselmis prolonga – see Altenburger et al. Citation2020), was less often recorded, and its lower abundance might reflect a potential life-cycle change, from the haploid to the diploid form, as SDC was established using nitrogen-rich culture media (Altenburger et al. Citation2020). The dependence of T. amphioxeia on high DIN concentrations likely leads to the summer dominance of Hemiselmis among the cryptophytes. While the high abundances of Hemiselmis in RF during summer match the reports for the Gulf of Naples (Cerino & Zingone Citation2006) and for Kattegat (Hill et al. Citation1992), it was in the latter region also recorded in other seasons. Observations of cultured Hemiselmis spp indicate that Hemiselmis can endure nutrient stress, making it suitable to survive in the DIN-limited RF during summer. Findings of the cryptophyte assemblage in RF corroborate previous studies (Hill et al. Citation1992; Cerino & Zingone Citation2006), all showing that the composition within a taxonomic group changes over time, most likely reflecting distinct environmental conditions. However, studies focusing on Hemiselmis ecology in nature are usually limited in time, being restricted to cruises or short-lived surveys such as this study, limiting the comprehension of key aspects of their ecology. Hemiselmis records by traditional monitoring in RF are scarce and most likely these algae end up being added to non-identified flagellates. This is likely the case for many other locations, highlighting a bottleneck to assess Hemiselmis over longer time scales.
Additionally, the identification of Hemiselmis to species level is challenging because of the small cell size and the lack of distinct morphological features. For many years, distinction of the marine Hemiselmis species was based on colouration (Lane & Archibald Citation2008). Such limitations make Hemiselmis one of the most enigmatic cryptophyte genera. We established five strains in culture but were able to identify only two species level: H. virescens and H. cf. cryptochromatica, the latter recorded for the first time in Europe. Apart from the phylogenetic support, the H. cf. cryptochromatica culture exhibited the same pale-green colour as stated in the original description of the species (Lane & Archibald Citation2008). No information on environmental conditions of the type material of H. cryptopchromatica has been obtained, and the only ecological information available is the maximum temperature conditions of cultures, up to 21°C (Lane & Archibald Citation2008), making it difficult to make ecological inferences on environmental preferences and distribution. Our material of H. cf. cryptochromatica was isolated in June, at a water temperature of c. 20°C, but the isolate also grew in the culture at 10°C, indicating potential plasticity in ecological requirements. The other three unidentified Hemiselmis strains have characteristics that resemble H. cryptochromatica, such as size and culture colouration (Lane & Archibald Citation2008), but one of the strains (Hemi_11) was isolated in November 2016 when the temperature was about 6°C. Whether those belong to same species or not, those observations highlight that marine Hemiselmis ecology and diversity remain largely unknown.
Prasinophytes: hidden diversity in RF
Prasinophytes were common in RF, but with a lower relative contribution to the total biomass when compared to the main groups, cryptophytes and euglenophytes. Prasinophytes were mainly important around spring, although they were also present during the following months, and this applied especially to Mantoniella squamata (Manton & Parke) Desikachary, a common species in Danish waters, which occurs all year round (Moestrup Citation1992). Pico-eukaryotes were present in all samples, and two strains, one collected in August and the other in December, were identified as two potentially distinct species of the prasinophyte genus Ostreococcus. Due to the small size and limited morphological characteristics, species identification based on morphology is not feasible, and organisms can be distinguished based on genome (Palenik et al. Citation2007), further supporting the ecotypes distinction proposed by Rodríguez et al. (Citation2005). Considering the environmental differences (temperature, light, nutrient availability) found between August and December in RF, it is likely that the two Ostreococcus strains are distinct species. Small prasinophytes can be subjected to intense grazing pressure, with reported abundance decline coinciding with blue mussel larval development in Oslo Fjord (Backe-Hansen & Throndsen Citation2002). In RF, ciliate grazing is very intense at all seasons, controlling especially small-sized phytoplankton populations (Haraguchi et al. Citation2018). As most prasinophytes recorded in this study were smaller than 10 µm, their lack of abundance after May () is probably related to grazing intensification towards summer.
