ABSTRACT
Objectives: Osteoarthritis (OA)-related chronic pain is associated with physical and psychosocial impairment as well as poorer quality of life. There is limited literature on long-term opioid therapy in OA patients. This post hoc analysis of OA patients assessed the long-term safety and effectiveness of a once-daily, single-entity, extended-release formulation of hydrocodone (HYD) with abuse-deterrent properties for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which other treatment options are inadequate.
Methods: This is a post hoc analysis of the 307 patients with OA pain from a primary open-label study. Following screening and dose titration, patients who achieved a stable HYD dose continued into a 52-week maintenance period. Supplemental non-opioid or short-acting opioid analgesics were allowed throughout the study. Safety was monitored. Effectiveness evaluations included “average pain over the last 24 hours” scores, “pain right now” scores, Brief Pain Inventory-Short Form and treatment satisfaction questionnaire.
Results: No new or unexpected safety concerns emerged during treatment with HYD. HYD demonstrated a safety profile consistent with other µ-opioid agonists with 22% discontinuations of treatment due to adverse events, a majority of which were related to the study drug. Clinically meaningful analgesia was achieved as mean “average pain over the last 24 hours”; scores decreased by 2.9 points from baseline to the end of maintenance. During the maintenance period, pain severity declined 2.7 points and interference by 2.5 points from baseline. Mean “pain right now” scores were similar at dosing and 12 hours later. A majority of patients reported satisfaction with HYD.
Conclusion: In OA patients, long-term HYD treatment was generally well tolerated and provided clinically important analgesia.
1. Introduction
Osteoarthritis (OA), the most common form of arthritis, develops as a result of complex interactions among biological, biomechanical, and genetic factors [Citation1,Citation2]. Although OA can affect any joint, it usually involves the knees, hips, hands, or spine and manifests as progressive deterioration of articular cartilage, pain, swelling, and reduced mobility of the affected joints [Citation2,Citation3]. The prevalence of OA increases with age; among adults in the United States, OA occurs in 14% of those 25 years or older and in 34% of those 65 years or older [Citation4]. Overall, the prevalence of hand, knee, or hip joint OA in the United States has increased from 21 million adults in 1995 to 27 million adults in 2005 [Citation5].
OA is an important cause of disability and impaired health-related quality of life. It imposes a significant economic burden on patients [Citation6–Citation10]. Estimates indicate that approximately 80% of OA patients have some degree of movement reduction, 25% cannot perform major activities of daily living, and 11% of adults with OA of the knee require assistance with personal care [Citation4]. In particular, OA-related pain is associated with impairment of physical functionality and psychosocial attributes, poorer quality of life, and an increase in symptoms of depression [Citation8,Citation11]. Reducing joint pain is a critical treatment goal for patients with OA [Citation12].
The American College of Rheumatology guidelines recommend the use of acetaminophen, topical or oral nonsteroidal anti-inflammatory drugs, topical capsaicin, corticosteroid injections, or tramadol as first-line pharmacological therapies for pain management of OA patients [Citation13] depending on the affected joint and individual patient characteristics. Other opioid analgesics are not recommended for patients with hand OA but are strongly recommended for patients with knee or hip OA who experience suboptimal pain relief with alternative therapies and who cannot undergo knee or hip arthroplasty [Citation13]. Although the prevalence of long-term opioid treatment for chronic pain – including OA-related pain – has increased [Citation14], few studies have examined the safety and effectiveness of prolonged opioid use in patients with chronic OA-associated pain [Citation15,Citation16].
Hysingla® ER (HYD; Purdue Pharma L.P., Stamford, CT, USA) is a once-daily, extended-release, single-entity formulation of the semisynthetic µ-opioid receptor agonist hydrocodone with abuse-deterrent properties approved for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate [Citation17]. A long-term open-label study demonstrated that treatment with HYD 20–120 mg was associated with improvements in pain relief and functional activities of daily living that were maintained over 52 weeks in patients with moderate-to-severe chronic noncancer and nonneuropathic pain [Citation18]. Data from a subset of patients with OA pain from the primary open-label study was evaluated for safety and effectiveness of HYD.
