ABSTRACT
Objective: Prescription opioid related abuse, suicide and death are significant public health problems. This study compares rates of poison center calls categorized as intentional abuse, suspected suicidal intent or fatality for the 7-day buprenorphine transdermal system/patch (BTDS) with other extended-release and long-acting (ER/LA) opioids indicated for chronic pain.
Methods: Retrospective 24-month cohort study using National Poison Data System data from July 2012 through June 2014. BTDS was introduced in the United States in January 2011. Numbers and rates of calls of intentional abuse, suspected suicidal intent and fatalities were evaluated for BTDS, ER morphine, ER oxycodone, fentanyl patch, ER oxymorphone and methadone tablets/capsules, using prescription adjustment to account for community availability. Rate ratios (RR) and 95% confidence intervals (CI) were calculated.
Results: Absolute numbers and prescription-adjusted rates of intentional abuse and suspected suicidal intent with BTDS were significantly lower (p < .0001) than for all other ER/LA opioid analgesics examined. No fatalities associated with BTDS exposure were reported.
Conclusion: This post-marketing evaluation of BTDS indicates infrequent poison center calls for intentional abuse and suspected suicidal intent events, suggesting lower rates of these risks with BTDS compared to other ER/LA opioids.
1. Introduction
Over the past decade, opioid analgesic abuse (i.e. non-analgesic use for psychoactive purposes) [Citation1] and misuse (i.e. analgesic use in a way other than prescribed or instructed by a healthcare professional) [Citation1] have emerged as significant public health problems, with accompanying serious increases in addiction, overdoses, and deaths [Citation2]. Estimates of the prevalence of problematic use of opioids cover a broad range from 1% to 81% and estimate of opioid addiction rates among pain patients vary from 8% to 12% [Citation3], though estimates of addiction may be complicated to assess in chronic pain patients using long-term opioid treatment with opioid dependence [Citation4]. However, opioid analgesics are an important treatment option for pain severe enough to require opioid treatment and for which alternative treatment options are inadequate. Opioid analgesics are prescribed as extended-release (ER) and immediate-release (IR) formulations to over 4 and 68 million pain patients annually in the United States (US), respectively (IMS health, unpublished data). Reduction of abuse and misuse and their consequences while preserving analgesic benefits of opioids to patients is an important goal [Citation5], and the development of new opioid analgesic formulations with reduced abuse potential is supported by the US Food and Drug Administration (FDA) [Citation6].
Buprenorphine is a partial mu-opioid receptor agonist with high mu receptor affinity [Citation7,Citation8] and slow receptor dissociation [Citation9]. It has been available for use in clinical practice in the US since 1981 [Citation10]. Buprenorphine is also an antagonist at kappa-opioid receptors, an agonist at delta-opioid receptors, and a partial agonist at ORL-1 (nociceptin) receptors [Citation11].
In early preclinical studies, a ceiling on the effects of buprenorphine across a range of pharmacodynamic measures was observed [Citation12–Citation14]. In subsequent studies in healthy volunteers and non-dependent opiate abusers [Citation15], a ceiling was demonstrated for respiratory depression along with a number of subjective effect measures (e.g. ‘drug-liking’) that have been used to predict abuse potential, consistent with its classification as a partial agonist. Clinically, buprenorphine acts as a full agonist for analgesia [Citation16,Citation17], with no apparent ceiling effect for analgesia at therapeutic doses [Citation18]. In part due to these characteristics, the Drug Enforcement Administration (DEA) [Citation19] in accordance with the Controlled Substances Act [Citation20] has designated buprenorphine as a Schedule III medicine, meaning that it has lower expected abuse or dependency potential than Schedule II medicines. In addition to these properties that may have an effect on abuse rates, some research has suggested anti-suicide potential of low doses of buprenorphine [Citation21,Citation22].
