ABSTRACT
Diabetes imparts a substantial increased risk for cardiovascular disease-related mortality and morbidity. Because of this, current medical guidelines recommend prophylactic treatment with once-daily, low-dose aspirin (acetylsalicylic acid) for primary and secondary prevention of cardiovascular (CV) events in high-risk patients. However, only modest reductions in CV events and mortality have been observed with once-daily aspirin treatment in patients with diabetes, including patients with a previous CV event, perhaps because of disparity between aspirin pharmacokinetics and diabetes-related platelet abnormalities. Once-daily aspirin irreversibly inactivates platelets for only a short duration (acetylsalicylic acid half-life, approximately 15–20 minutes), after which time newly generated, active platelets enter the circulation and weaken aspirin’s effect. Platelets from patients with diabetes are more reactive and are turned over more rapidly than platelets from normal individuals; the short inhibitory window provided by once-daily aspirin may therefore be insufficient to provide 24-h protection against CV events. Alternative conventional aspirin regimens (e.g. higher daily dose, twice-daily dosing, combination with clopidogrel) and newer formulations (e.g. 24-h, extended-release) have been proposed to overcome the apparent limited efficacy of conventional aspirin in patients with diabetes; however, tolerability concerns and limited clinical efficacy data need to be taken into account when considering the use of such regimens.
Introduction
In the United States, approximately 9% of the population (~29.1 million people) had diabetes in 2012, 21.0 million with diagnosed diabetes and 8.1 million believed to have undiagnosed diabetes.[Citation1,Citation2] There are numerous reasons for concern about these statistics, not the least of which is the increased risk of cardiovascular disease (CVD) and the poorer prognosis after cardiovascular (CV) events in patients with diabetes.[Citation3–Citation5] Indeed, 75–80% of all patients with diabetes will likely die from CVD-related causes.[Citation6]
Current guidelines from the American Diabetes Association (ADA) and collaborative recommendations from the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD) recommend once-daily low-dose (75–162 mg/day) aspirin, otherwise known as acetylsalicylic acid, for primary prevention of CV events in patients with diabetes with a high risk for a CV event and for secondary prevention of CV events.[Citation7,Citation8] However, these guidelines are based on poorly controlled or uncontrolled studies, are extrapolated from data obtained from other patient populations at high risk for coronary heart disease (CHD) [Citation7,Citation9] and are based on the assumption that aspirin administered once daily exerts 24-h CVD protection.
Aspirin reduces the risk of CV events through the irreversible inhibition of cyclooxygenase (COX)-1, a key enzyme involved in vascular clot formation, within circulating platelets [Citation10]; therefore, it has been assumed that with daily dosing, the efficacy of aspirin is maintained over a 24-h period for the duration of a platelet’s lifespan (i.e. ~10 days).[Citation11] However, recent advances in the understanding of the pathophysiological mechanisms of CVD in patients with diabetes challenge this assumption. This narrative review provides an assessment of the efficacy of aspirin for CVD prevention in patients with diabetes, discusses how the mechanism of action of aspirin relates to this risk reduction and addresses ways in which the challenges of aspirin use in patients with diabetes may be overcome.
Methodology
PubMed was searched to identify clinical trials published within the past 10 years that contained ‘diabetes’ as a medical subject heading and met the following search parameters: (‘aspirin’ OR ‘acetylsalicylic acid’) OR (‘clopidogrel’) AND ‘cardiovascular’. Eighty-one English-language articles were retrieved. Review of the reference lists in these publications identified 32 articles for further evaluation. Articles were included if they reported research results or provided other clinically relevant information about the use of aspirin in patients with diabetes.
Aspirin: antiplatelet effects and pharmacokinetics
Platelets play a pivotal physiological role in the clotting process and are also involved in the development of thrombotic and atherogenic complications.[Citation12] In healthy individuals, damage to the vascular endothelium as a result of subendothelial cholesterol accumulation and inflammation allows exposure of circulating platelets to endothelium- and vascular smooth muscle-derived prothrombotic factors (e.g. von Willebrand factor, tissue factor and collagen), which leads to platelet activation.[Citation13–Citation16] Activated platelets release additional prothrombotic mediators, such as adenosine diphosphate (ADP) and thromboxane A2 (TXA2), which activate additional platelets locally and release components necessary for clot formation (e.g. thrombin).[Citation13–Citation15] Activated platelets also produce pro-inflammatory signals that recruit inflammatory cells to the area.[Citation16] These inflammatory cells produce cytokines that stimulate endothelial and smooth muscle cells to produce tissue factor, the initiator of coagulation.[Citation17] Activation of the coagulation cascade results in a fibrin meshwork that (along with cross-linking of platelets, endothelial cells and leukocytes) blocks the vascular interior ().[Citation13–Citation16]
Figure 1. The role of platelets after vascular injury.[Citation13–Citation16] After plaque rupture, TF is released from endothelial cells and platelets are activated by collagen and vWF. TF is converted to thrombin, which activates platelets and facilitates formation of a fibrin meshwork. Activated platelets produce ADP and TXA2, which in turn augment platelet activation and recruit additional platelets to the area. Activated platelets also produce inflammatory signals that recruit leukocytes to the area. The leukocytes amplify platelet activation signals and cross-link to fibrin, facilitating clot formation. ADP: adenosine diphosphate; TF: tissue factor; TXA2: thromboxane A2; vWF: von Willebrand factor.