2. Methods
2.1. Study design and treatment
This report describes a post hoc analysis of data from a multicenter, open-label study (clinicalTrials.gov: NCT01400139) that evaluated the long-term safety and effectiveness of once-daily HYD 20–120 mg in patients with moderate-to-severe chronic noncancer and nonneuropathic pain [Citation18]. Patients experiencing chronic pain related to OA were included in this analysis. Study procedures have been previously described in detail [Citation18].
The primary study was approved by a central institutional review board. All patients provided written informed consent before undergoing any protocol-specific study procedures. The primary study comprised a screening period (≤14 days), a dose-titration period (≤45 days), a maintenance period (52 weeks), and an optional taper period (≤14 days). Patients satisfying study enrollment criteria during screening entered the dose-titration period and had their incoming daily opioid dosages converted to HYD 20, 40, 60, or 80 mg [Citation18]. During the titration period, the HYD dose was adjusted by single-dose-level increments (up to 120 mg) until patients achieved a stable dose, defined as one that provided acceptable tolerability and analgesia for ≥7 days. Patients attaining a stable HYD dose continued into the 52-week maintenance period on that dose. The HYD dose could be adjusted by single-dose-level changes as necessary throughout the maintenance period.
During the titration and maintenance periods, treatment with supplemental short-acting opioids or nonopioid analgesics, but not long-acting or controlled-release opioid formulations, was permitted as necessary.
2.2. Patients
Study participants included adults (≥18 years) with moderate-to-severe chronic noncancer, nonneuropathic pain that was present for at least 3 months prior to the screening visit. At screening, patients were required to have either pain that was controlled by a stable analgesic regimen equivalent to ≤120 mg oxycodone with an ‘average pain over the last 14 days’ score of ≤4 or pain that was uncontrolled with a stable analgesic regimen equivalent to ≤100 mg oxycodone with an ‘average pain over the last 14 days’ score of ≥5.
Pregnant or lactating women were not eligible for study entry. Patients were excluded if they had chronic pain of neuropathic or malignant causes; fibromyalgia; reflex sympathetic dystrophy or causalgia (complex regional pain syndrome); diabetic amyotrophy; meningitis; discitis; uncontrolled gout; pseudogout; psoriatic arthritis; active Lyme disease; rheumatoid arthritis or other inflammatory arthritis; trochanteric bursitis; ischial tuberosity bursitis; or the presence of significant cardiac, pulmonary, neurologic, hepatic, renal, gastrointestinal, or psychiatric conditions.
2.3. Safety assessments
Safety evaluations included monitoring for and recording adverse events (AEs), clinical laboratory assessments, electrocardiogram, physical examination, and vital sign measurement.
2.4. Effectiveness assessments
Study drug effectiveness was evaluated as change from baseline using measures including daily ‘average pain over the last 24 h’ scores reported on an 11-point numerical rating scale (NRS; 0 = no pain and 10 = pain as bad as you can imagine). Other effectiveness measures included ‘pain right now’ scores recorded daily immediately prior to HYD dosing and at approximately 8 pm, the Brief Pain Inventory-Short Form (BPI-SF) recording pain severity and functional pain interference on mood and daily activities, and the Treatment Satisfaction Questionnaire (TSQ) assessing the patient’s experience with the study drug relative to the prestudy pain regimen.
2.5. Statistics
The safety population for this analysis comprised all patients who received at least one dose of study medication and had OA-related pain. No imputation of efficacy data was performed, and no formal statistical hypothesis testing was conducted. Where applicable, safety and effectiveness variables were summarized using mean and standard deviation (SD) or 95% confidence intervals.
