ABSTRACT
Recent investigations into the species diversity of red blades in Hawai‘i have yielded several specimens of Kallymeniaceae from Hawaiian Mesophotic Coral Ecosystems. Our combined morphological and mitochondrial COI-5P and plastid rbcL phylogenetic analyses indicated widespread cryptic diversity among those specimens commonly identified as Kallymenia sensu lato based on morphology. These analyses resolved four unique genetic lineages of Hawaiian taxa in the genus Croisettea, which are all restricted to the lower mesophotic depths (c. 60–150 m). Croisettea currently includes three described species distributed in the North Atlantic, Indian and South Pacific Oceans, and the Mediterranean Sea. Croisettea is a new genus record for the Hawaiian Islands, expanding its biogeographic range to the North Pacific. The genus has now been enlarged to include seven species comprising previously described taxa as well as four new Hawaiian taxa (C. kalaukapuae sp. nov., C. haukoaweo sp. nov., C. ohelouliuli sp. nov. and C. pakualapa sp. nov.). The known distributions of the Hawaiian Croisettea species are restricted to areas around their type localities. Although this pattern hints at a remarkable degree of endemicity, both across depth gradients in a reef area and among islands, it is also linked to a limited sampling of the group, suggesting that additional species, and more accurate distributional ranges, remain to be detected not only in Hawai‘i but also worldwide.
INTRODUCTION
In the last several years, Hawaiian mesophotic algal collections have been a large source of new species. Recent and ongoing floristic surveys of Hawaiian marine habitats, including Mesophotic Coral Ecosystems (MCEs) – extending from 30 to at least 150 m depths (Hinderstein et al. Citation2010), are leading to a more accurate recognition of the diversity, especially among Rhodophyta. An astounding array of new generic (i.e. Ethelia, Halopeltis, Haraldiophyllum, Incendia, Leptofauchea, Meredithia, Psaromenia, Ramicrusta, Seiria, Sonderophycus, Umbraulva) and species records in the Hawaiian Archipelago have resulted from Hawaiian mesophotic collections (Spalding et al. Citation2016; Paiano et al. Citation2020; Sherwood et al. Citation2020Citationa, Citationb, Citationc; Alvarado Citation2021; Cabrera et al. Citation2021).
Despite increased research efforts, MCEs remain largely unexplored, and the taxonomy of many MCE-associated species requires clarification. Notably, many rarely recorded shallow reef members of the red blades, which often harbour cryptic species, are frequently observed and collected in Hawaiian MCEs (Spalding et al. Citation2019). Here, we follow Bickford et al. (Citation2007) in considering species to be cryptic when morphologically indistinguishable taxa representing distinct entities are classified under one taxonomic name.
Phylogenetic revisions of the long-established ‘red blade’ genus Kallymenia J. Agardh (D’Archino et al. Citation2010, Citation2011, Citation2012, Citation2016, Citation2017, Citation2018) led to the reinstatement of the genus Euhymenia Kützing, nom. illeg. (Saunders et al. Citation2017); however, Euhymenia is regarded as a superfluous name originally intended to replace Kallymenia, and the new genus Croisettea M.J. Wynne was proposed to accommodate the species in question (Wynne Citation2018). The emended description of the genus Croisettea comprises: expanded to lobed membranous red blades with 2–3-celled carpogonial branches (Norris & Womersley Citation1971; Wynne Citation2018). As currently circumscribed Croisettea includes three species: C. requienii (J. Agardh) M.J. Wynne (the generitype), C. australis (Womersley & R.E. Norris) M.J. Wynne and C. tasmanica (Harvey) M.J. Wynne. The genus has a wide and disjunct distribution in the North Atlantic, Pacific and Indian Oceans, as well as the Mediterranean (Guiry & Guiry Citation2021). The Southern Hemisphere has been hypothesized to be the centre of Croisettea diversity, with several Croisettea cryptic species complexes detected and yet to be described (Saunders et al. Citation2017). Additionally, the three recognized species of Croisettea occur both in the shallow and mesophotic – with C. requienii documented as low as 95 m depth (Agardh Citation1847), C. australis at 50 m and C. tasmanica at 40 m (Womersley Citation1994). Hence, the full extent of the diversity of the genus Croisettea remains incompletely known without inclusion of additional specimens from unrepresented geographical areas and depth ranges.
Through combined morphological and multi-gene molecular analyses, we characterized four novel species of Croisettea documented exclusively in the lower mesophotic (c. 60–150 m), adding to the long list of newly recorded genera in the Hawaiian Islands. It is essential to bring attention to such rarely seen mesophotic species and to provide a taxonomic (and especially, molecular) framework for future researchers to perform more extensive fieldwork, obtain more collections or describe further new taxa.
MATERIAL AND METHODS
Specimens were sampled during mesophotic surveys from 2006 to 2019 in the Papahānaumokuākea Marine National Monument (PMNM) (also referred to as the Northwestern Hawaiian Islands. NWHI) by National Oceanic and Atmospheric Administration (NOAA) divers using mixed gas closed-circuit rebreathers, and in the Main Hawaiian Islands (MHI) in the ‘Au‘Au Channel between the islands of Lānaʿi and Maui using the manned submersibles Pisces IV and Pisces V. The locations of the sampling sites are shown in Fig. S1 and the specimen collection details are presented in Table S1.
Morphological characterization
Anatomical and reproductive features were observed in material that was hand-sectioned with a razor blade. Sections were rehydrated in modified Pohl’s solution (Pohl Citation1965) for approximately 5 min, stained with 0.5% aniline blue for approximately 5 min, and then mounted in 30% Karo™. Sections of stipe and basal regions, which were generally thicker than apical cross sections, were rehydrated from herbarium sheets and stained for at least 10 min. Note that rehydration and staining longer than 20 min will cause the blades to disintegrate into a dense mass of cells. To illustrate the full view of the sections, several successive images from individual sections were combined using Autostitch free software (Ma et al. Citation2007).