![Figure 1. The role of platelets after vascular injury.[Citation13–Citation16] After plaque rupture, TF is released from endothelial cells and platelets are activated by collagen and vWF. TF is converted to thrombin, which activates platelets and facilitates formation of a fibrin meshwork. Activated platelets produce ADP and TXA2, which in turn augment platelet activation and recruit additional platelets to the area. Activated platelets also produce inflammatory signals that recruit leukocytes to the area. The leukocytes amplify platelet activation signals and cross-link to fibrin, facilitating clot formation. ADP: adenosine diphosphate; TF: tissue factor; TXA2: thromboxane A2; vWF: von Willebrand factor.](/cms/asset/ec9c22d6-5b0b-4093-b215-bf476a6740e0/ipgm_a_1131106_f0001_b.gif)
Aspirin reduces clot formation by decreasing production of TXA2, a key platelet activator.[Citation10,Citation18–Citation21] Aspirin irreversibly binds to and inhibits the COX enzymes [Citation22] (particularly COX-1), which are responsible for TXA2 synthesis and production of prostaglandins, which aid in vascular hemostasis, protection of the gut and regulation of renal blood flow ().[Citation10] Low doses of aspirin exert their antiplatelet effects presystemically [Citation20] by inhibiting COX-1 as platelets travel through the portal circulation,[Citation23] potentially sparing systemic prostaglandins.[Citation21] In contrast, the higher aspirin doses required for antiinflammatory and hyperalgesia purposes may also inhibit COX-2, potentially disrupting systemic physiologic processes and increasing the risk of mucosal damage and bleeding events (as indicated in Sostres et al. [Citation24]). Because of its original design as an antipyretic and pain reliever, aspirin – both immediate-release and enteric-coated formulations – has a rapid onset of effect but a brief therapeutic window.[Citation11,Citation19,Citation20] These conventional aspirin formulations are rapidly absorbed by the stomach and/or the upper small intestine and provide peak plasma aspirin concentrations within 40 min (immediate release) to 5 h (enteric coated) after administration [Citation25–Citation27]; however, the half-life of aspirin (acetylsalicylic acid) is only approximately 15–20 min,[Citation28] which limits the window of time during which it inhibits platelet COX-1. Consistent with this pharmacokinetic profile and aspirin’s irreversible inhibition of COX-1, aspirin’s antiplatelet effect (e.g. inhibition of TXA2 production) after a single dose in healthy individuals is rapid (because of its quick absorption) and temporarily sustained (because of its irreversible COX-1 inhibition) despite the quickly decreasing plasma aspirin concentrations ().[Citation19,Citation20] However, the antiplatelet effects [i.e. inhibition of TXA2 production () and of arachidonic acid–induced platelet aggregation] in healthy individuals begin to recover within 24 h,[Citation29,Citation30] as aspirin is metabolized to inactive antiplatelet substances and newly generated platelets with uninhibited COX enzymes enter the circulation.[Citation14,Citation30] This reduction in antiplatelet effects likely occurs even more quickly in patients with rapid platelet turnover, such as patients with diabetes.[Citation31]
Figure 2. Mechanism of action of aspirin. Aspirin inhibits production of TXA2 by preventing the conversion of arachidonic acid to PGH2 by COX-1 and COX-2. COX, cyclooxygenase; PG, prostaglandin; TXA2, thromboxane A2.
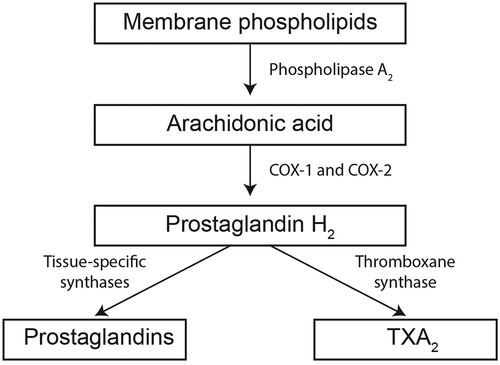
Figure 3. Antiplatelet effect of IR aspirin in healthy individuals.[Citation20,Citation29] Healthy male volunteers (n = 10) received a single dose of IR aspirin 20 mg orally (A). Vertical line represents time of aspirin administration. Serum TXB2 concentration (a surrogate marker of TXA2 production) in healthy volunteers (n = 50) who received non-enteric-coated aspirin 75 mg for 7 days (B). Serum TXB2 levels tended to increase 24 h after aspirin dosing, although this difference did not reach statistical significance. ASA: aspirin; IR: immediate release; TXA2: thromboxane A2; TXB2: thromboxane B2.
![Figure 3. Antiplatelet effect of IR aspirin in healthy individuals.[Citation20,Citation29] Healthy male volunteers (n = 10) received a single dose of IR aspirin 20 mg orally (A). Vertical line represents time of aspirin administration. Serum TXB2 concentration (a surrogate marker of TXA2 production) in healthy volunteers (n = 50) who received non-enteric-coated aspirin 75 mg for 7 days (B). Serum TXB2 levels tended to increase 24 h after aspirin dosing, although this difference did not reach statistical significance. ASA: aspirin; IR: immediate release; TXA2: thromboxane A2; TXB2: thromboxane B2.](/cms/asset/b3b941ab-eccb-4959-8239-73b732b9c550/ipgm_a_1131106_f0003_b.gif)
Efficacy of aspirin in patients with diabetes
Multiple studies have shown a reduction in CV events with aspirin in patients with CHD,[Citation32,Citation33] and because other conditions have similar pathophysiologic mechanisms (e.g. augmented platelet reactivity), it has been assumed that aspirin would provide cardioprotection in other patient populations at increased risk of CVD (e.g. patients with diabetes).[Citation9] However, although diabetes is often believed to confer an increase in the risk of a CV event equivalent to the increase observed in patients with a history of CVD, diabetes likely confers only an intermediate increase in the risk of a CV event.[Citation34,Citation35] Thus, the absolute benefits of aspirin therapy may not be as substantial for patients with diabetes as they are for patients with CHD.[Citation33] Indeed, direct evidence of aspirin’s efficacy and safety in randomized primary CVD prevention trials has been inconclusive for patients with diabetes, regardless of the patients’ baseline characteristics.[Citation36–Citation38] In patients with diabetes without known CHD, gender,[Citation36,Citation38] hypertensive status,[Citation36] lipid status,[Citation36] smoking status,[Citation36] and ankle brachial pressure index [Citation38] did not significantly affect response to once-daily aspirin (81–100 mg). Patients with diabetes without known CVD who are at higher risk of CV events (i.e. who have renal dysfunction,[Citation39] receive insulin therapy,[Citation37] display increased inflammation at baseline,[Citation40] or have elevated blood pressure [Citation41]) have also shown no clear benefit with once-daily aspirin therapy. Furthermore, aspirin (81–100 mg) provided no significant benefit in reducing the incidence of CV events in patients with diabetes and unknown CVD categorized as ‘high risk’ according to the ADA definition of high risk (i.e. men >50 years of age and women >60 years who had ≥1 additional CV risk factor).[Citation42]