In 2010, a buprenorphine transdermal system [BTDS, Butrans® Purdue Pharma L.P.] was approved in the US for the treatment of pain. It is indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment for which alternative treatment options are inadequate [Citation11]. BTDS continuously delivers dosages of 5, 7.5, 10, 15, or 20 mcg/h of buprenorphine for 7 days [Citation23,Citation24], as a steady over time, controlled release of the medication to patients in chronic pain [Citation25–Citation31]. Improvements in health-related quality of life have also been demonstrated in post hoc analyses of randomized clinical trials of patients with pain treated with BTDS [Citation32,Citation33] as well as improvements in sleep quality [Citation34] and reductions in pain interference in functioning [Citation35].
To date there are few published post-marketing data evaluating rates of intentional abuse or suicide related to buprenorphine exposure. This study used data from the National Poison Data System (NPDS). Calls to poison centers are strongly correlated with poisoning mortality as identified on death certificates for opioids and may be used for timely surveillance of mortality [Citation36]. The NPDS, which has been operational since 1985 and managed by the American Association of Poison Control Centers (AAPCC), contains case records from calls made to poison control centers in the US. The data in NPDS are nationally representative and have been used both directly [Citation37] and indirectly [Citation38–Citation42] to assess abuse trends. The NPDS is a poisoning exposure surveillance database that captures 99.8% of poison exposures reported to all poison centers in the US [Citation43,Citation44]. The extensive network of poison centers reporting into the NPDS allows for nationally representative coverage. In 2013, there were over 2.1 million human exposure cases and over 800,000 information calls (no human exposure) entered into the NPDS with 57 regional poison centers serving the entire population of US. Poison centers are open 24-h each day of the year, do not charge fees to users, and must be accredited by the AAPCC, which annually publishes an aggregate data report [Citation45]. Calls received at US poison centers are managed by healthcare professionals who have received specialized training in toxicology and managing exposure emergencies. Opioid exposures reported to poison centers are classified by these personnel into categories in accordance with a standardized data dictionary. For calls with more than one substance implicated in an exposure, the poison center staff chooses a primary substance of implication in accordance to relative contribution of the substance to the patient’s clinical outcome.
The aim of this study was to assess rates of intentional abuse, suspected suicidal intent, and fatalities associated with BTDS exposures reported to NPDS, and to compare them to other Schedule II ER opioid analgesics and methadone, a long-acting opioid.
2. Materials and methods
This was a retrospective observational study of data from poison centers in the US. Cases of human opioid exposures recorded in the NPDS as originating in the US during the 2-year period from July 2012 (18 months after BTDS became available in the US) to June 2014 were evaluated in this retrospective cohort study. The opioid analgesics in the evaluation included BTDS (Schedule III), fentanyl patch, ER oxymorphone, ER oxycodone and ER morphine (all Schedule II ER opioid analgesics), and methadone tablets/capsules (Schedule II and indicated for treatment of opioid addiction and analgesia). Poison centers use a national standardized data dictionary that has defined definitions for cases reported to the poison centers. These standardized definitions were used for the study as follows: intentional abuse is defined as an exposure resulting from the intentional, improper, or incorrect use of a substance in order to gain a high, euphoric effect or some other psychotropic effect. Suspected suicidal intent is defined as inappropriate use of a substance for self-destructive or manipulative reasons. Total cases were separated into intentional abuse, suspected suicidal intent, and other reasons. Absolute numbers of exposure cases for each individual opioid of interest were identified.
Prescription volume data were obtained from IMS (Collegeville, PA, USA). The prescription data sample includes cash, Medicaid, and third-party transactions for over 50,000 pharmacies in the US [Citation46]. Other potential channels of distribution, such as long-term care, hospital dispensaries, and mail order are included in this dataset. Prescription-adjusted rates and 95% confidence intervals of intentional abuse and suspected suicidal intent were calculated using dispensing data purchased from IMS Health in order to adjust for availability of drug in the community. Using BTDS as the reference, relative rate ratios (RR) with corresponding p-values were calculated.
This observational study involved assessment of purchased and anonymized data, which did not involve direct patient/practitioner involvement, and therefore was exempt from human subjects’ research ethics committee review.