3. Results
3.1. Patient characteristics
Among the 922 patients who received HYD 20–120 mg in the open-label primary study [Citation18], 307 (33.3%) with pain etiology or medical history of OA and associated arthralgia and joint pain at screening were included in this analysis. Overall, the mean age of patients in this analysis was 55.9 years, and the mean body mass index was 32.7 kg/m2 (). Joints affected by OA in this study included the knee (43.6%), spine (40.7%), hip (8.5%), shoulder (2.6%), ankle (0.01%), foot (0.01%), and hand (0.01%). In addition to OA-related pain, the most frequently occurring conditions prior to study entry were hypertension (56%), gastroesophageal reflux disease (34%), depression (30%), insomnia (28%), seasonal allergy (24%), anxiety (21%), obesity (19%), hypercholesterolemia (18%), constipation (16%), hyperlipidemia (15%), asthma (14%), type 2 diabetes mellitus (13%), drug hypersensitivity (11%), carpal tunnel syndrome (10%; not a specific exclusion criteria in the protocol), headache (10%), and migraine (10%).
Table 1. Demographic and baseline characteristics of patients with osteoarthritis pain.
On average, patients included in this analysis had their primary pain condition diagnosed 10.7 years (SD, 9.67) prior to study enrollment (). The mean ‘average pain over last 14 days’ score was 6.7 at screening, and the baseline mean ‘average pain over the last 24 h’ score was 6.5. At screening, most patients (59%) were opioid-experienced, and the mean incoming opioid dose for patients with OA was equivalent to 15.5 mg oxycodone. The most commonly used opioid medications prior to study initiation were hydrocodone/acetaminophen (39%), tramadol (13%), oxycodone/acetaminophen (9%), and oxycodone (6%). In addition, 81% of patients reported using nonopioids for analgesia at baseline, of which the most frequently used were ibuprofen (26%), naproxen (19%), and acetaminophen (12%). Other nonopioids used to manage pain such as cyclobenzaprine, meloxicam, celecoxib, bupropion, citalopram, diclofenac, gabapentin, duloxetine, and sertraline were also used individually by ≥5% of patients.
Of the 307 patients included in this analysis, 261 (85%) completed the titration period and 163 (62%) completed the 52-week maintenance period. The most common reasons for treatment discontinuation in this study were AEs (22%), patient’s choice (10%), and lack of therapeutic effect (5%) (). Treatment discontinuation also occurred due to administrative reasons (4%), lost to follow-up (3%), and confirmed or suspected diversion (2%). Two patients (1%) were discontinued from the study as they did not qualify for the maintenance period.
Table 2. Summary of patient disposition and reasons for discontinuation.
3.2. Safety
Opioid-related treatment-emergent AEs (TEAEs) were observed in 66% of patients (). The most commonly reported opioid-related TEAEs were constipation (27%), nausea (25%), somnolence (15%), dizziness (14%), vomiting (12%), and headache (10%). In total, 57 AEs occurring in 26 patients (8.5%) during the dose titration and 57 AEs occurring in 37 patients (14.2%) during the maintenance periods resulted in treatment discontinuation. Of those, 51 patients discontinued secondary to AEs that were deemed by the investigator to be related to study medication. The most common AEs leading to treatment discontinuation during the dose-titration period were nausea (3.6%), vomiting (1.6%), and somnolence (1.3%). For the maintenance period, AEs resulting in treatment discontinuation included nausea (1.9%), vomiting (1.5%), and constipation (1.5%). Among patients who discontinued the titration period due to AEs, HYD 20 mg was administered to 14 patients, 40 mg to 3 patients, 60 mg to 6 patients, 80 mg to 2 patients, and 120 mg to 1 patient at the time of AE onset. Among patients who discontinued the maintenance period due to AEs, 12 patients received HYD 20 mg, 12 patients received 40 mg, 5 patients received 60 mg, 8 patients received 80 mg, and 5 patients received 120 mg at AE onset.
Table 3. Incidence of opioid-related treatment-emergent adverse events (TEAEs).