DNA sequencing and phylogenetic reconstruction
Total genomic DNA was extracted from silica gel-preserved material or herbarium specimens using the OMEGA E.Z.N.A. Plant DNA Kit (OMEGA Biotek, Norcross, Georgia, USA) following the manufacturer’s protocol. The mitochondrial COI-5P region was amplified using the primers GazF1 and GazR1 and the recommended PCR profile (Saunders Citation2005), while the plastid rubisco large subunit (rbcL) gene was amplified as described in Xuan-Nguyen et al. (Citation2019). Successful PCR products were sequenced by Genewiz Inc. (South Plainfield, New Jersey, USA). Sequence data were edited and aligned with additional sequences downloaded from GenBank (Table S2) in Geneious Prime 2019.1.3 (http://www.geneious.com).
Sequence alignment was performed using MUSCLE plug-in (Edgar Citation2004) with default settings in Geneious Prime to construct alignments for each gene: COI-5P with 25 sequences of 664 base pairs (bp), and rbcL with 26 sequences of 1300 bp. These alignments used Dumontia simplex Cotton as the outgroup (Saunders et al. Citation2017). We analysed the rbcL and COI datasets individually and concatenated the congruent datasets (Figs S3, S4). PartitionFinder v1.1.1 analyses suggested the General Time Reversible model with gamma distributed rate variation among sites and a proportion of invariant sites for the concatenated data set (Lanfear et al. Citation2012). The concatenated dataset was used in phylogenetic reconstruction with Maximum Likelihood (ML) using RAxML (Stamatakis Citation2014) with 1,000 bootstrap replicates, and Bayesian Inference (BI) using MrBayes v3.2.6 (Ronquist et al. Citation2012) based on the nucleotide substitution models determined by the Akaike Information Criteria (AIC) in MrModeltest 2.3 (Nylander et al. Citation2008) through tree builder plugins in Geneious Prime. The Bayesian analysis was run with 2,000,000 generations of Markov Chain Monte Carlo iterations until the standard deviation of split frequencies was below 0.01. The first 10% of trees of each run were discarded as burn-in. Visualization of the trees was performed via the interactive Tree of Life (https://itol.embl.de/; Letunic & Bork Citation2019). All new sequences were submitted to GenBank (accession numbers: COI, OM509717–OM5097124; rbcL, OM621854–OM621863).
RESULTS
Phylogenetic analyses
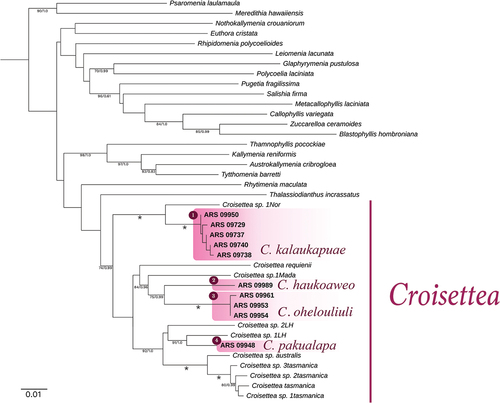
Ten rbcL and nine COI-5P sequences were newly generated in this study (Table S1). These sequences were compared to GenBank sequences (80 for rbcL and 74 for COI-5P) representing all available genera in the family Kallymeniaceae (Tables S1, S2; Figs S3, S4). The BA and ML analyses of the concatenated alignment resulted in the same tree topology, and only the ML tree is shown (). Phylogenetic analyses confirmed the placement of four lineages of Hawaiian specimens in three moderately- to well-supported Croisettea subclades. The first subclade with moderate support was composed of C. ohelouliuli as sister to an undescribed Croisettea from Madagascar (sp. 1 Mada). The second subclade with good support contained C. pakualapa and C. haukoaweo, as well as C. australis and C. tasmanica and undescribed Croisettea specimens from Lord Howe Island (LH), Australia. The third subclade with full support included C. kalaukapuae and an undescribed Croisettea from Norfolk Island, Australia. The concatenated COI+rbcL analyses demonstrated the distinctiveness of Hawaiian Croisettea from the other three recognized species in the genus. The four Hawaiian species exhibited some phenotypic variation (detailed in ), mostly with differences in blade thickness and sizes of vegetative characters; however, the current evidence for their recognition as distinct species lies overwhelmingly in their genetic distinctiveness. They are proposed below as new species.
Table 1. Comparison of morphological and anatomical characters among Croisettea species.
Fig. 1. RAxML phylogenies inferred from the combined alignment of COI and rbcL. Outgroup (Dumontia simplex) pruned to facilitate presentation. Support values at nodes >70% (ML bootstrap, first value) and >0.9 (Bayesian posterior probability, second value) are shown. Asterisk on branches indicates full support; asterisk on species names indicates the generitype (*). Scale bar = substitutions per site.
Croisettea kalaukapuae F.P. Cabrera & A.R. Sherwood sp. nov.
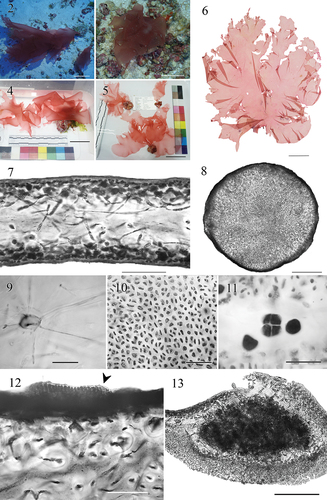
Figs 2–13. Habit, general morphology and anatomy of Croisettea kalaukapuae sp. nov.Fig. 2. Holotype specimen, male gametophyte (BISH 780911) in situ, collected at Papa’āpoho (Lisianski) at 84 m. Scale bar = 5 cm.Fig. 3. Paratype specimen (BISH 780917) in situ, collected at Lalo (French Frigate Shoals) at 83 m. Scale bar = 5 cm.Fig. 4. Live holotype specimen (BISH 780911), cleaned of epiphytes. Scale bar = 5 cm.Fig. 5. Live holotype specimen (BISH 780917), cleaned of epiphytes. Scale bar = 5 cm.Fig. 6. Voucher for BISH 780911 (holotype, tetrasporophyte). Scale bar = 5 cm.Fig. 7. Cross section through blade showing filamentous medulla and cortex, BISH 780911. Scale bar = 100 μm.Fig. 8. Cross section through stipe showing dense aggregation of narrow internal filaments, BISH 780910. Scale bar = 400 μm.Fig. 9. Squash preparation showing stellate cell. BISH 780912. Scale bar = 50 μm.Fig. 10. Cortical cells in surface view, BISH 780909. Scale bar = 50 μm.Fig. 11. Detail of cruciate tetrasporangia, BISH 780910. Scale bar = 50 μm.Fig. 12. Cross section through blade with arrows showing male nemathecium, BISH 780918. Scale bar = 100 μm.Fig. 13. Cross section through mature female cystocarp, BISH 780909. Scale bar = 200 μm.