3. Results
A total of 13,989 ER/LA opioid exposure calls of interest from July 2012 to June 2014 were identified including 117 for BTDS, 797 for ER oxymorphone, 2044 for the fentanyl patch, 2346 for ER morphine, 2830 for ER oxycodone, and 5855 for methadone tablets/capsules.
3.1. Abuse
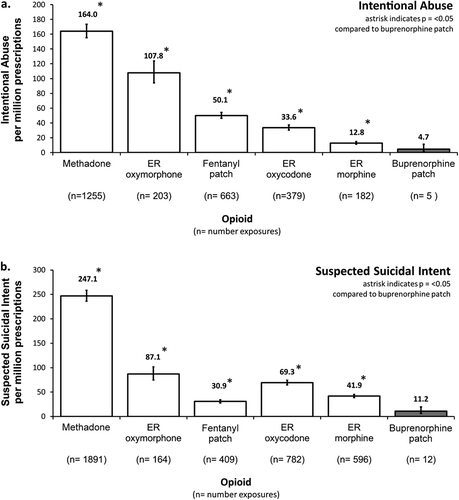
Of the 2687 calls to poison centers involving an opioid of interest categorized as intentional abuse during the 2-year post-marketing evaluation period, only 5 cases involved BTDS. In comparison, there were 663 calls involving the fentanyl patch, 182 involving ER morphine, 203 involving ER oxymorphone, 379 involving ER oxycodone and 1255 involving methadone tablets/capsules ()). These cases numbers correspond to a rate of 5 abuse calls per million prescriptions for BTDS, 50 for the fentanyl patch, 13 for ER morphine, 34 for ER oxycodone, 108 for ER oxymorphone, and 164 for methadone. The prescription-adjusted rates of abuse for the other opioid analgesics compared to BTDS were 10-fold higher for the fentanyl patch (RR = 10.8, 95% CI: 4.46–25.9, p < 0.001), twofold higher for ER morphine (RR = 2.7, 95% CI: 1.13–6.68, p = 0.0258), sevenfold higher for ER oxycodone (RR = 7.2, 95% CI: 2.99–17.4, p < 0.001), 23-fold higher for ER oxymorphone (RR = 23.2, 95% CI: 9.53–56.2, p < 0.001), and 35-fold higher for methadone (RR = 35.2, 95% CI: 14.6–84.8, p < 0.001) (). The rate of abuse calls to poison centers was statistically significantly lower for BTDS in comparison to the other ER/LA opioids.
Table 1. Absolute counts and prescription-adjusted rates of abuse, cases of suspected suicidal intent, and fatalities by opioid type (National Poison Data System, 3Q2012–2Q2014).
3.2. Suspected suicidal intent
Of the 3854 calls to poison centers involving opioids of interest categorized as suspected suicidal intent, only 12 involved BTDS while 409 calls involving the fentanyl patch, 596 involving ER morphine, 164 involving ER oxymorphone, 782 involving ER oxycodone, and 1891 involving methadone tablets/capsules were recorded ()). This corresponds to a rate of 11 per million prescriptions for BTDS, 31 for the fentanyl patch, 42 for ER morphine, 69 for ER oxycodone, 87 for ER oxymorphone, and 247 for methadone. For the other opioid analgesics compared to BTDS, this rate was twofold higher for the fentanyl patch (rate ratio, RR = 2.8, 95% CI: 1.56–4.91, p < 0.001), threefold higher for ER morphine (RR = 3.7, 95% CI: 2.12–6.64, p < 0.001), sixfold higher for ER oxycodone (RR = 6.2% CI: 3.51–11.0, p < 0.001), sevenfold higher for ER oxymorphone (RR = 7.8, 95% CI: 4.34–14.0, p < 0.001), and 22-fold higher for methadone (RR = 22.1, 95% CI: 12.53–39.0, p < 0.001) (). The rate of suspected suicidal intent calls was statistically significantly lower for BTDS in comparison to the other ER/LA opioids.
