ABSTRACT
Objectives: To evaluate utilization patterns in patients initiating buprenorphine transdermal system (BTDS), CIII, and estimate the proportion decreasing their total opioid dose over time.
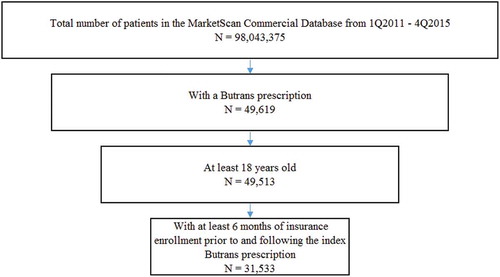
Methods: This retrospective cohort study used data from the Truven Health Analytics MarketScan® Commercial Claims and Encounters Database from 1 January 2011 through 31 December 2015. Eligible individuals were adults aged 18–64 years newly dispensed BTDS (index prescription) who had at least six months of insurance coverage prior to (baseline period) and following (study period) the index prescription.
Results: Back and neck pain was the most common pain condition in the study population (n = 31,533) and 88% were dispensed opioids in the baseline period. Nearly half (48%) received BTDS in a strength of 10 mcg/hour as their index prescription. Most (80%) patients prescribed BTDS had concomitant prescriptions for other opioids, chiefly immediate-release (IR) opioids (77%). During the baseline period, median opioid dose among patients prescribed opioids was 50 morphine-equivalent doses (MED), with 33% of patients using nonsteroidal anti-inflammatory drugs and 44% adjuvant analgesics. During the study period, BTDS use lasted a median 30 days and mean 100 days. Median dose of BTDS remained largely constant, and median dose of all opioids during continuous use of BTDS was 65.6 units MED. However, 24% of patients reduced total units MED from the baseline period (median mean dose, 74.5 units MED) until the end of the study period (42.8).
Conclusions: Most patients initiating treatment with BTDS had a history of treatment with IR opioids. Though the average change in total opioid daily dose after patients were prescribed BTDS was modest, an important subpopulation of approximately one-quarter of patients were able to markedly reduce their total units MED compared with prior opioid therapy. BTDS should be investigated as an option to help patients step down from higher opioid doses.
1. Introduction
Clinical challenges associated with safe opioid use and prescribing behaviors have prompted several federal agencies to increase efforts to curb opioid abuse in the USA The Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) have released updated resources to better inform patients and healthcare providers about appropriate treatment options for managing chronic pain [Citation1,Citation2]. In addition to new state-based prescribing and clinician training guidelines, the FDA has mandated changes to safety information in immediate-release (IR) opioid labeling [Citation3]. These changes include a new black-box warning about the serious risks of misuse, abuse, addiction, overdose and death for IR opioids similar to that of ER opioids. As part of its new initiative, the CDC published an opioid prescribing guideline in March 2016 [Citation2]. Within its recommendations for evaluating, selecting, and monitoring opioid use in the treatment of chronic pain, the CDC guideline prefers initial use of IR formulations and low morphine-equivalent doses (MED), and reserves use of extended-release (ER) formulations for opioid-tolerant patients [Citation2].
Buprenorphine is a partial mu-opioid agonist [Citation4,Citation5] with multiple formulations approved for medication-assisted treatment and pain management [Citation5–Citation7]. Butrans® (buprenorphine Transdermal System, or BTDS), CIII is an ER 7-day transdermal formulation of buprenorphine approved for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment for which alternative treatment options are inadequate [Citation8].
Further examination of the current use of BTDS in patients with chronic pain, and their concomitant use of other opioids and analgesics, may provide insight into current treatment behaviors in this population. The current retrospective cohort study had two objectives. The primary objective was to evaluate utilization patterns in patients initiating BTDS, focusing on patient demographic and clinical characteristics, history of prior and concomitant opioid, nonsteroidal anti-inflammatory drug (NSAID), and adjuvant analgesic use, as well as dosing information for BTDS and other opioids used during the study period. The secondary objective was to estimate the proportion of patients prescribed BTDS who decreased their total opioid dose over time, perhaps as part of a dose step down, another potential use of BTDS that may merit testing in future clinical trials.