description
Blades flat, thin, delicate, with smooth, pleated or undulate margins, blush to rose pink in colour, and with a soft, slippery consistency. Blades ranging from 1.5–35 cm long by 1.5–48 cm wide and 150–230 µm thick. Young blades vary in shape but typically slightly wider than high. Mature blades orbicular in shape and forming deep lobes. Blades single, erupting abruptly from a short, stiff stipe arising from a small discoidal holdfast that is usually attached to rhodoliths. Carposporophytes 600–900 µm in diameter, scattered over the blade. Tetrasporophytes and gametophytes isomorphic. Tetrasporangia scattered throughout the cortex, terminal, cruciately divided, 10–14 × 10–24 µm.
holotype
ARS 09739/BISH 780911, Kapou (Lisianski), Hawai‘i, USA (25°52.94ʹN, 173°57.73ʹW, 84 m depth, collected 15 September 2014 by R. Pyle and D. Wagner). GenBank accessions: rbcL, OM621858; COI, OM509720.
isotypes
BISH 780912 and BISH 780913, collection details as for the holotype.
etymology
The species epithet kalaukapuae honours Laura Kalaukapu Low Lucas Thompson (1925–2020) for her advocacy for Hawai‘i’s cultural and natural resources, especially her significant contributions to the creation of the Papahānaumokuākea Marine National Monument, including her role as a founding member of the NWHI Coral Reef Ecosystem Reserve (see Table S3 for more information on how specific nomenclature was developed using traditional Hawaiian naming practices in collaboration with the Papahānaumokuākea Native Hawaiian Cultural Working Group, CWG).
distribution
Throughout the Papahānaumokuākea Marine National Monument including Manawai (Pearl and Hermes Atoll), Kapou (Lisianski Island) and Lalo (French Frigate Shoals), and exclusively collected from mesophotic depths, at 83–85 m.
Morphology and ecology
Thalli are foliose, consisting of a single blade, 1.5–35 cm long and 1.5–48 cm wide, arising from a short, stiff, cartilaginous stipe, abruptly expanding into a broad, gelatinous blade (). Thalli ranging from blush to rose pink, sometimes tending to a pinkish brown colour. Blade margins are mostly pleated to undulate. Blades 150–230 µm thick in section () with peripheral cells ultimately bearing cortex of one or two layers of periclinally compressed inner cortical cells, 3–6 × 6–11 µm, and a cartilaginous stipe <0.6 cm in length and 1.0–1.5 mm in diameter (). Medulla lax with an interconnected network of darkly staining stellate cells, typically with central bodies 7–9 µm in diameter and long, thin arms 2–4 µm wide by 30–100 µm long (), extending parallel to the blade surface. Stellate cells connected to the cortex of one or two layers of small isodiametric outer cortical cells 1–3 µm wide by 5–10 µm long ().
Tetrasporangia scattered in the cortex and cruciately divided (), 5–7 × 5–12 µm, on both surfaces and terminal in blade. Thalli are dioecious. Spermatangia are formed in nemathecia, scattered across median parts of the thallus; nemathecia develop on both sides of the blade, are darkly staining, and are elongate, with irregular margins (). Spermatangia (3–6 µm in diameter) borne singly on spermatangial mother cells (10–30 µm long) in the outer cortex. Cystocarps are approximately 600–900 µm in diameter, slightly protruding from the thallus surface, and are distributed across the blade surfaces except in the basal region. Carpospores 10–15 µm in diameter, forming a singular dense mass ().
These blades are relatively abundant on the mesophotic reefs in the PMNM (Manawai, Kapou and Lalo). They have been so far only documented from mesophotic depths (83–85 m). Blades are typically attached at a single point to coral rubble on a sandy bottom and are often observed to have a sprawling habit.
Croisettea haukoaweo F.P. Cabrera & A.R. Sherwood sp. nov.
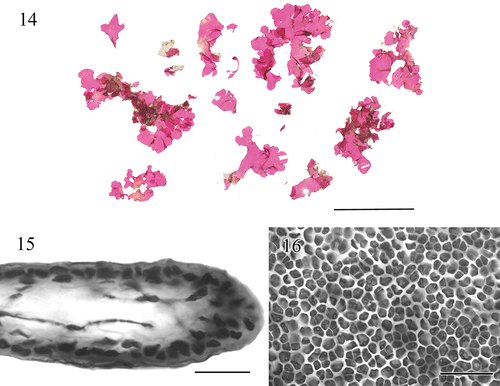
Figs 14–16. Morphology and anatomy of Croisettea haukoaweo sp. nov.Fig. 14. Pressed voucher for BISH 780919 (holotype, vegetative). Scale bar = 5 cm.Fig. 15. Cross section through apical portion of the blade showing inner cortical cells and medullary stellate cells, BISH 780919. Scale bar = 25 μm.Fig. 16. Cortical cells in surface view, BISH 780919. Scale bar = 50 μm.
description
Blades typically flabellate, single or clustered, 1–6 cm long by 1–8 cm wide and 40–80 µm thick, lobed with broadly crenate margin, magenta pink to rose red, with a soft, slippery consistency. One or more blades developing and producing in turn several to many marginal, subdichotomously highly lobed blades, often overtopping one another. Medulla uniform throughout with a sparse arrangement of elongated filamentous stellate cells with 4–6 arms; cortex 1–2 layers of ovoid cells, 1–3 µm wide by 5–10 µm high.