3.3. Fatalities
Among the 95 fatalities recorded for the ER/LA opioid drugs of interest, no BTDS exposure was associated with a fatal outcome (0 deaths out of 1,073,812 prescriptions dispensed) while methadone tablets/capsules had the highest (51 deaths out of 7,653,314 prescriptions dispensed) followed by the fentanyl patch (17 deaths out of 13,237,727 prescriptions dispensed) ().
3.4. Gender distribution of cases
Abuse cases were slightly more prevalent among females than males: 2 out of 5 buprenorphine patch cases were female, 9 of 21 fentanyl patch cases were female, 22 of 46 for ER morphine cases were female, 2 of 5 ER oxymorphone cases, 6 of 14 for ER oxcodone and 31 of 69 abuse cases for methadone. Similarly, suicide cases were slightly more prevalent among females than males: 7 out of 12 buprenorphine suicide cases were female, 19 of 31 fentanyl suicide cases were female, 43 of 87 ER morphine cases were female, 26 of 42 ER oxymorphone cases, 38 of 69 ER oxycodone cases, and 131 of 246 methadone cases were female.
4. Discussion
This analysis shows that calls to US poison centers for intentional abuse and suspected suicidal intent events associated with BTDS occurred rarely, with no BTDS exposure (for any reason) associated with a fatal outcome during the 2-year evaluation period. Prescription-adjusted rates of abuse and suspected suicidal intent were statistically significantly lower than that for other Schedule II ER/LA opioids during the study period. The findings of this study are consistent with that of other studies that have found lower rates of fatal overdoses and poisonings associated with buprenorphine use compared with other use of similar opioids including methadone [Citation47,Citation48]. Weigand et al. compared abuse rates of buprenorphine transdermal system with other opioids in the RADARS System and found that BTDS was abused and diverted at low rates compared with other buprenorphine products, ER tramadol, fentanyl patch, and an aggregate grouping of Schedule II ER opioids in all programs of the RADARS System [Citation49]. Our study used a different surveillance system and found low rates of BTDS abuse in the National Poison Data System compared to ER morphine, ER oxycodone, ER oxymorphone, methadone, and fentanyl patch. In addition, the rate of suicide and fatalities associated with BTDS in the National Poison Data System were low relative to the comparator opioid analgesics assessed in our study.
A number of factors may have contributed to the very low numbers of poison center calls of intentional abuse or suspected suicidal intent for BTDS in this study. The unique intrinsic properties of the buprenorphine molecule contributes to its classification as a partial mu-opioid receptor agonist with high mu receptor affinity [Citation7,Citation8], with tight binding to mu-opioid receptor and slow receptor dissociation [Citation9]. Buprenorpine has the potential for low respiratory depression, and can function as a possible barrier to pure mu-opioid agonists. The 7-day transdermal delivery system, incorporating buprenorphine into a drug/polymer adhesive matrix, may also contribute to less abuse because approximately 48 h are needed to reach maximum buprenorphine concentrations after initial patch application, which limits the ability for a user to gain a rapid high. These properties, along with observation in experimental studies of less drug liking [Citation50], contributed to the subsequent designation of BTDS as Schedule III by DEA, which is consistent with the observation of infrequent poison center calls categorized as intentional abuse.
The low rate of suspected suicidal intent attributed to BTDS may be due in part to its multiple receptor activity. Buprenorphine is also an antagonist at kappa-opioid receptors, an agonist at delta-opioid receptors, and a partial agonist at ORL-1 (nociceptin) receptors [Citation11]. Its kappa antagonist properties, have been postulated to be linked with antidepressant effects in small case reports/series of patients with major depressive disorder [Citation51–Citation53], and may contribute to resolving patients’ suicidal ideation [Citation22], and be involved in antagonist-agonist modulation [Citation54].