Several meta-analyses of aspirin data in patients with diabetes without known CVD and patients with diabetes and CHD have consistently shown only modest reductions in CV-related (1–9%),[Citation34,Citation43–Citation47] all-cause mortality (1–7%),[Citation34,Citation43–Citation49] stroke (2–30%),[Citation33,Citation34,Citation43–Citation50] myocardial infarction (MI) (5–10%),[Citation33,Citation34,Citation43–Citation50] and major CV events (8–11%) [Citation34,Citation43–Citation45,Citation47,Citation48] with aspirin (). In a single meta-analysis of seven randomized trials, the number needed to treat for primary prevention of a major adverse CV event with aspirin in patients with diabetes was 92, with an estimated likelihood of being helped versus harmed of 6 [Citation34]; similar information for patients with diabetes and CVD are not currently available. In general, with the possible exception of MI, relative risk (RR) reductions in patients with diabetes without known CHD and patients with diabetes and CHD are not substantially different.[Citation46] In a meta-analysis of randomized clinical trials in patients with diabetes with CHD (13 trials) or without known CHD (10 trials), the RR of CV-related mortality, MI and stroke ranged from 0.98 to 1.06 in patients with diabetes and no known CHD and from 0.68 to 0.96 in patients with CHD ().[Citation46] However, there was a significant reduction in all-cause mortality with aspirin in patients with a history of CHD (RR, 0.82; 95% CI, 0.69–0.98) that was not observed in patients with diabetes without known CHD (RR, 1.01; 95% CI, 0.85–1.19); this difference appeared to be associated with daily aspirin doses ≤325 mg, although this could not be firmly established because of substantial heterogeneity among the studies.[Citation46]
Figure 4. Risk of adverse cardiovascular outcomes in patients with diabetes who were receiving low-dose aspirin.[Citation34,Citation43–Citation50] Relative risk and 95% confidence interval with aspirin versus controls is provided for all studies except Stavrakis et al. [Citation45], for which the odds ratio versus controls is presented. CV: cardiovascular; MI: myocardial infarction.
![Figure 4. Risk of adverse cardiovascular outcomes in patients with diabetes who were receiving low-dose aspirin.[Citation34,Citation43–Citation50] Relative risk and 95% confidence interval with aspirin versus controls is provided for all studies except Stavrakis et al. [Citation45], for which the odds ratio versus controls is presented. CV: cardiovascular; MI: myocardial infarction.](/cms/asset/a5079d5c-be01-4382-a011-0b7888034608/ipgm_a_1131106_f0004_b.gif)
Figure 5. Relative risk of CV endpoints in patients with diabetes with and without comorbid CHD who received preventative aspirin therapy.[Citation46] Data from a meta-analysis of randomized controlled trials (n = 1 to 13 trials) that enrolled patients with diabetes alone or with diabetes and CHD who received aspirin (50–1300 mg/day) for prevention of CV events. CHD: coronary heart disease; CV: cardiovascular; MI: myocardial infarction.
![Figure 5. Relative risk of CV endpoints in patients with diabetes with and without comorbid CHD who received preventative aspirin therapy.[Citation46] Data from a meta-analysis of randomized controlled trials (n = 1 to 13 trials) that enrolled patients with diabetes alone or with diabetes and CHD who received aspirin (50–1300 mg/day) for prevention of CV events. CHD: coronary heart disease; CV: cardiovascular; MI: myocardial infarction.](/cms/asset/ce7c8ea7-117a-4b59-a60e-da9e7d0e7108/ipgm_a_1131106_f0005_b.gif)
The daily dose of aspirin (categorized as ≤100, 101–325 and >325 mg or ≥100 and <100 mg) does not appear to effect RR reductions in patients with diabetes,[Citation46,Citation51] although lower doses (<100 mg/day) were shown to reduce the risk of stroke compared with higher doses (>100 mg/day) in one meta-analysis of patients with diabetes and no known CVD.[Citation44] The ADA recommends aspirin doses of 75–162 mg/day [Citation7] because higher doses (162.5–325 mg/day) of aspirin have been shown to increase the risk of hemorrhagic events, such as intracranial or gastrointestinal bleeding, in patients receiving aspirin for primary or secondary prevention of CV events.[Citation52] However, meta-analyses of trials conducted specifically in patients with diabetes have demonstrated no significant effect of aspirin versus placebo on risk of bleeding, although the authors urge caution in interpreting these results because of statistical inadequacies.[Citation43–Citation45,Citation48]
Because of the lack of substantial evidence to the contrary, it has been suggested that aspirin does not provide any additional benefit compared with other therapies targeted at lowering CV risk (e.g. statins).[Citation33,Citation51,Citation53] Indeed, 21–44% of patients [Citation54–Citation58] with diabetes display reduced laboratory responses (e.g. reduced TXA2 production and platelet sensitivity) to antiplatelet therapies.[Citation31,Citation59–Citation66] The inability to substantially reduce the risk of CV events and the lack of laboratory responsiveness to aspirin in patients with diabetes may be related to a mismatch between the mechanism of action and pharmacology of aspirin and the platelet abnormalities observed in patients with diabetes.
Platelet abnormalities and high on-treatment platelet reactivity with aspirin in patients with diabetes
As mentioned previously, patients with diabetes have an approximately two- to fourfold increased risk of CHD (coronary death, non-fatal MI) and stroke (ischemic, hemorrhagic and unclassified) compared with individuals without diabetes.[Citation3,Citation67] The reasons for this increased risk are diverse and include genetic and environmental factors,[Citation68] metabolic abnormalities (e.g. hyperglycemia, insulin resistance),[Citation68] an overall pro-inflammatory state,[Citation17] comorbidity with other conditions that influence hemostatic clotting mechanisms (e.g. obesity),[Citation68] and high on-treatment platelet reactivity (HTPR) with aspirin, the traditional CVD preventive therapy, because of platelet abnormalities.