During the overall study, 43 treatment-emergent serious AEs (SAEs) were reported in 30 (10%) patients (). Of these SAEs, two occurred during the dose-titration period and were considered to be definitely related to the study drug; a severe esophageal obstruction was reported in a patient receiving HYD 20 mg, while medication abuse of moderate severity was documented in a patient receiving HYD 80 mg. Treatment was discontinued, and both patients were withdrawn from the study, while the remaining patients continued with the study. The remaining SAEs occurred during the maintenance period. In total, seven SAEs resulted in treatment discontinuation and study withdrawal during the maintenance period while the rest of the patients continued with the study. These included a severe AE of gastrointestinal bleeding; a severe AE of hypoxia; a severe AE of worsening right hip OA; a severe AE of spontaneous aging hematoma; a severe AE of worsening OA of the right knee; a moderate AE of increased right knee pain; and a mild AE of medication abuse; all of these SAEs were considered unrelated to HYD, except for the one SAE of medication abuse in a patient receiving HYD 40 mg. During the maintenance period, an SAE of oversedation was reported in a patient receiving HYD 60 mg, which was considered possibly related to the study drug. The patient recovered from this SAE following treatment. One patient died due to hypoxia, which was not considered to be treatment related.
Table 4. Incidence of treatment-emergent serious adverse events.
3.3. Effectiveness
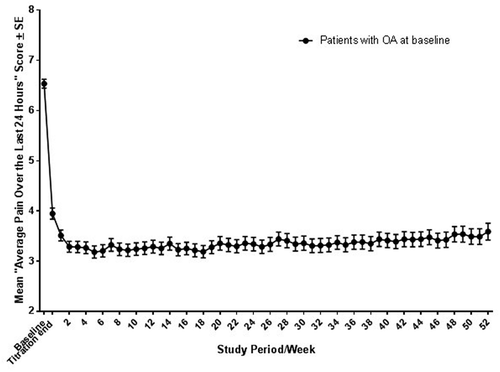
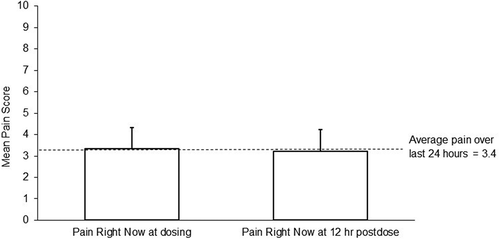
The mean (standard error [SE]) ‘average pain over the last 24 h’ score decreased from 6.5 (0.1) at baseline to 3.9 (0.1) at the end of the titration period (). An additional decrease in mean (SE) ‘average pain over the last 24 h’ scores occurred by the end of the week 1 of the maintenance period (3.5 [0.1]), which persisted for the duration of the 52-week period (final score of 3.6 [0.2]) for those who could continue treatment for 52 weeks. In addition, mean (SE) pain severity, as assessed by BPI-SF, decreased by 2.7 points from 6.17 (0.1) at baseline to 3.5 (0.1) points. Similarly, the mean (SE) pain interference subscale of the BPI-SF declined by 2.54 points from 5.07 (0.1) at baseline to 2.5 (0.1) points for the overall maintenance period. During the maintenance period, mean (SD) ‘pain right now’ scores were comparable at dosing (3.3 [1.8]) and at 12 h post-dose (3.2 [1.9]) ().
3.4. HYD dose adjustments
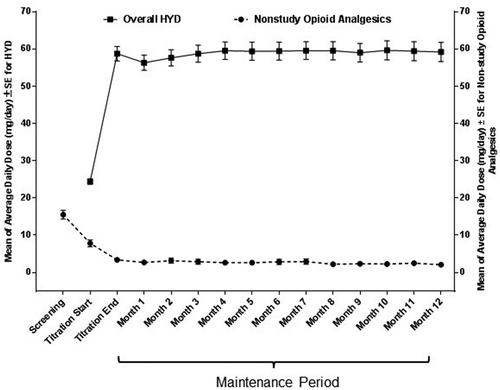
At the end of the titration period, patients were receiving a mean (SE) daily dose of HYD of 58.8 (1.9) mg (). HYD dosage was stable for the duration of the maintenance period, with patients receiving a mean daily dose of 59.2 (2.6) mg at the end of the 52-week maintenance period.
Figure 3. Mean daily dose of HYD and nonstudy opioids during the titration period and the 52-week maintenance period in patients with osteoarthritis-related pain.a HYD: hydrocodone bitartrate; SE: standard error.
aOne patient reported unusually high use of nonstudy opioids (162 mg/day) during month 9 of the Maintenance Period. These data have not been included in this figure.