There are some limitations of the current study. Interpretations of the results of this study are limited by the design of the study. The number of prescriptions for BTDS (approximately 1 million) was relatively small compared to the other ER/LA opioids in the study, thereby limiting statistical power. However, trends in both the absolute numbers of BTDS exposures and the prescription-adjusted rates of these exposures were similar. Adjustment for prescription volume of the different ER opioids is a valuable method for accounting for availability for abuse. However, a potential bias in the interpretation of rates based on prescription dispensing is unequal diversion of individual opioid products; it is possible that prescription volume data underestimates the amount of some of these opioids available for abuse. In addition, prescription adjustment is not ideal because it does not adjust for the dosage strength of the units prescribed using a shared metric such as morphine-equivalent milligrams. Data on milligrams dispensed was not available for this analysis. However, the dosage strength of BTDS patches available for abuse after extraction is much higher than ER opioid tablets: the number of milligrams of buprenorphine contained in the highest and lowest patch strength of BTDS is 20 and 5 mg, respectively, which has a morphine-equivalent potency of approximately 2000 and 500 mg respectively (since the morphine-equivalence of buprenorphine is 85–100 times more potent than morphine). The highest dosage strengths of tablets of ER morphine are 200 mg and of ER oxycodone are 120 mg morphine equivalents. We could have used the number of dosing units dispensed instead of prescriptions dispensed, but a comparison of the dosing unit of a 7-day patch to a dosing unit of an ER opioid tablet that is taken twice a day could create imprecision and distortion of rates.
Another limitation is that calls to poison centers represent individuals seeking assistance for an acute medical problem, and therefore do not represent all exposures to a product, providing the potential for underreporting (e.g. individuals may not have time to know how to contact a poison center). However, a strong correlation between rates of calls to poison centers and mortality rates assessed using Medical Examiner database deaths related to methadone exists [Citation36]. In addition, a strong correlation between rates of abuse calls to poison centers and rates of emergency department visits in the Drug Abuse and Warning Network [Citation55] suggests that the outcomes from this study represent rates of abuse nationally in the US as well as clinical practice patterns. Although misclassification of opioid product exposures is possible, trained personnel use precise National Drug Codes (NDC) [Citation56] to record drug exposures in accordance with a standardized data dictionary when asking callers to describe the product associated with the call. Since BTDS is the only transdermal buprenorphine formulation available in the US market, misidentification is unlikely.
5. Conclusion
Calls and reports to US poison centers of intentional abuse, suspected suicidal intent, and fatalities attributed to BTDS exposure were infrequent, with prescription-adjusted rates of such events being significantly lower than that for other ER/LA opioids. These results suggest that BTDS provides a safety advantage for the risks assessed in this surveillance system for patients requiring daily, around-the-clock opioid treatment.
Methadone was the opioid that had the highest rate of abuse, suicides and fatalities in the National Poison Data System among the opioids studied. We included methadone because it is considered by the FDA as a therapeutic equivalent to BTDS, as indicated by both sharing a common indication in the FDA-approved label of management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate [Citation11,Citation57]. Methadone is also designated as part of the ER/LA opioid Risk Evaluation and Mitigation Strategy (REMS) by the FDA [Citation58]. However, methadone’s use as an opioid dependence/addiction treatment medication results in substantial community availability of population exposure that is not captured or controlled for by IMS prescription volume data. To limit the contribution of methadone used for opioid dependence treatment, we restricted the data for analysis to only methadone tablets, and excluded methadone formulations used for addiction treatment, e.g. oral solutions, in both the National Poison Data System and the IMS prescription data.
This study used an ecological design assessing national abuse and suicide rates in the US. The poison center database has the strength of assessing abuse and suicide cases in the general US population, including patients prescribed opioids and non-patient people abusing opioids to get high or stave off withdrawal. A weakness of the database is that it cannot link individual patient characteristics to the risk of abuse or suicide, and therefore cannot control for demographic or dose covariates. The majority of people dispensed the buprenorphine patch use it concomitantly with other opioid analgesics [Citation59], so that the overall prescribed daily opioid dose of patients prescribed buprenorphine patch is similar to that of other extended-release opioids (unpublished IMS data). However future studies may wish to use additional databases to control for patient-level differences, although such database studies will probably be limited to abuse and suicide among patients prescribed opioids rather than the substantial proportion who misused diverted opioids obtained from friends, family or black market dealers [Citation60].