holotype
ARS 09989/BISH 780919, ‘Au‘Au Channel, Maui, Hawai‘i, USA (104 m depth), collected 29 September 2006 by H. Spalding and T. Kerby. GenBank accession: rbcL, OM621863.
etymology
The species epithet haukoaweo refers to “the vibrant limu entwined with pūko’ako’a (Halimeda sp.) found in the cool deep waters”. The term ‘hau’ in the name also honours Mr. Skippy Hau, conservationist and retired State of Hawai‘i Division of Aquatic Resources staff on Maui, for his lifetime dedication to the ocean and his community (see Table S3 for more details on how the CWG developed the species name).
distribution
A single collection, from ‘Au‘Au Channel, Maui, Hawai‘i, USA; mesophotic depth of 104 m.
Morphology and ecology
Blades single or clustered, 1–6 cm in height, 1–8 cm wide, lobed with broadly crenate margins (). Blades in cross section uniformly 40–80 µm thick. Stipe and mode of attachment to substrate not observed. The medulla consisting primarily of sparse filaments and stellate cells with central bodies ranging from 5–10 µm wide by 1–3 µm high, and elongate, slender arms (4–6 in number) that are 2–3 µm wide by 15–50 µm long (). Surface view of outer cortical ovoid cells 1–3 µm wide by 5–10 µm high (). Tetrasporangial and gametangial reproduction not observed.
Although mode of attachment of blades was not identified, parts of blades were found entwined with species of mounding, prostrate species of Halimeda J.V. Lamouroux, which are abundant in the ‘Au‘Au Channel, Maui (see Spalding et al. Citation2019, fig. 29.1b). Blades of C. haukoaweo are only documented in the MHI in the ‘Au‘Au Channel, Maui at 104 m depth.
Croisettea ohelouliuli F.P. Cabrera & A.R. Sherwood sp. nov.
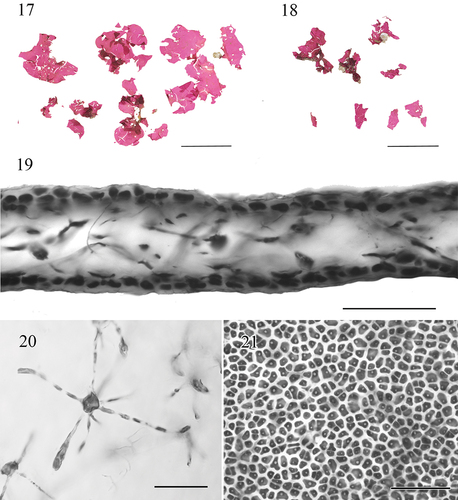
Figs 17–21. Morphology and anatomy of Croisettea ohelouliuli sp. nov.Fig. 17. Pressed voucher for BISH 780920 (holotype, vegetative). Scale bar = 5 cm.Fig. 18. Pressed voucher for BISH 780921 (paratype, vegetative). Scale bar = 5 cm.Fig. 19. Cross section through blade, BISH 780920. Scale bar = 50 μm.Fig. 20. Detail of a stellate cells in a squash preparation. BISH 780920. Scale bar = 50 μm.Fig. 21. Cortical cells in surface view, BISH 780921. Scale bar = 50 μm.
description
Blades foliose, magenta pink to rose red, blades sometimes wider than high, 5–9 cm in height and 0.5–7 cm wide. Blades are smooth-surfaced, irregularly lobed, membranous, and attached with a short stipe to the substratum by a small discoid holdfast. Blades uniformly 40–80 µm thick, the medulla composed primarily of sparse filaments and stellate cells with central bodies 5–9 µm wide by 7–13 µm high, and elongate, slender arms (4–6 in number), 2–4 µm wide by 30–100 µm long. The outer cortical layer subtending 1–2 layers of refractive isodiametric cells, 3–7 µm wide by 5–12 µm high.
holotype
ARS 09953/BISH 780920, ‘Au‘Au Channel, Maui, Hawai‘i, USA (113 m depth), collected 29 September 2006 by H. Spalding and T. Kerby. GenBank accessions: rbcL, OM621861; COI, OM509723.
isotype
ARS 09954/BISH 780921, ‘Au‘Au Channel, Maui, Hawai‘i, USA (94 m depth), collected 29 September 2006 by H. Spalding and T. Kerby.
etymology
The species epithet ohelouliuli refers to “the dark and vibrant ‘ōhelo” (or algae with no known species attached to it). ‘Ōhelo also describes the colour of the limu in its reference to a mauka (land) plant, Vaccinium reticulatum Smith, and its deep, red-coloured berries and endemicity to Hawai‘i (see Table S3 for more details on how the species name was developed by the CWG).
distribution
Two specimens collected from the ‘Au‘Au Channel, Maui, Hawai‘i, USA; depth range of 94–113 m.
Morphology and ecology
Thalli are bright fuchsia to dark red, soft and fleshy, irregularly lobed or with smooth margins, 1.5–9 cm in length and 0.5–7 cm in width (). Blades emerge from a short stipe with a small discoid holdfast that attaches to the substratum, beset with numerous perforations and small protuberances, and are uniformly 40–80 µm thick (). The medulla is composed primarily of sparse filaments with darkly staining stellate cells with large central bodies 5–9 µm wide by 7–13 µm, bearing high and elongate, slender, radiating arms (4–6 in number), 2–4 µm wide by 30–100 µm long (). Cortex compact with 1–2 layers of isodiametric cells, 3–7 µm wide by 5–12 µm high (). Tetrasporangial and gametangial reproduction not observed.
Blades found growing attached either to dense assemblages of Halimeda spp or coral rubble. Numerous perforations and small protuberances on blades either ontogenetic or marks of grazing pressure from mesophotic herbivores. So far, this species has only been documented in the MHI, ‘Au‘Au Channel, Maui at a depth range of 94–113 m.