Multiple mechanisms facilitate a hyper-reactive, ‘angry’ platelet phenotype in patients with diabetes (e.g. persistent hyperglycemia, hyperlipidemia, insulin resistance and oxidative stress; ).[Citation17,Citation69,Citation70] This ‘angry’ phenotype encompasses a variety of prothrombotic characteristics. The thromboxane pathway is activated by high exogenous and/or endogenous insulin levels in patients with diabetes.[Citation71–Citation73] Platelets from patients with diabetes have fewer insulin receptors and the affinity of these receptors to insulin is reduced compared with normal individuals.[Citation74] This reduced insulin sensitivity increases platelet responsiveness to platelet activators, such as thrombin.[Citation74] Platelets from patients with diabetes tend to have a larger mean platelet volume,[Citation75–Citation77] which allows the platelets to be more metabolically and enzymatically active [Citation74]; thus, metabolism of arachidonic acid and subsequent production of TXA2 are increased in platelets from patients with diabetes.[Citation72–Citation74,Citation78] Larger platelets also contain more prothrombotic mediators and have a greater surface area over which to display adhesion molecules.[Citation74] Membrane fluidity, which may disrupt intracellular signaling and increase platelet adhesion, is decreased in platelets from patients with diabetes.[Citation74,Citation79,Citation80] Expression of adhesion molecules (e.g. CD32) and platelet surface receptors [e.g. glycoprotein (GP) IIb/IIIa [Citation81]] that facilitate endothelial–platelet; platelet–platelet; and platelet–leukocyte binding, cross-talk and clot formation (e.g. fibrinogen binding) is augmented.[Citation74,Citation78,Citation82–Citation84] Production of inflammatory cytokines, mitogenic mediators, and reactive oxygen species is also increased and may disrupt intracellular communication and cross-communication with other cell types (e.g. endothelial cells).[Citation74] In addition to the increase in platelet reactivity, patients with diabetes exhibit increased platelet turnover,[Citation85] which increases the proportion of new platelets with uninhibited COX-1 that enter the circulation. Newly formed, immature platelets likely contribute to the overall ‘angry’ platelet phenotype associated with diabetes because they have a greater mean volume and are more reactive than mature platelets.[Citation11,Citation15,Citation86,Citation87] They also contain denser granules, possess mRNA for de novo enzyme synthesis, secrete a higher concentration of prothrombotic factors (e.g. TXA2) and have increased expression of adhesion receptors (e.g. GPIb and GPIIb/IIIa).[Citation14,Citation15]
Figure 6. Mechanisms underlying increased clot formation in patients with diabetes.[Citation17] Altered glucose metabolism and insulin signaling increase platelet activation, inflammation and vasoconstriction, all of which increase clot formation. PG: prostaglandin; VSMC: vascular smooth muscle cell.
![Figure 6. Mechanisms underlying increased clot formation in patients with diabetes.[Citation17] Altered glucose metabolism and insulin signaling increase platelet activation, inflammation and vasoconstriction, all of which increase clot formation. PG: prostaglandin; VSMC: vascular smooth muscle cell.](/cms/asset/d37bcebc-4d76-4ee0-865f-ba02d83d7f85/ipgm_a_1131106_f0006_b.gif)
Because of these platelet abnormalities and resulting HTPR, once-daily low-dose conventional aspirin may not reduce the reactivity of the ‘angry’ diabetic platelet to the level required to observe clinical efficacy (i.e. reduced production of TXA2 ≥ 95%).[Citation10,Citation88] For example, the increased platelet turnover in patients with diabetes [Citation75,Citation76,Citation85] combined with the short half-life of aspirin means that newly synthesized, hyper-reactive immature platelets remain uninhibited during a considerable portion of the 24-h window of time between once-daily aspirin doses.[Citation11] Indeed, markers of immature platelets (i.e. mean platelet volume and thiazole-orange-positive platelets) independently predicted recovery of thromboxane B2 (TXB2) production during a 24-h period in patients with diabetes (with or without known CVD) who had received 7 days of enteric-coated aspirin 100 mg/day.[Citation31] A separate study also reported a positive, but non-significant, correlation between immature platelet count and increased platelet aggregation 24 h after immediate-release aspirin 75 mg administration in patients with diabetes and coronary artery disease (CAD).[Citation66]
Overcoming HTPR with aspirin in patients with diabetes
Practical approaches to surmounting HTPR with aspirin (e.g. increasing aspirin dose, altering dosing frequency and combination with other antiplatelet therapies) have been somewhat successful at overcoming laboratory-defined HTPR (i.e. aspirin resistance), but data are somewhat sparse and at times contradictory. For example, increasing the aspirin dose (from 30 mg/day to up to 320 mg/day) significantly reduced platelet aggregation in patients with diabetes and CAD in most,[Citation89–Citation91] but not all,[Citation61,Citation92] studies and production of TXB2 or 2,3-dinor-TXB2 (markers of actual COX inhibition) has been reported to decrease [Citation61,Citation91,Citation92] or remain unaltered.[Citation90] Although increasing the daily dose of aspirin may augment the antiplatelet effect of aspirin,[Citation18,Citation21] it also decreases prostaglandin production [Citation18] and increases the potential risk for adverse effects (e.g. gastrointestinal and intracranial bleeding [Citation52,Citation93]); thus, the risk-to-benefit ratio for aspirin needs to be taken into account when considering higher-dose regimens. However, as mentioned above, there is little evidence that increased daily aspirin doses provide any additional clinical benefit (i.e. reduced RR of CV events).[Citation32,Citation46,Citation51]
Twice-daily administration of low aspirin doses (which provides an additional window of time for COX inhibition during a 24-h period) is another possible therapeutic option and has provided more consistent results. In patients with diabetes and CAD, twice-daily dosing of aspirin 100 mg (total dose, 200 mg/day) or 75 mg (total dose, 150 mg/day) improved platelet aggregation compared with once-daily administration of aspirin 100 or 75 mg/day, respectively.[Citation89,Citation94] Twice-daily administration of aspirin 100 mg (total dose, 200 mg/day) also slowed recovery of TXB2 concentrations during a 12- to 24-h period compared with once-daily aspirin 100 mg in patients with diabetes and prior vascular disease. However, improved aspirin responsiveness has not been observed with twice-daily dosing of higher doses [i.e. 162 mg once daily vs. 162 mg twice daily (324 mg/day total dose) or 200 mg once daily vs. 200 mg twice daily (400 mg/day total dose)],[Citation92,Citation95] suggesting that this effect may plateau at certain daily dosages.[Citation92,Citation95] This plateau effect, combined with the possibility of dose-related aspirin adverse events, suggests that twice-daily dosing that augments the overall total dose of aspirin administered may not be ideal. However, studies in patients with diabetes and CVD who were on twice-daily aspirin dosing regimens without an increase in total daily dose (e.g. 75 mg twice daily vs. 150 mg once daily) also demonstrated reductions in platelet aggregation with twice-daily versus once-daily dosing.[Citation91,Citation94] Unfortunately, twice-daily aspirin may present an adherence issue for some patients. Patient non-adherence to CHD preventive regimens (e.g. angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, statins and thiazide diuretics) tends to be high (~43% of patients).[Citation96] In addition, approximately 27–33% of patients with diabetes are non-adherent with their diabetes medication regimens, especially when they are on oral regimens requiring multiple doses per day.[Citation97–Citation99]