2. Methods
2.1. Study design
This retrospective cohort study used data from the Truven Health Analytics MarketScan® Commercial Claims and Encounters Database from 1 January 2011 through 31 December 2015. This database contains demographic, prescription, inpatient and outpatient diagnoses, and procedure data on more than 100 million commercially insured, de-identified patients in the USA.
2.2. Inclusion criteria
The study population included individuals aged 18–64 years with a new (index) prescription for BTDS and at least 6 months of insurance coverage prior to the index prescription. This period serves as a baseline to allow for evaluation of prescription history and medical comorbidities. Individuals were also required to have at least 6 months of insurance coverage following the index prescription, to assess study measures following initiation with BTDS.
2.3. Study measures
Demographic characteristics were defined on the date of the index prescription and included age, age group, gender, and geographic region. Several pain conditions were identified in the 6 months before or 1 month following the index prescription, using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes. Specific conditions included abdominal pain, acute pain, arthropathies and related disorders (excluding osteoarthritis and rheumatoid arthritis), back and neck pain, chronic pain, fibromyalgia, fractures, dislocations, sprains and strains, headache, neuropathic pain, osteoarthritis, osteopathies, chondropathies, and acquired musculoskeletal deformities, psychiatric conditions, rheumatism (excluding fibromyalgia and neuralgia), rheumatoid arthritis, substance use disorder, other injuries, and other pain. Use of adjuvant medications sometimes used as part of an analgesic strategy was also captured; these included duloxetine, gabapentin, milnacipran, and pregabalin. Use of NSAIDs was recorded, specifically aspirin, diclofenac, diflunisal, etodolac, fenoprofen, flurbiprofen, ibuprofen, indomethacin, ketoprofen, ketorolac, meclofenamate, mefenamic acid, nabumetone, naproxen, oxaprozin, piroxicam, salsalate, sulindac, and tolmetin. Both IR and ER formulations of opioids were identified. IR opioids included buprenorphine, butorphanol, codeine, dihydrocodeine, fentanyl, hydrocodone, hydromorphone, meperidine, morphine, nalbuphine, oxycodone, oxymorphone, pentazocine, propoxyphene, tapentadol, and tramadol. ER formulations included buprenorphine, fentanyl, hydrocodone, hydromorphone, levorphanol, methadone, morphine, oxycodone, oxymorphone, tapentadol, and tramadol.
In identifying and classifying exposures and outcomes, it was assumed that prescriptions for BTDS were used as dispensed. Episodes of continuous use of BTDS were calculated using a 15-day allowable gap between subsequent prescriptions. However, only the first episode of continuous use was considered.
Dose of BTDS and concomitant opioids was computed across the first 6 months following the index prescription. Dose was calculated for the first episode of continuous use, and average monthly dose for each patient was calculated by summing the dose from the BTDS prescription (or concomitant opioid) in a given 30-day period and dividing the sum by the number of days covered by that drug. Both mean and median dose on days covered were obtained for the study cohort.
Total opioid use was expressed in MED to account for dose from both BTDS and other opioid treatments. Baseline opioid use was defined and quantified as average dose on days used for the sum of all concomitantly prescribed opioids. For BTDS, a MED conversion factor of 1 mcg/h of patch for a day to 2.4 mg morphine was used. Opioid tolerance was defined as having at least one prescription for an IR or ER opioid prior to initiating BTDS. Additionally, in order to evaluate different timeframes for opioid tolerance, baseline opioid use was examined across several additional time periods prior to the index prescription, with 6 months set as the standard. Baseline use of NSAIDs and other adjuvant pain treatments was also determined in different time windows before the index prescription to inform assessment of the patient’s overall history of pain treatment, though dose change for these drugs were not evaluated as part of this study. Use of concomitant opioids, NSAIDs, and adjuvants was determined using information contained in the prescription drug files.
To determine whether patients were using BTDS to down-titrate their opioid dose, for each patient the following difference was calculated:
For patients with a reduction in dose from the baseline period, the percent and median dose reduction were calculated.
The results were analyzed using descriptive statistics. Percentages, mean, median, and distribution were calculated for all patient characteristics and utilization variables of interest.