Croisettea pakualapa F.P. Cabrera & A.R. Sherwood sp. nov.
Figs 22–26. Morphology and anatomy of Croisettea pakualapa sp. nov.Fig. 22. Live holotype specimen, BISH 780907. Scale bar = 1 cm.Fig. 23. Pressed voucher (visible cut from obtaining tissue for DNA extraction, BISH 780907 (holotype, tetrasporophyte). Scale bar = 1 cm.Fig. 24. Cross section through blade showing medullary filaments, BISH 780907. Scale bar = 50 μm.Fig. 25. Cortical cells and tetrasporangia in surface view, BISH 780907. Scale bar = 100 μm.Fig. 26. Detail of a stellate cell. BISH 780907. Scale bar = 50 μm.
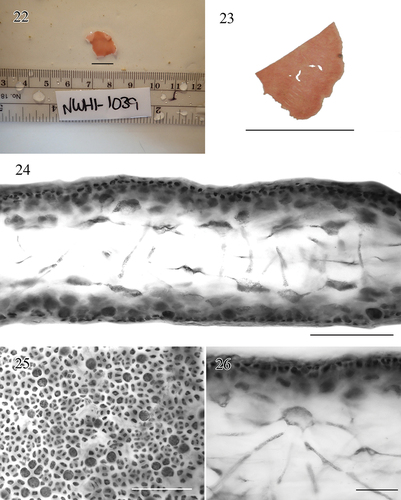
description
Thalli single, composed of non-perforate blades with smooth to minutely dentate margins, rose pink in colour. Thalli with a soft, slippery consistency, 1.0 cm long by 0.6 cm wide and 50–100 µm thick, and orbicular in shape. Cruciately divided tetrasporangia scattered in the cortex of both blade surfaces, typically spherical and regularly cruciate, 14–18 × 14–18 µm. Medulla with a loose arrangement of elongate stellate cells 30–50 µm in diameter, bearing 6–8 radiating arms 2–4 × 50–100 μm; cortex of 1–2 layers of small isodiametric cells, 2–5 μm in diameter.
holotype
ARS 09948/ BISH 780907, Manawai (Pearl and Hermes Atoll), Hawai‘i, USA (27°44.48ʹN, 175°57.50ʹW, 84 m depth, collected 15 September 2014 by B. Hauk). GenBank accessions: rbcL, OM621860; COI, OM509722.
etymology
The species epithet pakualapa refers to “a sprawling, tapered limu found on the ridge” (see Table S3 for more details on how the species name was developed by the CWG).
distribution
A single specimen collected from Manawai (Pearl and Hermes Atoll), Hawai‘i, USA; at a depth of 85 m.
Morphology and ecology
Only a single blade was collected, non-stipitate, 1.0 cm in height, 0.6 cm wide, with smooth to minutely dentate margins (). Blade cross section 50–100 µm thick (). Tetrasporangia scattered in the cortex of both blade surfaces, typically spherical and regularly cruciate, 14 × 14 to 18 × 18 µm (). The inner cortex is composed of 1–2 layers of small, isodiametric cells, 2–5 μm in diameter, which subtends one or two layers of periclinally elongated outer cortical cells covering the subsurface cells (). Medullary stellate cells 30–50 µm in diameter, bearing 6–8 radiating arms 2–4 × 50–100 μm (). Gametangial reproduction not observed.
The single blade was collected at Manawai (Pearl and Hermes Atoll, PMNM) at a depth of 85 m. Although mode of attachment was not identified, this small blade was collected on a flat sandy surface, adjacent to a ridge.
DISCUSSION
Genus-level relationships within the family Kallymeniaceae remain equivocal because of varying degrees of phylogenetic support (Selivanova et al. Citation2020; Skriptsova Citation2021). The placement of Croisettea has not been fully resolved in our molecular phylogenetic analyses, and support is lacking for the Croisettea clade. Our phylogenetic analyses allied the four newly proposed species as distinct lineages within Croisettea. While relationships among Croisettea species remain largely unresolved or weakly supported in our analyses, our expanded phylogeny suggests that there are potentially many more genera in Kallymeniaceae. With the scale of phylogenetic studies continuing to grow and more taxa, particularly in the understudied MCEs, being included, additional independent lineages are likely to emerge. Further studies revisiting the morphology of this group and providing robust resolution of the molecular phylogenies will be necessary to better understand evolutionary relationships in the group and help define the taxonomic status of Croisettea.
There is considerable overlap in the morphological characters of C. kalaukapuae, C. ohelouliuli and C. pakualapa, such that it would be impossible to distinguish them in the field. Only C. haukoaweo has a suite of characters, particularly its deeply lobed blades, that clearly distinguishes it from the other Hawaiian Croisettea species. However, the young blades of C. haukoaweo, lacking lobes, also have overlapping morphological characters with all congeners. The potential for misidentification is incredibly high when preserved or juvenile specimens are the only material available for comparison. For example, C. pakualapa is easily confused with C. kalaukapuae or C. ohelouliuli. Overall, our phylogenetic and morphological analyses corroborate previous reports that cryptic speciation is rampant among red blades, which exhibit extremely high phylogenetic diversity (Rodríguez-Prieto et al. Citation2019; D’Archino & Zuccarello Citation2020).
Although cosmopolitan in distribution, kallymeniacean diversity is concentrated in the temperate regions of the world (Saunders et al. Citation2017). Abbott (Citation1999) reported her earliest encounters with Kallymenia, which she previously identified as Pugetia Kylin (Abbott Citation1996), in the tropical Pacific to be an ‘unusual occurrence’. Tropical Hawaiian Croisettea species are locally restricted to lower temperature and irradiance levels (Spalding et al. Citation2019), similar to temperate congeners. Yet, in contrast to its congeners distributed across shallow to mesophotic depths, Hawaiian Croisettea is exclusively documented in the lower mesophotic. For these reasons, we believe these new species to be endemic. Moreover, having distinct genetic differences in spite of overlapping in their geographical distribution (i.e. C. haukoaweo and C. ohelouliuli in ‘Au‘Au Channel; and C. kalaukapuae and C. pakualapa at Manawai) hints at a remarkable degree of endemicity. Thus, the Hawaiian MCEs appears to be a diversity hotspot for Croisettea.