Another potential option for reducing CV events in patients with diabetes is the P2Y12 inhibitor clopidogrel (Plavix; Sanofi-Aventis, Bridgewater, NJ, USA). However, a lack of antiplatelet effects similar to that observed with aspirin has been observed in patients with diabetes [Citation100] and, in patients at high risk of a CV event (i.e. evidence of a previous occlusive event or other predisposing condition), clopidogrel alone provided no additional CV protection compared with aspirin.[Citation32] Because clopidogrel and aspirin exert their antiplatelet effects through two separate pathways (i.e. reduced TXA2 production via COX inhibition with aspirin [Citation10] and ADP-induced activation of GPIIb-GPIIIa with clopidogrel [Citation100–Citation103]), it was hypothesized that combination therapy with both of these antiplatelet agents might provide additional protection against CVD. In patients with type 2 diabetes, combination therapy with aspirin 81 mg/day and clopidogrel 75 mg/day for 1 month significantly reduced ADP-, epinephrine- and collagen-stimulated platelet aggregation compared with aspirin 81 mg/day alone; however, no significant difference in arachidonic acid-induced aggregation was observed between treatment groups.[Citation104]
In patients with diabetes and CAD who displayed HTPR with aspirin, treatment with combination aspirin 100 mg/day plus clopidogrel 75 mg/day significantly reduced the percentage of patients who exhibited HTPR with combination antiplatelet therapy compared with treatment with aspirin 300 mg/day. However, this effect was driven primarily by a reduction in ADP-induced platelet aggregation; other aggregators (e.g. collagen) did not have significantly altered platelet aggregation compared with aspirin 300 mg/day.[Citation105] Furthermore, subanalyses of patients with diabetes from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial did not demonstrate a significant reduction in a composite endpoint of MI, stroke and CV-related mortality or in overall and CV-related mortality with aspirin (75–162 mg/day) plus clopidogrel (75 mg/day) over aspirin (75–162 mg/day) alone [Citation106,Citation107]; a significant increase in the risk of moderate bleeding events was observed with combination therapy.[Citation107] On the basis of these data, it is unclear whether the combination of aspirin and clopidogrel is safe and provides consistent benefit above the benefit observed with aspirin alone in patients with diabetes.
In addition, as with twice-daily aspirin dosing, patient adherence may be a concern with the two-drug combination of aspirin and clopidogrel. Dosing complexity, including the daily number of pills and increased dosing frequency, has been shown to reduce adherence in patients with diabetes.[Citation108,Citation109] In a systematic review of loose-pill combination therapies for the treatment of type 2 diabetes, switching from monotherapy to a loose-pill combination of multiple medications reduced adherence by up to 12%.[Citation110]
A final option for overcoming HTPR with aspirin may be the availability of a prescription, extended-release formulation of aspirin 162.5 mg/day (Durlaza®, New Haven Pharmaceuticals, Inc., North Haven CT). This unique aspirin formulation provided constant and prolonged (24-h) release of aspirin following a single dose in healthy volunteers compared with a conventional, immediate-release aspirin formulation.[Citation111] The extended-release formulation provides a protracted period during which aspirin may inactivate platelets. The prolonged inhibitory period may be especially beneficial for patients with diabetes or other conditions associated with high platelet turnover because it would allow for inactivation of new, immature platelets without the need for twice-daily dosing or polypharmacy. The prescription, extended-release, low-dose aspirin was approved as secondary prevention for CVD in September 2015; however, the impact of this new therapy on CVD prophylaxis in patients with diabetes remains to be determined.
Conclusions
Despite advances in treatment and preventive interventions, diabetes continues to confer an additional risk of CV events.[Citation3] The ADA and ESC/EASD guidelines recommend once-daily, low-dose aspirin for the primary and secondary prevention of CVD in patients with diabetes at high risk of CV events,[Citation7,Citation8] and if this guidance were followed, most patients with diabetes would be receiving a daily aspirin regimen. However, less than half of physicians (42–44%) report prescribing an antiplatelet therapy to patients with diabetes and vascular disease and only 20% recommend treatment to patients with diabetes without known vascular disease.[Citation112] The reasons for physician non-adherence to preventive aspirin guidelines likely involve multiple factors, such as insufficient knowledge of current guidelines, anticipation of patient non-adherence,[Citation112] and experience with HTPR with aspirin in their patients. Unfortunately, adherence and HTPR are both difficult to monitor in the clinical setting, which prevents optimization of aspirin regimens. Given the new insights into the pathophysiology underlying HTPR with aspirin in patients with diabetes, it is recommended that physicians consider alternative aspirin dosing regimens for appropriate patients with diabetes. Given the lack of outcomes data, it is suggested that the dose and frequency of aspirin administration should be made empirically, on the basis of the laboratory results of prospective, randomized trials [Citation91,Citation94,Citation95,Citation104,Citation105] and patient potential for adherence and potential susceptibility to gastrointestinal upset. Unfortunately, outcomes, adherence and safety data from large, prospective studies of alternative aspirin dosing regimens are not currently available.
Financial & competing interests disclosure
This paper has been supported by New Haven Pharmaceuticals. Technical editorial and medical writing support, under direction of the author, was provided by Mary Beth Moncrief, PhD, and Jillian Gee, PhD, Synchrony Medical Communications, LLC, West Chester, PA, with support from New Haven Pharmaceuticals. DSH Bell has been a consultant and been on the Speakers’ Bureau for Novo Nordisk, Janssen, AstraZeneca, and Takeda. He has also been a consultant for New Haven Pharmaceuticals. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- American Diabetes Association. Statistics About Diabetes Data from the National Diabetes Statistics Report, 2014 (released June 10, 2014) Overall Numbers, Diabetes and Prediabetes. 2014. [ cited 2014 Nov 26] Available from: http://www.diabetes.org/diabetes-basics/statistics/.
- Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312:1218–1226.
- Sarwar N, Gao P, Seshasai SR, et al The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222.
- Cubbon RM, Abbas A, Wheatcroft SB, et al. Diabetes mellitus and mortality after acute coronary syndrome as a first or recurrent cardiovascular event. PLoS One. 2008;3:e3483.
- Leander K, Wiman B, Hallqvist J, et al. Primary risk factors influence risk of recurrent myocardial infarction/death from coronary heart disease: results from the Stockholm Heart Epidemiology Program (SHEEP). Eur J Cardiovasc Prev Rehabil. 2007;14:532–537.
- Bonow RO, Bohannon N, Hazzard W. Risk stratification in coronary artery disease and special populations. Am J Med. 1996;101:4A17S–22S.
- American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37:S14–S80.
- Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035–3087.
- Nicolucci A, De BG, Sacco M, et al. AHA/ADA vs. ESC/EASD recommendations on aspirin as a primary prevention strategy in people with diabetes: how the same data generate divergent conclusions. Eur Heart J. 2007;28:1925–1927.
- Tantry US, Mahla E, Gurbel PA. Aspirin resistance. Prog Cardiovasc Dis. 2009;52:141–152.
- Grove EL. Antiplatelet effect of aspirin in patients with coronary artery disease. Dan Med J. 2012;59:B4506.
- Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494.
- Di Minno MN, Guida A, Camera M, et al. Overcoming limitations of current antiplatelet drugs: a concerted effort for more profitable strategies of intervention. Ann Med. 2011;43:531–544.
- Freynhofer MK, Bruno V, Wojta J, et al. The role of platelets in athero-thrombotic events. Curr Pharm Des. 2012;18:5197–5214.
- Vazzana N, Ranalli P, Cuccurullo C, et al. Diabetes mellitus and thrombosis. Thromb Res. 2012;129:371–377.
- Wallace EL, Smyth SS. Targeting platelet thrombin receptor signaling to prevent thrombosis. Pharmaceuticals (Basel). 2013;6:915–928.
- Hess K. The vulnerable blood. Coagulation and clot structure in diabetes mellitus. Hamostaseologie. 2015;35:25–33.
- FitzGerald GA, Oates JA, Hawiger J, et al. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest. 1983;71:676–688.
- Patrono C, Ciabattoni G, Pinca E, et al. Low dose aspirin and inhibition of thromboxane B2 production in healthy subjects. Thromb Res. 1980;17:317–327.
- Pedersen AK, FitzGerald GA. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. N Engl J Med. 1984;311:1206–1211.
- Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest. 1982;69:1366–1372.
- Roth GJ, Stanford N, Majerus PW. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci U S A. 1975;72:3073–3076.
- Patrono C. Aspirin as an antiplatelet drug. N Engl J Med. 1994;330:1287–1294.
- Sostres C, Lanas A. Gastrointestinal effects of aspirin. Nat Rev Gastroenterol Hepatol. 2011;8:385–394.
- Rainsford KD. Aspirin and related drugs. New York, NY: Taylor & Francis; 2004.
- Tantry US, Bliden KP, Gurbel PA. Overestimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulation. J Am Coll Cardiol. 2005;46:1705–1709.
- Patrignani P, Tacconelli S, Piazuelo E, et al. Reappraisal of the clinical pharmacology of low-dose aspirin by comparing novel direct and traditional indirect biomarkers of drug action. J Thromb Haemost. 2014;12:1320–1330.
- Patrono C, García Rodríguez LA, Landolfi R, et al. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353:2373–2383.
- Würtz M, Hvas AM, Jensen LO, et al. 24-hour antiplatelet effect of aspirin in patients with previous definite stent thrombosis. Int J Cardiol. 2014;175:274–279.
- Perneby C, Wallen NH, Rooney C, et al. Dose- and time-dependent antiplatelet effects of aspirin. Thromb Haemost. 2006;95:652–658.
- Rocca B, Santilli F, Pitocco D, et al. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low-dose aspirin in patients with and without diabetes. J Thromb Haemost. 2012;10:1220–1230.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86.
- Baigent C, Blackwell L, Collins R, et al; Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860.
- Butalia S, Leung AA, Ghali WA, et al. Aspirin effect on the incidence of major adverse cardiovascular events in patients with diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol. 2011;10:25.
- Bulugahapitiya U, Siyambalapitiya S, Sithole J, et al. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis. Diabet Med. 2009;26:142–148.
- Ogawa H, Nakayama M, Morimoto T, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134–2141.
- Okada S, Morimoto T, Ogawa H, et al. Differential effect of low-dose aspirin for primary prevention of atherosclerotic events in diabetes management: a subanalysis of the JPAD trial. Diabetes Care. 2011;34:1277–1283.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840.
- Saito Y, Morimoto T, Ogawa H, et al. Low-dose aspirin therapy in patients with type 2 diabetes and reduced glomerular filtration rate: subanalysis from the JPAD trial. Diabetes Care. 2011;34:280–285.
- Soejima H, Ogawa H, Morimoto T, et al. Aspirin possibly reduces cerebrovascular events in type 2 diabetic patients with higher C-reactive protein level: subanalysis from the JPAD trial. J Cardiol. 2013;62:165–170.
- Soejima H, Ogawa H, Morimoto T, et al. Aspirin reduces cerebrovascular events in type 2 diabetic patients with poorly controlled blood pressure. Subanalysis from the JPAD trial. Circ J. 2012;76:1526–1532.
- Okada S, Morimoto T, Ogawa H, et al. Effect of low-dose aspirin on primary prevention of cardiovascular events in Japanese diabetic patients at high risk. Circ J. 2013;77:3023–3028.
- Zhang C, Sun A, Zhang P, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: A meta-analysis. Diabetes Res Clin Pract. 2010;87:211–218.
- De Berardis G, Sacco M, Strippoli GF, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ. 2009;339:b4531.
- Stavrakis S, Stoner JA, Azar M, et al. Low-dose aspirin for primary prevention of cardiovascular events in patients with diabetes: a meta-analysis. Am J Med Sci. 2011;341:1–9.