3. Results
3.1. Patient demographics and clinical characteristics
A total of 31,533 patients met the study criteria (). Approximately 65% were female, and most were aged 45–64 years (). summarizes the pain conditions identified in patients 6 months before or 1 month following the index BTDS prescription. Back and neck pain was the most common condition, affecting approximately 80% of the population, followed by arthropathies (45%), rheumatism (excluding fibromyalgia) (42%), other pain (33%), and psychiatric conditions (31%) ().
Table 1. Demographic characteristics of the BTDS Cohort at index.
Table 2. Pain conditions 6 months before or 1 month following index BTDS prescription.
3.2. Utilization
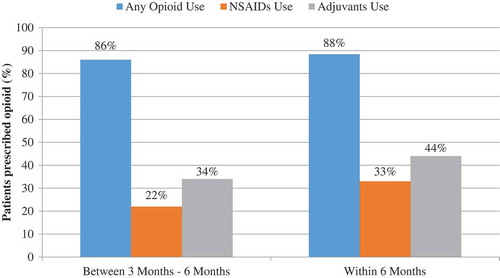
Approximately 40% of the study population received as their index prescription a buprenorphine patch with a strength of 5 mcg/h, the lowest strength available and indicated for opioid naïve patients (). Additionally, nearly half of the study population had an index prescription of a buprenorphine patch with a strength of 10 mcg/h (48%). Most patients initiating on Butrans are opioid experienced, taking >30 mg/day MED, therefore the 10 mcg/h is the correct starting dose for these patients, per labeled dosing instructions. Within the 6-month baseline period, 88% of patients were prescribed an opioid, including 86% 3–6 months prior to index, suggesting long-term use of opioids before initiating BTDS (). Forty-four percent (44%) had evidence of an adjuvant analgesic prescription during the 6-month baseline period, and an additional 33% had a prescription for at least one NSAID (). A large proportion (80%) of patients prescribed BTDS had concomitant prescriptions for other opioids. Specifically, among BTDS patients, 77% had at least one claim for an IR opioid and 18% had at least one claim for an ER opioid ().
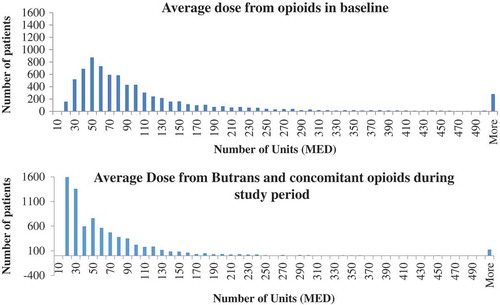
Median opioid dose among patients prescribed opioids during the baseline period was 50 units MED. With the exception of some high-dose outliers, the distribution of average opioid dose during the baseline period revealed a fairly normal distribution and a median of approximately 50 units MED, 25th percentile of 32 units MED, 75th percentile of 82 units MED, and 90th percentile of 251 units MED ().
Within the sample of patients prescribed Butrans, 33% of patients had NSAID (N = 10,393) and 44% had adjuvant analgesic (N = 13,810) prescriptions in the 6 months prior to BTDS initiation (). During the period of continuous BTDS use, 23% of patients were prescribed NSAIDs and 40% adjuvant analgesics. Additionally, most patients who initiated BTDS without a preexisting opioid prescription started treatment on the lowest patch or tablet strength available. Among 3933 opioid-naïve patients, 51% received an index BTDS prescription for 5 mcg/h.
Table 3. Opioid dose and non-opioid pain treatments at baseline in the BTDS cohort.
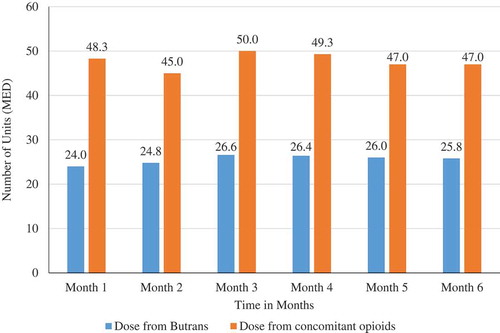
BTDS was used for a median of 30 days, with a mean (SD) of 100.4 days (SD 150.7). Approximately, one-quarter (8772, 28%) of patients used BTDS continuously for more than 90 days, with 15.1% of patients having at least 181 days of continuous use. During the study period, the median dose was 24.0 units MED, which remained largely constant over the first six months of use. After a median dose of 24.0 units MED at month one, patients who continued to receive prescriptions had a median dose that had increased only to 25.8 units MED at month 6 ().