Our study of Hawaiian Croisettea, similar to a study of Hawaiian mesophotic Ulvaceae (Spalding et al. Citation2016), does not support the Deep Reef Refuge Hypothesis, which postulates that mesophotic reefs function as refugia when there is considerable species overlap with shallow-water counterparts (Bongaerts & Smith Citation2019). In both of these studies, novel species were only documented at mesophotic depths. Yet, species overlap across spatial ranges differs by genus and species. For instance, among Hawaiian representatives of the genus Martensia K. Hering, M. tsudae A.R. Sherwood & Showe M. Lin and M. hawaiiensis A.R. Sherwood & Showe M. Lin occur both at shallow and mesophotic depths, whereas M. abbottiae A.R. Sherwood & Showe M. Lin and M. lauhiekoeloa A.R. Sherwood & Showe M. Lin are only documented in MCEs (Sherwood et al. Citation2019). At present, the Hawaiian endemic flora associated with the MCEs suggests two ecotypes: depth generalists that occur both in the shallow and mesophotic, and depth specialists usually in the lower mesophotic zone. With ample evidence for the existence of depth-specialists compared to depth-generalist algae, there is lower empirical support of the DRRH, and thus a lower likelihood of Hawaiian MCEs serving as refugia. During the course of field collections for this study, the PMNM experienced two major coral bleaching events (Couch et al. Citation2017) and a direct hit from a major hurricane (Pascoe et al. Citation2021), demonstrating that MCEs are not without vulnerabilities.
Describing new species from limited specimens cannot represent the whole picture of phenotypic diversity for a species, and this affects the completeness and utility of species descriptions. In this study, we describe C. haukoaweo and C. pakualapa from single specimens and C. ohelouliuli based on two specimens. Despite numerous expeditions to the ‘Au‘au Channel and PMNM being one of the most extensively collected MCEs globally, the current distribution ranges of these taxa cannot be determined due to lack of access to MCEs with manned ROV or submersibles and consistent funding for mesophotic expeditions. These limitations greatly hinder a comprehensive sampling of marine algae from these unique habitats and, in turn, our ability to describe comprehensively spatial distributions, diversity and endemicity of the mesophotic flora. Nevertheless, expedient formal taxonomic recognition of C. haukoaweo, C. pakualapa and C. ohelouliuli is beneficial in providing a taxonomic (and especially, molecular) framework for future researchers to compare future collections or describe further new taxa, and ensures the diversity is recognized, as a starting point for future research. Schneider et al. (Citation2019) used single mesophotic algal specimens to describe new species that corresponded morphologically to old herbarium collections, which is a good workaround for limited collections that are non-cryptic. Description of some mesophotic organisms (i.e. fishes, decapods, etc.) based on single specimens are justified by the low likelihood for timely acquiring of additional samples from the logistically challenging mesophotic reefs (Shepherd et al. Citation2018; Felder & Lemaitre Citation2020).
The description of C. kalaukapuae, C. haukoaweo, C. ohelouliuli and C. pakualapa raises the total number of Croisettea species from three to seven. Notably, these new species represent half of the currently recognized diversity of the Hawaiian Kallymeniaceae and contribute to the broader knowledge of MCE algal biodiversity. Our present knowledge of diversity of the Hawaiian macroalgal flora is far from exhaustive, and further species are to be expected among red blades. Given the continuing interest in Kallymeniaceae as model species for palaeobiology and biogeography (Bringloe & Saunders Citation2018), further investigation of its evolutionary history will be critical.
Supplemental Material
Download MS Word (3.1 MB)ACKNOWLEDGEMENTS
We thank the NOAA Papahānaumokuākea Native Hawaiian Cultural Working Group for their invaluable contributions in developing specific epithets for the Hawaiian species of Croisettea. G. McFall, J. Leonard, K. Lopes, S. Matadobra, B. Hauk, T. Efird, A. Fukunaga, D. Wagner and H. Owen assisted with diving and small boat operations. Our gratitude goes to Terry Kirby and the Hawai‘i Undersea Research Laboratory (HURL) Pisces IV and V submersible and RCV-150 pilots and crew, as well as the crew of the R/V Ka’imikai-o-Kanaloa, for access to these great depths. Fieldwork and specimen collections in the Northwestern Hawaiian Islands were authorized under Papahānaumokuākea Marine National Monument research permits PMNM-2014-15, PMNM 2015-029 and PMNM-2018-029 issued to R. Kosaki.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the authors.
SUPPLEMENTARY MATERIAL
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00318884.2022.2096823
Additional information
Funding
REFERENCES
- Abbott I.A. 1996. New species and notes on marine algae from Hawaii. Pacific Science 50: 142–156.
- Abbott I.A. 1999. Marine red algae of the Hawaiian Islands. Bishop Museum Press, Honolulu, Hawaii, USA. 465 pp.
- Agardh J.G. 1847. In historiam algarum symbolae. Linnaea 15: 443–457.
- Alvarado E.A. 2021. Uncovering diversity in the mesophotic zone of Hawaii: species new to science in the genera Halopeltis and Leptofauchea (Rhodymeniales, Rhodophyta). MSc thesis. University of Hawaii at Mānoa, Honolulu, Hawaii, USA. 59 pp.
- Bickford, D., Lohma, D.J., Sodhi, N.S., Ng, P.K., Meier, R., Winker, K., Ingram, K.K. & Das, I. 2007. Cryptic species as a window on diversity and conservation. Trends in Ecology & Evolution 22: 148–155. DOI:10.1016/j.tree.2006.11.004.
- Bongaerts P. & Smith T.B. 2019. Beyond the “deep reef refuge” hypothesis: a conceptual framework to characterize persistence at depth. In: Mesophotic coral ecosystems (Ed. by Y. Loya, K. Puglise & T. Bridge), pp 881–895. Springer, New York, New York, USA.