- Simpson SH, Gamble JM, Mereu L, et al. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med. 2011;26:1336–1344.
- Xie M, Shan Z, Zhang Y, et al. Aspirin for primary prevention of cardiovascular events: meta-analysis of randomized controlled trials and subgroup analysis by sex and diabetes status. PLoS One. 2014;9:e90286.
- Younis N, Williams S, Ammori B, et al. Role of aspirin in the primary prevention of cardiovascular disease in diabetes mellitus: a meta-analysis. Expert Opin Pharmacother. 2010;11:1459–1466.
- Calvin AD, Aggarwal NR, Murad MH, et al. Aspirin for the primary prevention of cardiovascular events: a systematic review and meta-analysis comparing patients with and without diabetes. Diabetes Care. 2009;32:2300–2306.
- Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395–1402.
- Seshasai SR, Wijesuriya S, Sivakumaran R, et al. Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:209–216.
- McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med. 2006;119:624–638.
- Machlus KR, Italiano JE Jr. The incredible journey: from megakaryocyte development to platelet formation. J Cell Biol. 2013;201:785–796.
- Tasdemir E, Toptas T, Demir C, et al. Aspirin resistance in patients with type II diabetes mellitus. Ups J Med Sci. 2014;119:25–31.
- Fateh-Moghadam S, Plockinger U, Cabeza N, et al. Prevalence of aspirin resistance in patients with type 2 diabetes. Acta Diabetol. 2005;42:99–103.
- Simpson SH, Abdelmoneim AS, Omran D, et al. Prevalence of high on-treatment platelet reactivity in diabetic patients treated with aspirin. Am J Med. 2014;127:95, e1–95, e9.
- Cohen HW, Crandall JP, Hailpern SM, et al. Aspirin resistance associated with HbA1c and obesity in diabetic patients. J Diabetes Complications. 2008;22:224–228.
- Ertugrul DT, Tutal E, Yildiz M, et al. Aspirin resistance is associated with glycemic control, the dose of aspirin, and obesity in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95:2897–2901.
- Watala C, Boncler M, Gresner P. Blood platelet abnormalities and pharmacological modulation of platelet reactivity in patients with diabetes mellitus. Pharmacol Rep. 2005;57(Suppl):42–58.
- Salama MM, Morad AR, Saleh MA, et al. Resistance to low-dose aspirin therapy among patients with acute coronary syndrome in relation to associated risk factors. J Clin Pharm Ther. 2012;37:630–636.
- DiChiara J, Bliden KP, Tantry US, et al. The effect of aspirin dosing on platelet function in diabetic and nondiabetic patients: an analysis from the aspirin-induced platelet effect (ASPECT) study. Diabetes. 2007;56:3014–3019.
- Watala C, Golanski J, Pluta J, et al. Reduced sensitivity of platelets from type 2 diabetic patients to acetylsalicylic acid (aspirin)-its relation to metabolic control. Thromb Res. 2004;113:101–113.
- Watala C, Pluta J, Golanski J, et al. Increased protein glycation in diabetes mellitus is associated with decreased aspirin-mediated protein acetylation and reduced sensitivity of blood platelets to aspirin. J Mol Med (Berl). 2005;83:148–158.
- Singla A, Antonino MJ, Bliden KP, et al. The relation between platelet reactivity and glycemic control in diabetic patients with cardiovascular disease on maintenance aspirin and clopidogrel therapy. Am Heart J. 2009;158:784–786.
- Mylotte D, Kavanagh GF, Peace AJ, et al. Platelet reactivity in type 2 diabetes mellitus: a comparative analysis with survivors of myocardial infarction and the role of glycaemic control. Platelets. 2012;23:439–446.
- Christensen KH, Grove EL, Wurtz M, et al. Reduced antiplatelet effect of aspirin during 24 hours in patients with coronary artery disease and type 2 diabetes. Platelets. 2015;26:230–235.
- Luscher TF, Creager MA, Beckman JA, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part II. Circulation. 2003;108:1655–1661.
- Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, et al. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5:444–470.
- Vaidyula VR, Boden G, Rao AK. Platelet and monocyte activation by hyperglycemia and hyperinsulinemia in healthy subjects. Platelets. 2006;17:577–585.
- Keating FK, Sobel BE, Schneider DJ. Effects of increased concentrations of glucose on platelet reactivity in healthy subjects and in patients with and without diabetes mellitus. Am J Cardiol. 2003;92:1362–1365.
- Lubitz SA, Benjamin EJ, Ellinor PT. Atrial fibrillation in congestive heart failure. Heart Fail Clin. 2010;6:187–200.
- Davì G, Catalano I, Averna M, et al. Thromboxane biosynthesis and platelet function in type II diabetes mellitus. N Engl J Med. 1990;322:1769–1774.
- Davì G, Rini GB, Averna M, et al. Thromboxane B2 formation and platelet sensitivity to prostacyclin in insulin-dependent and insulin-independent diabetics. Thromb Res. 1982;26:359–370.
- Kim JH, Bae HY, Kim SY. Clinical marker of platelet hyperreactivity in diabetes mellitus. Diabetes Metab J. 2013;37:423–428.
- Zaccardi F, Rocca B, Pitocco D, et al. Platelet mean volume, distribution width, and count in type 2 diabetes, impaired fasting glucose, and metabolic syndrome: a meta-analysis. Diabetes Metab Res Rev. 2015;31:402–410.
- Lee EY, Kim SJ, Song YJ, et al. Immature platelet fraction in diabetes mellitus and metabolic syndrome. Thromb Res. 2013;132:692–695.
- Shah B, Sha D, Xie D, et al. The relationship between diabetes, metabolic syndrome, and platelet activity as measured by mean platelet volume: the National Health And Nutrition Examination Survey, 1999–2004. Diabetes Care. 2012;35:1074–1078.
- DiMinno G, Silver MJ, Cerbone AM, et al. Increased binding of fibrinogen to platelets in diabetes: the role of prostaglandins and thromboxane. Blood. 1985;65:156–162.
- Winocour PD, Bryszewska M, Watala C, et al. Reduced membrane fluidity in platelets from diabetic patients. Diabetes. 1990;39:241–244.
- Watala C, Boncer M, Golanski J, et al. Platelet membrane lipid fluidity and intraplatelet calcium mobilization in type 2 diabetes mellitus. Eur J Haematol. 1998;61:319–326.