Among the prasinophytes from RF, an undescribed species belonging to the genus Pyramimonas was found. This new species possessed morphological characteristics of Pyramimonas subg. Vestigiferae, such as small squared underlayer scales with perforations in the flagellar pit (corresponding to type 1 from McFadden et al. Citation1986), the excentric pyrenoid with invading thylakoids, the square synistosome, and two bi-layered eyespots (McFadden et al. Citation1986). Since the description of the subgenus Vestigiferae by McFadden et al. in 1986 (invalidly published for lack of type; validated in McFadden et al. Citation1987) these features have been consistently reported for all species within the group, therefore representing stable features (Daugbjerg et al. Citation2020). The swimming behaviour in culture is similar to what has been described from other species of this subgenus, with cells swimming forward, rotating along the cell axis and stopping to change direction (Daugbjerg & Moestrup Citation1992; Daugbjerg et al. Citation2020; Harðardóttir et al. Citation2014). In LM, live cells resembled P. orientalis, but the characteristics of the large body scales (observed in EM), which are determinant for species identification (Norris & Pienaar Citation1978), indicated a different identity. Eight round perforations ornamented the box scales at the base, arranged around the centre in a square with rounded corners. Furthermore, the crown scale had distinctive characteristics, such as the intricate pattern on its base. Pyramimonas cyclotetra Daugbjerg & Moestrup is the only other Pyramimonas species with box scales possessing round holes in the base plate (Daugbjerg & Moestrup Citation1992). Pyramimonas octopora box scales differ from the arctic P. cyclotreta by 1) the way the holes are arranged around the centre (in a square with round corners in P. octopora and in circle in P. cyclotreta); 2) not having striations; and 3) the lack of a central spine. The crown scales of P. cyclotreta are simpler than those of P. octopora, especially regarding the crown scale base pattern. The crown scales most similar to P. octopora were recorded in an organism similar to P. orientalis (P. aff. orientalis CCAP 4, in fi. 12 of Pennick Citation1984). However, Pennick’s drawing did not show the two smaller structures arranged in a nearly 45° angle in relation to the mid cross, as in the RF material. In the past years, two new species of P. subg. Vestigiferae were described, the arctic P. diskoicola (Harðardóttir et al. Citation2014) and P. tatianae (Daugbjerg et al. Citation2020). Morphologically, P. octopora differs from those two species by the more anterior position of the eyespot and by the morphology of the large body scales (as discussed above). While the phylogenetic SSU analyses placed P. octopora within the Vestigiferae clade, the closest organism was an uncultured chlorophyte (AY180034) sampled in a tidal pool (at 10°C and salinity c. 30) at Cape Cod, USA (Stoeck & Epstein Citation2003). Our phylogenetic tree supports a distinction between P. disomata and P. octopora as belonging to separate clades within the subgenus Vestigiferae. The general SSU phylogeny confirmed previous studies (Suda et al. Citation2013; Harðardóttir et al. Citation2014).
Final considerations
Our results exemplify a latent diversity in the small-sized phytoplankton in RF. Annual changes were observed between distinct taxonomic groups but also within the same taxonomic group, such as cryptophytes. Such changes might not be evident at coarser taxonomic levels, yet the hidden diversity probably reflects a range of ecological strategies related to seasonal changes in nutrient supply and grazing. The study allowed us to record Hemiselmis cf. cryptochromatica for the first time in Europe, and to describe a new Pyramimonas species in a well-studied site such as RF. These findings indicate that the known diversity on the smaller phytoplankton fraction is still expanding, impacting the knowledge of phytoplankton ecology and monitoring, and directly affecting diversity indices and reference databases used for metabarcoding. Current knowledge of diversity and functioning of phytoplankton communities is obviously lacking in detail. This applies not only to poorly studied areas but also to areas such as Roskilde Fjord, which have been studied and monitored for many years.
Supplemental Material
Download MS Word (1.2 MB)ACKNOWLEDGEMENTS
We thank Nikolaj Toftemark Nielsen for drawing Pyramimonas octopora scales.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the authors.
SUPPLEMENTARY MATERIAL
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
REFERENCES
- Altenburger A., Blossom H.E., Garcia-Cuetos L., Jakobsen H.H., Carstensen J., Lundholm N., Hansen P.J., Moestrup Ø. & Haraguchi L. 2020. Dimorphism in cryptophytes – the case of Teleaulax amphioxeia/Plagioselmis prolonga and its ecological implications. Science Advances 6: Article eabb1611. DOI: https://doi.org/10.1126/sciadv.abb1611.
- Altschul S.F., Madden T.L., Schäffer A.A., Zhang J., Zhang Z., Miller W. & Lipman D.J. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25: 3389–3402. http://www.ncbi.nlm.nih.gov/pubmed/9254694.