- Bringloe T.T. & Saunders G.W. 2018. Mitochondrial DNA sequence data reveal the origins of postglacial marine macroalgal flora in the Northwest Atlantic. Marine Ecology Progress Series 589: 45–58. DOI: 10.3354/meps12496.
- Cabrera F.P., Huisman J.M., Spalding H.L., Kosaki R.K. & Sherwood A.R. 2021. Diversity of Kallymeniaceae (Gigartinales, Rhodophyta) associated with Hawaiian mesophotic reefs. European Journal of Phycology 57: 1–11.
- Couch C.S., Burns J.H.R., Liu G., Steward K., Gutlay T.N., Kenyon J., Eakin C.M. & Kosaki R.K. 2017. Mass coral bleaching due to unprecedented marine heatwave in Papahānaumokuākea Marine National Monument (Northwestern Hawaiian Islands). PLOS One 12: Article e0185121. DOI: 10.1371/journal.pone.0185121.
- D’Archino R., Nelson W.A. & Zuccarello G.C. 2010. Psaromenia (Kallymeniaceae, Rhodophyta): a new genus for Kallymenia berggrenii. Phycologia 49: 73–85. DOI: 10.2216/08-29.1.
- D’Archino R., Nelson W.A. & Zuccarello G.C. 2011. Diversity and complexity in New Zealand Kallymeniaceae (Rhodophyta): recognition of the genus Ectophora and description of E. marginata sp. nov. Phycologia 50: 241–255. DOI: 10.2216/10-14.1.
- D’Archino R., Nelson W.A. & Zuccarello G.C. 2012. Stauromenia australis, a new genus and species in the family Kallymeniaceae (Rhodophyta) from southern New Zealand. Phycologia 51: 451–460. DOI: 10.2216/11-87.1.
- D’Archino R., Lin S.M., Gabrielson P.W. & Zuccarello G.C. 2016. Why one species in New Zealand, Pugetia delicatissima (Kallymeniaceae, Rhodophyta), should become two new genera, Judithia gen. nov. and Wendya gen. nov. European Journal of Phycology 51: 83–98. DOI: 10.1080/09670262.2015.1104557.
- D’Archino R., Nelson W.A. & Sutherland J.E. 2017. Neither Callophyllis nor Gelidium: Blastophyllis gen. nov. and Zuccarelloa gen. nov. (Kallymeniaceae, Rhodophyta) for three New Zealand species. Phycologia 56: 549–560. DOI: 10.2216/16-115.1.
- D’Archino R., Lin S.M. & Zuccarello G.C. 2018. Fulgeophyllis (Kallymeniaceae, Gigartinales), a new genus to accommodate two New Zealand species. Phycologia 57: 422–431. DOI: 10.2216/17-120.1.
- D’Archino R. & Zuccarello G.C. 2020. Foliose species of New Zealand red algae: diversity in the genus Tsengia (Tsengiaceae, Halymeniales), including T. northlandica sp. nov. Phycologia 59: 437–448. DOI: 10.1080/00318884.2020.1796107.
- Edgar, R.C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. DOI:10.1093/nar/gkh340.
- Felder D.L. & Lemaitre R. 2020. A new species of the hermit crab genus Cancellus H. Milne Edwards, 1836 from a mesophotic deep bank in the northwestern Gulf of Mexico (Crustacea: Decapoda: Diogenidae). Zootaxa 4890: 589–598. DOI: 10.11646/zootaxa.4890.4.10.
- Guiry M.D. & Guiry G.M. 2021. AlgaeBase. Worldwide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 5 August 2021.
- Hinderstein L.M., Marr J.C.A., Martinez F.A., Dowgiallo M.J., Puglise K.A., Pyle R.L., Zawada D.G. & Appeldoorn R. 2010. Theme section on “mesophotic coral ecosystems: characterization, ecology, and management”. Coral Reefs 29: 247–251. DOI: 10.1007/s00338-010-0614-5.
- Lanfear R., Calcott B., Ho S.Y. & Guindon S. 2012. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29: 1695–1701. DOI: 10.1093/molbev/mss020.
- Letunic I. & Bork P. 2019. Interactive Tree of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Research 47: W256–W259. DOI: 10.1093/nar/gkz239.
- Ma B., Zimmermann T., Rohde M., Winkelbach S., He F., Lindenmaier W. & Dittmar K.E. 2007. Use of autostitch for automatic stitching of microscope images. Micron 38: 492–499. DOI: 10.1016/j.micron.2006.07.027.
- Norris R.E. & Womersley H.B.S. 1971. The morphology and taxonomy of Australian Kallymeniaceae (Rhodophyta). Australian Journal of Botany Supplementary Series 2: 1–62.
- Nylander J.A., Wilgenbusch J.C., Warren D.L. & Swofford D.L. 2008. AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24: 581–583. DOI: 10.1093/bioinformatics/btm388.
- Paiano M.O., Huisman J.M., Cabrera F.P., Spalding H.L., Kosaki R.K. & Sherwood A.R. 2020. Haraldiophyllum hawaiiense sp. nov. (Delesseriaceae, Rhodophyta): a new mesophotic genus record for the Hawaiian Islands. Algae 35: 337–347. DOI: 10.4490/algae.2020.35.11.5.
- Pascoe K.H., Fukunaga A., Kosaki R.K. & Burns J.H. 2021. 3D assessment of a coral reef at Lalo Atoll reveals varying responses of habitat metrics following a catastrophic hurricane. Scientific Reports 11: Article 120150. DOI: 10.1038/s41598-021-91509-4.
- Pohl R.W. 1965. Dissecting equipment and materials for the study of minute plant structures. Rhodora 67: 95–96.
- Rodríguez-Prieto C. & Hommersand M.H. 2009. Behaviour of the nuclei in pre- and postfertilization stages in Kallymenia (Kallymeniaceae, Rhodophyta). Phycologia 48: 138–155. DOI: 10.2216/08-75.1.