Declaration of interest
PM Coplan, NE Sessler and V Harikrishnan are employees of Purdue Pharma L.P. R Singh was a consultant for Purdue Pharma L.P. at the time of the study. C Perkel is an assistant professor of addiction psychiatry, Mount Sinai Beth Israel, NY, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Smith SM, Dart RC, Katz NP, et al. Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. Pain. 2013;154:2287–2296.
- Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States,2010. JAMA. 2013;309:657–659.
- Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569–576.
- Ballantyne JC. Assessing the prevalence of opioid misuse, abuse, and addiction in chronic pain. Pain. 2015;156:567–568.
- Frieden TR, Houry D. Reducing the risks of relief–the CDC opioid-prescribing guideline. N Engl J Med. 2016;374:1501–1504.
- Guidance for industry. Abuse-deterrent opioids – evaluation and labeling. final guidance [Internet]. Silver Spring (MD): U.S. Food and Drug Administration; 2015 [cited 2016 Oct 30]. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm334743.pdf
- Dum J, Blasig J, Herz A. Buprenorphine: demonstration of physical dependence liability. Eur J Pharmacol. 1981;70:293–300.
- Lee KO, Akil H, Woods JH, et al. Differential binding properties of oripavines at cloned m- and d-opioid receptors. Eur J Pharmacol. 1999;378:323–330.
- Greenwald M, Johanson CE, Bueller J, et al. Buprenorphine duration of action: mu-opioid receptor availability and pharmacokinetic and behavioral indices. Biol Psychiatry. 2007;61:101–110.
- Johnson RE, Fudala PJ, Payne R. Buprenorphine: considerations for pain management. J Pain Symptom Manag. 2005;29:297–326.
- Butrans® [full prescribing information]. Stamford (CT): Purdue Pharma L.P.; 2014 [cited 2016 Nov 30]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021306s015s019lbl.pdf
- Cowan A, Doxey JC, Harry EJR. The animal pharmacology of buprenorphine, an oripavine analgesic agent. Br J Pharmacol. 1977;60:547–554.
- Lizasoain I, Leza JC, Lorenzo P. Buprenorphine: bell-shaped dose-response curve for its antagonist effects. Gen Pharmacol. 1991;22:297–300.
- Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580.
- Umbricht A, Huestis MA, Cone EJ, et al. Effects of high-dose intravenous buprenorphine in experienced opioid abusers. J Clin Psychopharmacol. 2004;24:479–487.
- Pergolizzi J, Aloisi AM, Dahan A, et al. Current knowledge of buprenorphine and its unique pharmacological profile. Pain Pract. 2010;10:428–450.
- Christoph T, Kogel B, Schiene K, et al. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol. 2005;507:87–98.
- Kress HG. Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine. Eur J Pain. 2009;13:219–230.
- Schedule of controlled substances: proposed rule: rescheduling of buprenorphine from schedule V to schedule III [Internet]. Springfield (VA): DEA Office of Division Control; [cited 2016 Oct 30]. Available from: http://www.deadiversion.usdoj.gov/fed_regs/rules/2002/fr0321.htm
- Federal Food, Drug and Cosmetic Act - Title 21: food and drugs, chapter 13: drug abuse prevention and control, subchapter 1: control and enforcement [Internet]. Springfield (VA): DEA Office of Division Control; [cited 2016 Oct 30]. Available from: http://www.fda.gov/regulatoryinformation/legislation/ucm148726.htm#cntlsbb
- Striebel JM, Kalapatapu RK. The anti-suicidal potential of buprenorphine: a case report. Int J Psychiatry Med. 2014;47:169–174.
- Yovell Y, Bar G, Mashiah M, et al. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled trial. Am J Psychiatr. 2016;173:491–498.