Nonstudy opioid analgesic dosages are given in oxycodone equivalents.
3.5. Rescue medication
The mean (SE) daily dose of supplemental nonstudy opioid analgesics declined from 7.8 (0.9) mg at the start of the titration period to 3.4 (0.6) mg at the end of the titration, and this decrease persisted throughout the 52-week maintenance period (final dose 2.1 [0.5] mg).
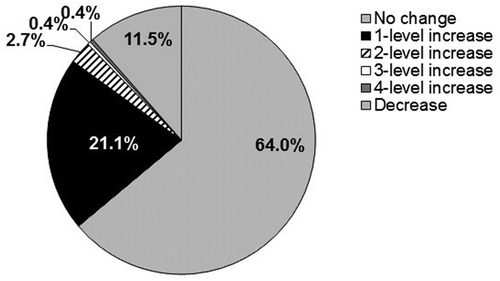
At the start of the maintenance period, 21% of patients received HYD 20 mg, 26% received HYD 40 mg, 18% received HYD 60 mg, 18% received HYD 80 mg, and 17% received HYD 120 mg. Most patients (64%) who entered the maintenance period remained on the same HYD dose for the duration of treatment (), while a dose increase of one level (e.g. HYD dose increase from 60 to 80 mg) was required for 21.1% of patients and of two levels (e.g. HYD dose increase from 60 to 120 mg) or more levels was required for fewer than 4% of patients. HYD dose was decreased for 11.5% of patients during the maintenance period. A similar distribution of dose adjustments was observed among patients who completed 6 and 12 months of maintenance although no dose increases of three or four levels were administered to those completing 12 months of maintenance treatment.
3.6. Treatment satisfaction
Of the 261 patients entering the maintenance period, 203 (22%) patients completed the TSQ, of whom 93% were satisfied with HYD and its ability to manage pain (). Overall, 100% of patients were satisfied with the frequency of HYD use, 99% found it easy to plan HYD use and found HYD convenient to treat pain, and 98% found it easy to use HYD to treat pain.
Table 5. Treatment satisfaction questionnaire.
4. Discussion
We evaluated the safety and effectiveness of long-term therapy with once-daily HYD among patients with moderate-to-severe chronic OA-related pain in this post hoc analysis of patients who experienced adequate pain relief and could tolerate the drug. Treatment with once-daily HYD for 52 weeks was well tolerated and was not associated with unexpected safety concerns among patients with OA. The observed safety profile of HYD was typical of a µ-opioid receptor agonist [Citation19,Citation20]. Although 22% of patients discontinued treatment due to AEs, this proportion was within the range (6.4–25%) reported in previous analyses that evaluated opioid therapy in OA patients for shorter durations (median duration of ≤12 weeks) [Citation21,Citation22].
HYD provided effective and consistent pain control in patients predominantly affected by knee, spine, and hip OA. A clinically meaningful reduction in mean ‘average pain over the last 24 h’ score (≥2 points) [Citation23] was established during the titration period and was sustained throughout the 52-week maintenance period. Similarly, clinically important changes were observed in pain interference scores during this study [Citation24], indicating that analgesia provided by HYD was associated with functional improvements in general activity, walking, work, mood, enjoyment of life, relations with others, and/or sleep. Importantly, the mean daily dose of HYD administered remained stable for the duration of the maintenance period, with the majority of patients not requiring dose adjustments. In addition to the 52-week maintenance period, analgesia provided by HYD was also consistent across the 24-h dosing interval. Furthermore, patients expressed a high degree of satisfaction with HYD for the management of their chronic pain. Cumulatively, these findings indicate that HYD treatment provided effective and sustained pain relief for 52 weeks among patients with OA.