- Tschoepe D, Roesen P, Kaufmann L, et al. Evidence for abnormal platelet glycoprotein expression in diabetes mellitus. Eur J Clin Invest. 1990;20:166–170.
- Cabeza N, Li Z, Schulz C, et al. Surface expression of collagen receptor Fc receptor-gamma/glycoprotein VI is enhanced on platelets in type 2 diabetes and mediates release of CD40 ligand and activation of endothelial cells. Diabetes. 2004;53:2117–2121.
- Hu H, Li N, Yngen M, et al. Enhanced leukocyte-platelet cross-talk in Type 1 diabetes mellitus: relationship to microangiopathy. J Thromb Haemost. 2004;2:58–64.
- Razmara M, Hjemdahl P, Ostenson CG, et al. Platelet hyperprocoagulant activity in Type 2 diabetes mellitus: attenuation by glycoprotein IIb/IIIa inhibition. J Thromb Haemost. 2008;6:2186–2192.
- Mijovic R, Kovacevic N, Zarkov M, et al. Reticulated platelets and antiplatelet therapy response in diabetic patients. J Thromb Thrombolysis. 2015;40:203–210.
- Ingram M, Coopersmith A. Reticulated platelets following acute blood loss. Br J Haematol. 1969;17:225–229.
- Karpatkin S. Heterogeneity of human platelets. II. Functional evidence suggestive of young and old platelets. J Clin Invest. 1969;48:1083–1087.
- DiMinno G, Silver MJ, Cerbone AM, et al. Trial of repeated low-dose aspirin in diabetic angiopathy. Blood. 1986;68:886–891.
- Addad F, Chakroun T, Elalamy I, et al. Antiplatelet effect of once- or twice-daily aspirin dosage in stable coronary artery disease patients with diabetes. Int J Hematol. 2010;92:296–301.
- Lemkes BA, Bahler L, Kamphuisen PW, et al. The influence of aspirin dose and glycemic control on platelet inhibition in patients with type 2 diabetes mellitus. J Thromb Haemost. 2012;10:639–646.
- Spectre G, Arnetz L, Östenson CG, et al. Twice daily dosing of aspirin improves platelet inhibition in whole blood in patients with type 2 diabetes mellitus and micro- or macrovascular complications. Thromb Haemost. 2011;106:491–499.
- Capodanno D, Patel A, Dharmashankar K, et al. Pharmacodynamic effects of different aspirin dosing regimens in type 2 diabetes mellitus patients with coronary artery disease. Circ Cardiovasc Interv. 2011;4:180–187.
- Serebruany VL, Steinhubl SR, Berger PB, et al. Analysis of risk of bleeding complications after different doses of aspirin in 192,036 patients enrolled in 31 randomized controlled trials. Am J Cardiol. 2005;95:1218–1222.
- Dillinger JG, Drissa A, Sideris G, et al. Biological efficacy of twice daily aspirin in type 2 diabetic patients with coronary artery disease. Am Heart J. 2012;164:600–606.
- Bethel MA, Harrison P, Sourij H, et al. Randomized controlled trial comparing impact on platelet reactivity of twice-daily with once-daily aspirin in people with Type 2 diabetes. Diabet Med. 2015 Jun 4. DOI:10.1111/dme.12828. [Epub ahead of print].
- Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012;125:882–887.
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310.
- Paes AH, Bakker A, Soe-Agnie CJ. Impact of dosage frequency on patient compliance. Diabetes Care. 1997;20:1512–1517.
- Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218–1224.
- Hall HM, Banerjee S, McGuire DK. Variability of clopidogrel response in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2011;8:245–253.
- Bhavaraju K, Georgakis A, Jin J, et al. Antagonism of P2Y(1)(2) reduces physiological thromboxane levels. Platelets. 2010;21:604–609.
- Armstrong PC, Dhanji AR, Tucker AT, et al. Reduction of platelet thromboxane A2 production ex vivo and in vivo by clopidogrel therapy. J Thromb Haemost. 2010;8:613–615.
- Shankar H, Garcia A, Prabhakar J, et al. P2Y12 receptor-mediated potentiation of thrombin-induced thromboxane A2 generation in platelets occurs through regulation of Erk1/2 activation. J Thromb Haemost. 2006;4:638–647.
- Serebruany VL, Malinin AI, Pokov A, et al. Effects of clopidogrel and aspirin in combination versus aspirin alone on platelet activation and major receptor expression in diabetic patients: the PLavix use for treatment of diabetes (PLUTO-Diabetes) trial. Am Heart J. 2008;155:93–97.
- Duzenli MA, Ozdemir K, Aygul N, et al. Comparison of increased aspirin dose versus combined aspirin plus clopidogrel therapy in patients with diabetes mellitus and coronary heart disease and impaired antiplatelet response to low-dose aspirin. Am J Cardiol. 2008;102:396–400.
- Dasgupta A, Steinhubl SR, Bhatt DL, et al. Clinical outcomes of patients with diabetic nephropathy randomized to clopidogrel plus aspirin versus aspirin alone (a post hoc analysis of the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance [CHARISMA] trial). Am J Cardiol. 2009;103:1359–1363.
- Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717.
- Donnan PT, MacDonald TM, Morris AD. Adherence to prescribed oral hypoglycaemic medication in a population of patients with Type 2 diabetes: a retrospective cohort study. Diabet Med. 2002;19:279–284.
- Mateo JF, Gil-Guillen VF, Mateo E, et al. Multifactorial approach and adherence to prescribed oral medications in patients with type 2 diabetes. Int J Clin Pract. 2006;60:422–428.
- Hutchins V, Zhang B, Fleurence RL, et al. A systematic review of adherence, treatment satisfaction and costs, in fixed-dose combination regimens in type 2 diabetes. Curr Med Res Opin. 2011;27:1157–1168.
- Patrick J, Dillaha L, Armas D, et al. A randomized trial to assess the pharmacodynamics and pharmacokinetics of a single dose of an extended-release aspirin formulation. Postgrad Med. 2015;127:573–580.
- Frieden TR. Use of selected clinical preventive services among adults–United States, 2007–2010. MMWR Morb Mortal Wkly Rep. 2012;61(Suppl):1–2.