While most patients had total opioid doses that remained relatively constant over time, as shown in , a subpopulation of 7537 (24%) patients prescribed BTDS had a reduction in total units MED (median reduction 25.5 units, mean 64.0) from the baseline until the end of the study period. A graphical depiction of the opioid dose down-titration in the subset of patients with continuous use indicated that the normalcy of the distribution disappeared and a new modal dose of approximately 20 units MED surfaced. The median dose from BTDS and other opioids during continuous use of BTDS was 65.6 units MED (interquartile range, 59.9). Dose fell substantially among patients who had a dose reduction, with a median mean dose during the baseline period of 74.5 units MED (mean 143.0), falling to a median dose during the study period of 42.8 units MED (mean 79.0).
4. Discussion
4.1. Limitations
This analysis was subject to several limitations. First, we were unable to determine if some opioid prescriptions were used as needed (PRN) rather than on a fixed dosing schedule, as there was no PRN indicator available in the database. As such, it was assumed that all prescriptions were used as dispensed. Second, the data was derived from a large convenience sample sourced primarily from large employers. Because medium- and small-sized employers are poorly represented, the results may not be generalizable to individuals covered by these types of employers, or to those with Medicare, Medicaid, or no insurance. Similarly, the majority of patients prescribed Butrans were in the South. This is due in part to two factors. First, there are more employers that provide data to Marketscan in this region, and second, there has been higher managed care uptake of Butrans and inclusion of the drug on formulary among health plans that are common in the southern states. Third, we were not able to directly determine indication from claims data, but instead examined pain-related diagnoses proximal to the prescription claim. Fourth, no assessment of safety events was conducted, due to the nature of the healthcare claims dataset used. Last, while opioid down-titration was observed in the data for a subpopulation of patients, the reasons for its initiation remain unclear. Whether the decrease is due to analgesic needs being met or whether Butrans is being used as part of a HCP’s efforts to down-titrate opioid patients is unclear. It is also not known whether BTDS was specifically or coincidentally selected for use in down-titration. This observation presents an opportunity for future research examining opioid down-titration patterns across multiple therapies to identify the most productive strategies for managing the use of this challenging drug class.
5. Conclusion
Most patients initiating treatment with BTDS in this analysis had a prior history of treatment with IR opioids. Though the average change in total opioid daily dose after patients were prescribed BTDS was modest (with most remaining at consistent dose or experiencing an expected modest increase during the initial months as the drug was titrated to effect), a substantial subpopulation of approximately one-quarter of patients who used this drug markedly reduced their total units MED. This is not expected for patients newly initiating an ER opioid, particularly those who have a history of previous treatment with other IR or ER opioid formulations. These findings suggest that it may be warranted to conduct future studies that explore a potential role for BTDS as an option for patients to reduce opioid doses.
Declaration of interest
L Wallace and A Kadakia are employees of Purdue Pharma L.P. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Writing assistance was provided by Luke Boulanger (Truven Health Analytics) and funded by Purdue Pharma L.P.
Additional information
Funding
References
- FDA announces safety labeling changes and postmarket study requirements for extended-release and long-acting opioid analgesics. Silver Spring (MD): US Food and Drug Administration; 2013 [cited 2016 Oct 28]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnoucements/ucm367726.htm
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. MMWR Recomm Rep. 2016;65(No. RR–1):1–49.
- FDA announces enhanced warnings for immediate-release opioids pain medications related to risks of misuse, abuse, addiction, overdose, and death. Silver Spring (MD: US Food and Drug Administration; 2016 [cited 2016 Oct 28]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnoucements/ucm491739.htm
- Tallarida RJ, Cowan A, Raffa RB. On deriving the dose-effect relation of an unknown second component: an example using buprenorphine preclinical data. Drug Alcohol Depend. 2010;109:126–129.
- Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1–11.
- Suboxone, [package insert]. Richmond (VA): Reckitt Benckiser Pharmaceuticals; 2016.
- Subutex, [package insert]. Richmond (VA): Reckitt Benckiser Pharmaceuticals; 2016.
- Butrans [package insert]. Stamford (CT): Purdue Pharma L.P.; 2014.