- Andersen P. & Throndsen J. 2003. Estimating cell numbers. In: Manual on harmful marine microalgae (Ed. by G.M. Hallegraeff, D.M. Anderson & A.D. Cembella), pp 99–129. UNESCO Publishing, Paris, France.
- Backe-Hansen P. & Throndsen J. 2002. Occurrence of pico- and smaller nanoplanktonic flagellates in the Inner Oslofjord, Eastern Norway, during the breeding season of the blue mussel (Mytilus edulis L.). Sarsia 87: 65–74. DOI: https://doi.org/10.1080/003648202753631749.
- Banse K. 1982. Cell volumes, maximal growth rates of unicellular algae and ciliates, and the role of ciliates in the marine pelagial. Limnology and Oceanography 27: 1059–1071. DOI: https://doi.org/10.4319/lo.1982.27.6.1059.
- Bergesch M., Odebrecht C. & Moestrup Ø. 2008. Nanoflagellates from coastal waters of Southern Brazil (32°S). Botanica Marina 51: 35–50. DOI: https://doi.org/10.1515/BOT.2008.003.
- Cerino F. & Zingone A. 2006. A survey of cryptomonad diversity and seasonality at a coastal Mediterranean site. European Journal of Phycology 41: 363–378. DOI: https://doi.org/10.1080/09670260600839450.
- Cullen J.J. & MacIntyre H.L. 2016. On the use of serial dilution culture method to enumerate viable phytoplankton in natural communities of plankton subjected to ballast water treatment. Journal of Applied Phycology 28: 279–298. DOI: https://doi.org/10.1007/s10811-015-0601-x.
- Daugbjerg N., Fassel N.M.D. & Moestrup Ø. 2020. Microscopy and phylogeny of Pyramimonas tatianae sp. nov. (Pyramimonadales, Chlorophyta), a scaly quadriflagellate from Golden Horn Bay (eastern Russia) and formal description of Pyramimonadophyceae classis nova. European Journal of Phycology 55: 49–63. DOI: https://doi.org/10.1080/09670262.2019.1638524.
- Daugbjerg N. & Moestrup Ø. 1992. Fine structure of Pyramimonas cyclotreta sp. nov. (Prasinophyceae) from Northern Foxe Basin, Arctic Canada, with some observations on growth rates. European Journal of Protistology 28: 288–298. DOI: https://doi.org/10.1016/S0932-4739(11)80235-X.
- Edwards K.F., Thomas M.K., Klausmeier C.A. & Litchman E. 2012. Allometric scaling and taxonomic variation in nutrient utilization traits and maximum growth rate of phytoplankton. Limnology and Oceanography 57: 554–566. DOI: https://doi.org/10.4319/lo.2012.57.2.0554.
- Ekelund F., Daugbjerg N. & Fredslund L. 2004. Phylogeny of Heteromita, Cercomonas and Thaumatomonas based on SSU rDNA sequences, including the description of Neocercomonas jutlandica sp. nov., gen. nov. European Journal of Protistology 40: 119–135. DOI: https://doi.org/10.1016/J.EJOP.2003.12.002.
- Grasshof K. [Ed] 1976. Methods of seawater analysis. Verlag Chemie, Weinheim, Germany. 317 pp. DOI: https://doi.org/10.4319/lo.1977.22.6.1103.
- Guillard R.R.L. & Hargraves P.E. 1993. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 32: 234–236. DOI: https://doi.org/10.2216/i0031-8884-32-3-234.1.
- Hall T.A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98.
- Hansen B., Bjornsen P.K. & Hansen P.J. 1994. The size ratio between planktonic predators and their prey. Limnology and Oceanography 39: 395–403. DOI: https://doi.org/10.4319/lo.1994.39.2.0395.
- Hansen P.J., Bjornsen P.K. & Hansen B.W. 1997. Zooplankton grazing and growth: scaling within the 2-2,000-mm body size range. Limnology and Oceanography 42: 687–704. DOI: https://doi.org/10.4319/lo.1997.42.4.0687.
- Haraguchi L. 2018. Function assessment of coastal ecosystem based on phytoplankton community structure. Ph.D thesis. Aarhus University, Roskilde, Denmark. 162 pp.
- Haraguchi L., Carstensen J., Abreu P.C. & Odebrecht C. 2015. Long-term changes of the phytoplankton community and biomass in the subtropical shallow Patos Lagoon Estuary, Brazil. Estuarine, Coastal and Shelf Science 162: 76–87. DOI: https://doi.org/10.1016/j.ecss.2015.03.007.