- Kapil R, Cipriano A, Friedman K, et al. Once-weekly transdermal buprenorphine application results in sustained and consistent steady-state plasma levels. J Pain Symptom Manag. 2013;46:65–75.
- Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–1268.
- Steiner D, Munera C, Hale M, et al. Efficacy and safety of buprenorphine transdermal system (BTDS) for chronic moderate to severe low back pain: a randomized, double-blind study. J Pain. 2011;12:1163–1173.
- Steiner DJ, Sitar S, Wen W, et al. Efficacy and safety of the seven-day buprenorphine transdermal system in opioid-naïve patients with moderate to severe chronic low back pain: an enriched, randomized, double-blind, placebo-controlled study. J Pain Symptom Manag. 2011;42:903–917.
- Gordon A, Callaghan D, Spink D, et al. Buprenorphine transdermal system in adults with chronic low back pain: a randomized, double-blind, placebo-controlled crossover study, followed by an open-label extension phase. Clin Ther. 2010;32:844–860.
- Gordon A, Rashiq S, Moulin DE, et al. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res Manag. 2010;15:169–178.
- James IG, O’Brien CM, McDonald CJ. A randomized, double-blind, double-dummy comparison of the efficacy and tolerability of low-dose transdermal buprenorphine (BuTrans seven-day patches) with buprenorphine sublingual tablets (Temgesic) in patients with osteoarthritis pain. J Pain Symptom Manag. 2010;40:266–278.
- Munera C, Drehobl M, Sessler NE, et al. A randomized, placebo-controlled, double-blinded, parallel-group, 5-week study of buprenorphine transdermal system in adults with osteoarthritis. J Opioid Manag. 2010;6:193–202.
- Landau CJ, Carr WD, Razzetti AJ, et al. Buprenorphine transdermal delivery system in adults with persistent noncancer-related pain syndromes who require opioid therapy: a multicenter, 5-week run-in and randomized, double-blind maintenance-of-analgesia study. Clin Ther. 2007;29:2179–2193.
- Yarlas A, Miller K, Wen W, et al. A randomized, placebo-controlled study of the impact of the 7-day buprenorphine transdermal system on health-related quality of life in opioid-naïve patients with moderate-to-severe chronic low back pain. J Pain. 2013;14:14–23.
- Miller K, Yarlas A, Wen W, et al. Buprenorphine transdermal system and quality of life in opioid-experienced patients with chronic low back pain. Expert Opin Pharmacother. 2013;14:269–277.
- Miller K, Wen W, Lynch SY, et al. Buprenorphine transdermal system improves sleep quality and reduces sleep disturbance in patients with moderate-to-severe chronic low back pain: results from two randomized controlled trials. Pain Pract. 2016;16:345–358.
- Yarlas A, Miller K, Wen W, et al. Buprenorphine transdermal system compared with placebo reduces interference in functioning for chronic low back pain. Postgrad Med. 2015;127:38–45.
- Dasgupta N, Davis J, Jonsson Funk M, et al. Using poison center exposure calls to predict methadone poisoning deaths. PLoS One. 2012;7:e41181.
- Coplan PM, Kale H, Sandstrom L, et al. Changes in oxycodone and heroin exposures in the National Poison Data System after introduction of extended-release oxycodone with abuse deterrent characteristics. Pharmacoepidemiol Drug Saf. 2013;22:1274–1282.
- Hughes AA, Bogdan GM, Dart RC. Active surveillance of abused and misused prescription opioids using poison center data: a pilot study and descriptive comparison. Clinical Toxicol. 2007;45:144–151.
- Reifler LM, Droz D, Bailey JE, et al. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 2012;13:434–442.
- Smith MY, Schneider MF, Wentz A, et al. Quantifying morbidity associated with the abuse and misuse of opioid analgesics: a comparison of two approaches. Clinical Toxicol. 2007;45:23–30.