The heterogeneous nature of pain in OA, a complex and diverse condition, underscores the need for an individualized therapeutic approach in patients with OA [Citation8,Citation25]. Optimal pharmacological management of OA-related pain entails achieving effective analgesia while minimizing safety concerns associated with the therapeutic agent. Among those with hip or knee OA, opioids may be considered for the management of pain if the patient does not adequately respond to more conservative pharmacological and nonpharmacological therapies and cannot undergo arthroplasty [Citation13]. Relative to placebo, opioid therapy is associated with a significant reduction in pain intensity and a modest improvement in physical function among patients with OA-related pain [Citation21]. However, few clinical trials have evaluated long-term therapy with hydrocodone in the OA population. This study provides evidence that HYD treatment was well tolerated and resulted in clinically meaningful pain relief that was maintained for 52 weeks in patients with moderate-to-severe OA-related pain. Additionally, these OA patients achieved improvements in functional activities with a stable daily dose of HYD. The results from this study demonstrate that HYD may be suitable for the management of carefully selected patients with moderate-to-severe chronic pain associated with OA.
This analysis is limited by its post hoc nature. In addition, patients were included in this analysis on the basis of their self-reported OA, which was not confirmed by clinical measures. Furthermore, the primary open-label study did not include a control group, thereby precluding comparisons with other treatment modalities. While this study evaluated the effectiveness of HYD at providing analgesia, OA-specific assessments to monitor the severity or progression of OA were not performed. This study did not address correlation of reduction in pain intensity compared with baseline and ability to continue with treatment for those patients. Additional studies will need to address the limitations of this analysis.
5. Conclusion
In summary, HYD had a safety profile similar to that of other µ-opioid analgesics and provided clinically important and stable long-term analgesia for up to 52 weeks in patients with OA. Notably, 52 weeks of HYD treatment were associated with improvements in the functional activities of patients. Thus, HYD therapy may be considered as a treatment option for select patients with moderate-to-severe chronic OA-related pain.
Declaration of interest
L Taber was a consultant for Purdue Pharma L.P. S Ripa, S Baldridge and E He are employees at Purdue Pharma L.P. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386:376–387.
- Loeser RF, Goldring SR, Scanzello CR, et al. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707.
- Taruc-Uy RL, Lynch SA. Diagnosis and treatment of osteoarthritis. Prim Care. 2013;40:821–36, vii.
- Centers for Disease Control and Prevention. Osteoarthritis. 2015 Oct 28 [cited 2016 Jan 17]. Available from: http://www.cdc.gov/arthritis/basics/osteoarthritis.htm
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35.
- Gabriel SE, Crowson CS, Campion ME, et al. Indirect and nonmedical costs among people with rheumatoid arthritis and osteoarthritis compared with nonarthritic controls. J Rheumatol. 1997;24:43–48.
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112:S13–9.
- Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21:1145–1153.
- Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10:437–441.
- Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–1330.
- Bookwala J, Harralson TL, Parmelee PA. Effects of pain on functioning and well-being in older adults with osteoarthritis of the knee. Psychol Aging. 2003;18:844–850.
- Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–162.
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465–474.
- Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18:1166–1175.
- Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276–286.
- Manchikanti L, Ailinani H, Koyyalagunta D, et al. A systematic review of randomized trials of long-term opioid management for chronic non-cancer pain. Pain Physician. 2011;14:91–121.
- HYSINGLATM ER (hydrocodone bitartrate) extended release tablets for oral use, CII [package insert]. Stamford (CT): Purdue Pharma L.P.; 2015.
- Wen W, Taber L, Lynch SY, et al. 12-Month safety and effectiveness of once-daily hydrocodone tablets formulated with abuse-deterrent properties in patients with moderate to severe chronic pain. J Opioid Manag. 2015;11:339–356.
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–20.
- Furlan AD, Sandoval JA, Mailis-Gagnon A, et al. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. Can Med Assoc J. 2006;174:1589–1594.
- Avouac J, Gossec L, Dougados M. Efficacy and safety of opioids for osteoarthritis: a meta-analysis of randomized controlled trials. Osteoarthritis Cartilage. 2007;15:957–965.
- da Costa BR, Nuesch E, Kasteler R, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2014;9:CD003115.
- Farrar JT, Young JP Jr., LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158.
- Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121.
- Mease PJ, Hanna S, Frakes EP, et al. Pain mechanisms in osteoarthritis: understanding the role of central pain and current approaches to its treatment. J Rheumatol. 2011;38:1546–1551.