- Haraguchi L., Jakobsen H.H., Lundholm N. & Carstensen J. 2018. Phytoplankton community dynamic: a driver for ciliate trophic strategies. Frontiers in Marine Science 5: 272. DOI: https://doi.org/10.3389/fmars.2018.00272.
- Harðardóttir S., Lundholm N., Moestrup Ø. & Nielsen T.G. 2014. Description of Pyramimonas diskoicola sp. nov. and the importance of the flagellate Pyramimonas (Prasinophyceae) in Greenland sea ice during the winter–spring transition. Polar Biology 37: 1479–1494. DOI: https://doi.org/10.1007/s00300-014-1538-2.
- Hasle G.R. 1978. The inverted microscope method. In: Phytoplankton manual. – monographs on oceanographic methodology, vol. 6 (Ed. by A. Sournia), pp 88–96. UNESCO publishing, Paris, France.
- Henriksen P., Riemann B., Kaas H., Sorensen H.M. & Sorensen H.L. 2002. Effects of nutrient-limitation and irradiance on marine phytoplankton pigments. Journal of Plankton Research 24: 835–858. DOI: https://doi.org/10.1093/plankt/24.9.835.
- Higgins H.W., Wright S.W. & Schlüter L. 2011. Quantitative interpretation of chemotaxonomic pigment data. In: Phytoplankton pigments: characterization, chemotaxonomy and applications in oceanography (Ed. by C.A. Llewellyn, E.S. Egeland, G. Johnsen & S. Roy), pp 257–313. Cambridge University Press, Cambridge, UK.
- Hill D.R.A., Moestrup Ø. & Vørs N. 1992. Rekylalger (Cryptophyceae). In: Plankton i de indre danske farvande (Ed. by H.A. Thomsen), pp 251–265. Havforskning fra Miliøstyrelsen, Copenhagen, Denmark.
- Hoef-Emden K. 2008. Molecular phylogeny of phycocyanin-containing cryptophytes: evolution of biliproteins and geographical distribution. Journal of Phycology 44: 985–993. DOI: https://doi.org/10.1111/j.1529-8817.2008.00530.x.
- Jakobsen H.H. & Markager S. 2016. Carbon-to-chlorophyll ratio for phytoplankton in temperate coastal waters: seasonal patterns and relationship to nutrients. Limnology and Oceanography 61: 1853–1868. DOI: https://doi.org/10.1002/lno.10338.
- Jeffrey S.W., Mantoura R.F.C. & Wright S.W. [Eds] 1997. Phytoplankton pigments in oceanography: guidelines to modern methods. UNESCO Publishing, Paris, France. 661 pp.
- Johnson Z.I. & Martiny A.C. 2015. Techniques for quantifying phytoplankton biodiversity. Annual Review of Marine Science 7: 299–324. DOI: https://doi.org/10.1146/annurev-marine-010814-015902.
- Kaas H. & Markager S. 1998. Technical guidelines for marine monitoring. http://bios.au.dk/videnudveksling/til-myndigheder-og-saerligt-interesserede/fagdatacentre/fdcmarintny/tekniske-anvisninger-nova–1998; searched on 23 February 2016.
- Kamp-Nielsen L. 1992. Benthic-pelagic coupling of nutrient metabolism along an estuarine eutrophication gradient. Hydrobiologia 235: 457–470. DOI: https://doi.org/10.1007/BF00026234.
- Lane C.E. & Archibald J.M. 2008. New marine members of the genus Hemiselmis (Cryptomonadales, Cryptophycea). Journal of Phycology 44: 439–450. DOI: https://doi.org/10.1111/j.1529-8817.2008.00486.x.
- Laza-Martínez A., Arluzea J., Miguel I. & Orive E. 2012. Morphological and molecular characterization of Teleaulax gracilis sp. nov. and T. minuta sp. nov. (Cryptophyceae). Phycologia 51: 649–661. DOI: https://doi.org/10.2216/11-044.1.
- Levasseur M., Therriault J.-C. & Legendre L. 1984. Hierarchical control of phytoplankton succession by physical factors. Marine Ecology Progress Series 19: 211–222. DOI: https://doi.org/10.3354/meps019211.