- Smith MY, Irish W, Wang J, et al. Detecting signals of opioid analgesic abuse: application of a spatial mixed effect poisson regression model using data from a network of poison control centers. Pharmacoepidemiol Drug Saf. 2008;17:1050–1059.
- Bailey JE, Barton PL, Lezotte D, et al. The effect of FDA approval of a generic competitor to OxyContin (oxycodone HCl controlled-release) tablets on the abuse of oxycodone. Drug Alcohol Depend. 2006;84:182–187.
- National Poison Data System (NPDS) [Internet]. Alexandria (VA): American Association of Poison Control Centers; [cited 2016 Oct 30]. Available from: http://www.aapcc.org/data-system/
- CDC Fact Sheet: Using the National Poisoning Data System for Public Health Surveillance. A collaborative effort of the Centers for Disease Control and Prevention and the American Association of Poison Control Centers [Internet]. Atlanta (GA): Department of Health and Human Services Centers for Disease Control and Prevention; 2005 [cited 2016 Oct 30]. Available from: http://www.cdc.gov/nceh/hsb/chemicals/pdfs/npds.pdf
- Mowry JB, Spyker DA, Cantilena LR, et al. 2013 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st annual report. Clin Toxicol. 2014;52:1032–1283.
- HSRN data brief: National Prescription Audit [Internet]. Collegeville (PA): IMS Health Informatics; 2011 [cited 2016 Oct 30]. Available from: https://www.imshealth.com/files/web/IMSH%20Institute/NPA_Data_Brief-.pdf
- Marteau D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open. 2015;5:e007629.
- Paone D, Tuazon E, Stajic M, et al. Buprenorphine infrequently found in fatal overdose in New York city. Drug Alcohol Depend. 2015;155:298–301.
- Wiegand TJ, Le Lait MC, Bartelson BB, et al. Analysis of the abuse and diversion of the buprenorphine transdermal delivery system. J Pain. 2016;17:745–752.
- Comer SD, Sullivan MA, Vosburg SK, et al. Abuse liability of intravenous buprenorphine/naloxone and buprenorphine alone in buprenorphine-maintained intravenous heroin abusers. Addiction. 2010;105:709–718.
- Bodkin JA, Zornberg GL, Lukas SE, et al. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15:49–57.
- Emrich HM, Vogt P, Herz A, et al. Antidepressant effects of buprenorphine. Lancet. 1982;320:709.
- Nyhuis PW, Gastpar M, Scherbaum N. Opiate treatment in depression refractory to antidepressants and electroconvulsive therapy. J Clin Psychopharmacol. 2008;28:593–595.
- Ehrich E, Turncliff R, Du Y, et al. Evaluation of opioid modulation in major depressive disorder. Neuropsychopharmacol. 2015;40:1448–1455.
- Davis JM, Severtson SG, Bucher-Bartelson B, et al. Using poison center exposure calls to predict prescription opioid abuse and misuse-related emergency department visits. Pharmacoepidemiol Drug Saf. 2014;23:18–25.
- National drug code database background information [Internet]. Silver Spring (MD): US Food and Drug Administration; 2012 [cited 2016 Oct 30]. Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/UCM070829
- Dolophine (methadone hydrochloride) [full prescribing information]. Columbus (OH): Roxane Laboratories, Inc; [cited 2016 Nov 30]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/006134s038lbl.pdf
- US Food and Drug Administration (FDA). What are extended-release and long-acting (ER/LA) opioid analgesics products? [cited 2016 Nov 30]. Available from: http://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=17
- Pergolizzi JV Jr, Ben-Joseph R, Chang CL, et al. US practitioner prescribing practices and patient characteristics of those newly treated with a buprenorphine transdermal patch system. Curr Med Res Opin. 2014;30:1579–1587.
- SAMHSA, Center for Behavioral Health Statistics and Quality. 2014 National survey on drug use and health: detailed tables. Rockville (MD): Substance Abuse and Mental Health Services Administration; 2015 [cited 2016 Nov 30]. Available from: http://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2014/NSDUH-DetTabs2014.pdf