- Rodríguez-Prieto C., DeClerck O., Huisman J.M. & Lin S.M. 2019. Characterisation of Nesoia latifolia (Halymeniaceae, Rhodophyta) from Europe with emphasis on cystocarp development and description of Nesoia mediterranea sp. nov. Phycologia 58: 393–404. DOI: 10.1080/00318884.2019.1591879.
- Ronquist F., Teslenko M., Van Der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A. & Huelsenbeck J.P. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. DOI: 10.1093/sysbio/sys029.
- Saunders, G.W. 2005. Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Philosophical Transactions of the Royal Society B: Biological Sciences 360: 1879–1888. DOI:10.1098/rstb.2005.1719.
- Saunders G.W., Huisman J.M., Vergés A., Kraft G.T. & Le Gall L. 2017. Phylogenetic analyses support recognition of ten new genera, ten new species and 16 new combinations in the family Kallymeniaceae (Gigartinales, Rhodophyta). Cryptogamie, Algologie 38: 79–132. DOI: 10.7872/crya/v38.iss2.2017.79.
- Schneider C.W., Popolizio T.R., Kraft L.G. & Saunders G.W. 2019. New species of Galene and Howella gen. nov. (Halymeniaceae, Rhodophyta) from the mesophotic zone off Bermuda. Phycologia 58: 690–697. DOI: 10.1080/00318884.2019.1661158.
- Selivanova O.N., Zhigadlova G.G. & Saunders G.W. 2020. Commanderella gen. nov. and new insights into foliose Kallymeniaceae (Rhodophyta) from the Russian Pacific coast based on molecular studies. Phycologia 59: 200–207. DOI: 10.1080/00318884.2020.1732150.
- Shepherd B., Phelps T., Pinheiro H.T., Pérez-Matus A. & Rocha L.A. 2018. Plectranthias ahiahiata, a new species of perchlet from a mesophotic ecosystem at Rapa Nui (Easter Island) (Teleostei, Serranidae, Anthiadinae). ZooKeys 762: 105–116. DOI: 10.3897/zookeys.762.24618.
- Sherwood A.R., Lin S.M., Wade R.M., Spalding H.L., Smith C.M. & Kosaki R.K. 2019. Characterization of Martensia (Delesseriaceae; Rhodophyta) from shallow and mesophotic habitats in the Hawaiian Islands: description of four new species. European Journal of Phycology 55: 172–185. DOI: 10.1080/09670262.2019.1668062.
- Sherwood A.R., Paiano M.O., Spalding H.L. & Kosaki R.K. 2020. Biodiversity of Hawaiian Peyssonneliales (Rhodophyta): Sonderophycus copusii sp. nov., a new species from the Northwestern Hawaiian Islands. Algae 35: 145–155. DOI: 10.4490/algae.2020.35.5.20.
- Sherwood A.R., Cabrera F.P., Spalding H.L., Alvarado E.A., Smith C.M., Hauk B.B., Matadobra S., Kosaki R.K. & Paiano M.O. 2021a. Biodiversity of Hawaiian Peyssonneliales (Peyssonneliaceae, Rhodophyta): new species in the genera Incendia and Seiria. Phytotaxa 524: 14–26. DOI: 10.11646/phytotaxa.524.1.2.
- Sherwood A.R., Paiano M.O., Wade R.M., Cabrera F.P., Spalding H.L. & Kosaki R.K. 2021b. Biodiversity of Hawaiian Peyssonneliales (Rhodophyta). 1. Two new species in the genus Ramicrusta from Lehua Island. Pacific Science 75: 185–195. DOI: 10.2984/75.2.2.
- Sherwood A.R., Paiano M.O., Cabrera F.P., Spalding H.L., Hauk B.B. & Kosaki R.K. 2021c. Ethelia hawaiiensis (Etheliaceae, Rhodophyta), a new mesophotic marine alga from Manawai (Pearl and Hermes Atoll), Papahānaumokuākea Marine National Monument, Hawaii. Pacific Science 75: 237–246. DOI: 10.2984/75.2.6.
- Skriptsova A.V. 2021. The systematics and current problems in the taxonomy of algae of the order Gigartinales (Rhodophyta) from the Far Eastern Seas of Russia. Russian Journal of Marine Biology 47: 73–83. DOI: 10.1134/S1063074021020103.
- Spalding H.L., Conklin K.Y., Smith C.M., O’Kelly C.J. & Sherwood A.R. 2016. New Ulvaceae (Ulvophyceae, Chlorophyta) from mesophotic ecosystems across the Hawaiian Archipelago. Journal of Phycology 52: 40–53. DOI: 10.1111/jpy.12375.
- Spalding H.L., Copus J.M., Bowen B.W., Kosaki R.K., Longenecker K., Montgomery A.D., Padilla-Gamiño J.L., Parrish F.A., Roth M.S., Rowley S.J. et al. 2019. The Hawaiian Archipelago. In: Mesophotic coral ecosystems (Ed. by Y. Loya, K. Puglise & T. Bridge), pp 445–464. Springer, New York, New York, USA.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. DOI: 10.1093/bioinformatics/btu033.
- Womersley H.B.S. 1994. The marine benthic flora of southern Australia - part IIIA - Bangiophyceae and Florideophyceae (Acrochaetiales, Nemaliales, Gelidiales, Hildenbrandiales and Gigartinales sensu lato). Australian Biological Resources Study, Canberra, Australia. 508 pp.
- Wynne M.J. 2018. Regarding Kallymenia J.Agardh, 1842, Euhymenia Kützing nom. illeg. 1843, and the proposal of Croisettea gen. nov. (Kallymeniaceae, Rhodophyta). Notulae Algarum 76: 1–4.
- Xuan-Nguyen X.V., Nguyen T.H., Dao V.H. & Liao L. 2019. New record of Grateloupia taiwanensis S.-M. Lin et H.-Y. Liang in Vietnam: evidence of morphological observation and rbcL sequence analysis. Biodiversitas, Journal of Biological Diversity 20: 669–688. DOI: 10.13057/biodiv/d200311.