- Lundholm N., Daugbjerg N. & Moestrup Ø. 2002. Phylogeny of the Bacillariaceae with emphasis on the genus Pseudo-nitzschia (Bacillariophyceae) based on partial LSU rDNA. European Journal of Phycology 37: 115–134. DOI: https://doi.org/10.1017/S096702620100347X.
- MacIntyre H.L., Cullen J.J., Rastin S., Waclawik M., Franklin K.J., Poulton N., Lubelcyk L., McPhee K., Richardson T.L., Van Meerssche E. et al. 2019. Inter-laboratory validation of the serial dilution culture – most probable number method for enumerating viable phytoplankton. Journal of Applied Phycology 31: 491–503. DOI: https://doi.org/10.1007/s10811-018-1541-z.
- Massana R., Gobet A., Audic S., Bass D., Bittner L., Boutte C., Chambouvet A., Christen R., Claverie J.-M., Decelle J. et al. 2015. Marine protist diversity in European coastal waters and sediments as revealed by high-throughput sequencing. Environmental Microbiology 17: 4035–4049. DOI: https://doi.org/10.1111/1462-2920.12955.
- McFadden G.I., Hill D.R.A. & Wetherbee R. 1986. A study of the genus Pyramimonas (Prasinophyceae) from Southeastern Australia. Nordic Journal of Botany 6: 209–234. DOI: https://doi.org/10.1111/j.1756-1051.1986.tb00875.x.
- McFadden G.I., Hill D.R.A. & Wetherbee R. 1987. Electron microscopic observations on Pyramimonas olivacea N. Carter (Prasinophyceae, Chlorophyta). Phycologia 26: 322–327. DOI:https://doi.org/10.2216/i0031-8884-26-3-322.1.
- Menden-Deuer S. & Lessard E.J. 2000. Carbon to volume relationships for dinoflagellates, diatoms, and other Protist plankton. Limnology and Oceanography 45: 569–579. DOI: https://doi.org/10.4319/lo.2000.45.3.0569.
- Moestrup Ø. 1992. Prasinophyceae og andre grønne flagellater. In: Plankton i de indre danske farvande (Ed. by H.A. Thomsen), pp 267–310. Havforskning fra Miliøstyrelsen, Copenhagen, Denmark.
- Norris R.E. & Pienaar R.N. 1978. Comparative fine-structural studies on five marine species of Pyramimonas (Chlorophyta, Prasinophyceae). Phycologia 17: 41–51. DOI: https://doi.org/10.2216/i0031-8884-17-1-41.1.
- Palenik B., Grimwood J., Aerts A., Rouzé P., Salamov A., Putnam N., Dupont C., Jorgensen R., Derelle E., Rombauts S. et al. 2007. The tiny eukaryote Ostreococcus provides genomic insights into the paradox of plankton speciation. Proceedings of the National Academy of Sciences 104: 7705–7710. DOI: https://doi.org/10.1073/pnas.0611046104.
- Pennick N.C. 1984. Comparative ultrastructure and occurrence of scales in Pyramimonas (Chlorophyta, Prasinophyceae). Archiv Für Protistenkunde 128: 3–11. DOI: https://doi.org/10.1016/S0003-9365(84)80022-6.
- Percopo I., Siano R., Cerino F., Sarno D. & Zingone A. 2011. Phytoplankton diversity during the spring bloom in the Northwestern Mediterranean Sea. Botanica Marina 54: 243–267. DOI: https://doi.org/10.1515/BOT.2011.033.
- Piredda R., Sarno D., Lange C.B., Tomasino M.P., Zingone A. & Montresor M. 2017. Diatoms resting stages in surface sediments: a pilot study comparing next generation sequencing and serial dilution cultures. Cryptogamie, Algologie 38: 31–46. DOI: https://doi.org/10.7872/crya/v38.iss1.2017.31.
- Piwosz K. 2019. Weekly dynamics of abundance and size structure of specific nanophytoplankton lineages in coastal waters (Baltic Sea). Limnology and Oceanography 64: 2172–2186. DOI: https://doi.org/10.1002/lno.11177.
- Posada D. & Crandall K.A. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14: 817–818. http://www.ncbi.nlm.nih.gov/pubmed/9918953.
- Ptacnik R., Solimini A.G., Andersen T., Tamminen T., Brettum P., Lepistö L., Willén E. & Rekolainen S. 2008. Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proceedings of the National Academy of Sciences of the United States of America 105: 5134–5138. DOI:https://doi.org/10.1073/pnas.0708328105.
- R Core Team. 2015. R: a language and environment for statistical computing. version 3.6.0. http://R-project.org.
- Rodríguez F., Derelle E., Guillou L., Le Gall F., Vaulot D. & Moreau H. 2005. Ecotype diversity in the marine picoeukaryote Ostreococcus (Chlorophyta, Prasinophyceae). Environmental Microbiology 7: 853–859. DOI: https://doi.org/10.1111/j.1462-2920.2005.00758.x.
- Ronquist F. & Huelsenbeck J.P. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574.
- Schlüter L., Møhlenberg F., Havskum H. & Larsen S. 2000. The use of phytoplankton pigments for identifying and quantifying phytoplankton groups in coastal areas: testing the influence of light and nutrients on pigment/chlorophyll a ratios. Marine Ecology Progress Series 192: 49–63. DOI: https://doi.org/10.3354/MEPS192049.
- Smetacek V. 1981. The annual cycle of protozooplankton in the Kiel Bight. Marine Biology 63: 1–11. DOI: https://doi.org/10.1007/BF00394657.
- Staehr P.A., Testa J. & Carstensen J. 2017. Decadal changes in water quality and net productivity of a shallow Danish estuary following significant nutrient reductions. Estuaries Coasts 40: 63–79. DOI: https://doi.org/10.1007/s12237-016-0117-x.
- Stoeck T. & Epstein S. 2003. Novel eukaryotic lineages inferred from small-subunit rRNA analyses of oxygen-depleted marine environments. Applied and Environmental Microbiology 69: 2657–2663. DOI: https://doi.org/10.1128/AEM.69.5.2657-2663.2003.
- Suda S., Bhuiyan M.A.H. & Faria D.G. 2013. Genetic diversity of Pyramimonas from Ryukyu Archipelago, Japan (Chlorophyceae, Pyramimonadales). Journal of Marine Science and Technology 21: 285–296. DOI: https://doi.org/10.6119/JMST-013-1220-16.
- Sun J. & Liu D. 2003. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research 25: 1331–1346. DOI: https://doi.org/10.1093/plankt/fbg096.
- Swofford D.L. 2003. Phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland, Massachusetts, USA.
- Thomsen H.A. & Buck K.R. 1998. Nanoflagellates of the central California waters: taxonomy, biogeography and abundance of primitive, green flagellates (Pedinophyceae, Prasinophyceae). Deep Sea Research Part II: Topical Studies in Oceanography 45: 1687–1707. DOI: https://doi.org/10.1016/S0967-0645(98)80013-1.
- Thomsen H.A. [Ed] 1992. Plankton i de indre danske farvande. Havforskning fra Miliøstyrelsen, Copenhagen, Denmark. 336 pp
- Throndsen J. 1978b. The dilution culture method. In: Phytoplankton manual. – monographs on oceanographic methodology, vol. 6 (Ed. by A. Sournia)), pp 218–224. UNESCO Publishing, Paris, France.
- Throndsen J. 1978a. Preservation and storage. In: Phytoplankton manual. – monographs on oceanographic methodology, vol. 6 (Ed. by A. Sournia), pp 69–74. UNESCO publishing, Paris, France.
- Throndsen J. 1993. The planktonic marine flagellates. In: Marine phytoplankton. A guide to naked flagellates and coccolithophorids (Ed. by C.R. Tomas), pp 7–145. Academic Press, San Diego, California, USA. DOI: https://doi.org/10.1016/B978-012693018-4/50007-0.
- Van Den Meersche K., Soetaert K. & Middelburg J.J. 2008. A Bayesian compositional estimator for microbial taxonomy based on biomarkers. Limnology and Oceanography – Methods 6: 190–199. DOI: https://doi.org/10.4319/lom.2008.6.190.
- Wright S.W., Jeffrey S.W., Mantoura R.F.C., Llewellyn C.A., Bjørnland T., Repeta D. & Welshmeyer N. 1991. Improved HPLC method for the analysis of chlorophylls and carotenoids from marine phytoplankton. Marine Ecology Progress Series 77: 183–196. DOI: https://doi.org/10.3354/meps077183.
- Zingone A., Harrison P.J., Kraberg A., Lehtinen S., McQuatters-Gollop A., O’Brien T., Sun J. & Jakobsen H.H. 2015. Increasing the quality, comparability and accessibility of phytoplankton species composition time-series data. Estuarine, Coastal and Shelf Science 162: 151–160. DOI: https://doi.org/10.1016/j.ecss.2015.05.024.