ABSTRACT
Periodontal diseases, such as chronic periodontitis, share common inflammatory risk factors with other systemic and chronic inflammatory disorders. Mucosal tissues, such as oral epithelia, are exposed to environmental stressors, such as tobacco and oral bacteria, that might be involved in promoting a systemic inflammatory state. Conversely, chronic disorders can also affect oral health. This review will summarize recent evidence for the interrelationship between chronic periodontitis and other prevalent chronic diseases such as cardiovascular diseases, diabetes, cancer and chronic respiratory diseases. The association with pregnancy is also included due to possible obstetric complications. We will focus on inflammatory cytokines such as TNF-alpha, IL-1, and IL-6, because they have been shown to be increased in patients with chronic periodontitis, in patients with chronic systemic diseases, and in patients with both chronic periodontitis and other chronic diseases. Therefore, an imbalance towards a proinflammatory immune response could underline a bidirectional link between chronic periodontitis and other chronic diseases. Finally, we highlight that a close coordination between dental and other health professionals could promote oral health and prevent or ameliorate other chronic diseases.
Introduction
Periodontal disease contributes significantly to the global burden of oral diseases and shares common risk factors with several chronic diseases. Recently, the World Health Organization (WHO) highlighted the importance of strengthening the control of periodontal disease worldwide [Citation1,Citation2]. According to the WHO, chronic noncommunicable diseases, including cardiovascular diseases, cancer, chronic respiratory diseases, and diabetes, remain the leading causes, about 70%, of death globally [Citation3]. In addition, periodontal disease is one of the most important oral diseases contributing to the global burden of chronic diseases and therefore represents a major public health problem [Citation4]. In the present review, we will first summarize the current classification of periodontal diseases, and lately, we will focus the discussion on chronic periodontitis and cytokines in the context of common systemic chronic inflammatory diseases.
Classification of periodontal diseases
Periodontal disease is a broad term for the spectrum of inflammatory diseases affecting the periodontium which comprises a set of structures that support the teeth: gingiva, cementum, periodontal ligament, and alveolar bone. In the 1999 classification system for periodontal diseases and conditions, over 40 different gingival diseases were listed, that were either dental-plaque induced or not associated with dental plaque [Citation5]. In addition, other major categories of destructive periodontal diseases were listed and periodontitis can also be a manifestation of systemic diseases. An abbreviated version of the 1999 classification of periodontal diseases and conditions is shown in . Chronic periodontitis refers to the progression of the disease over time without treatment while aggressive periodontitis shows rapid attachment loss and bone destruction, and possible familial aggregation of disease. According to that consensus, both are subcategorized in local or generalized forms, depending on the percentage of tooth affected sites (above or below 30%) and regarding the severity of attachment loss (slight: 1 or 2 mm, moderate: 3 or 4 mm; severe ≥5 mm) [Citation5,Citation6]. The distinction between chronic and aggressive periodontitis is also based on several clinical features, namely age of onset, rates of progression, patterns of destruction, signs of inflammation, and relative amounts of plaque, and calculus [Citation7]. However, the features of chronic periodontitis have been partially updated in 2014 by the American Academy of Periodontology (AAP) who have also announced that an update would commence in 2017 [Citation8]. This AAP task force report addressed three specific areas of concern with the current classification: attachment level, chronic versus aggressive periodontitis, and localized versus generalized periodontitis [Citation8]. Accordingly, diagnosis of periodontitis is based on multiple clinical and radiographic parameters (), all of which may not be required. In general, patients would have periodontitis when one or more sites had inflammation (bleeding on probing, BOP), radiographic bone loss, and increased probing depth (>3 mm) or clinical attachment loss (CAL ≥1 mm) [Citation8].
Table 2. Guidelines for determining severity of periodontitis [Citation8].
Patients with gingival recession or on periodontal maintenance therapy could present attachment loss, probing depth ≤3 mm, but with no signs of inflammation (no bleeding on probing). These patients should be diagnosed with a healthy but reduced periodontium [Citation8]. However, if inflammation (bleeding on probing) is present, the diagnosis should be a reduced periodontium with inflammation, i.e. gingivitis. However, in patients with probing depth >3 mm and with inflammation, the diagnosis should be periodontitis with severity according to guidelines shown in [Citation8]. This report recognized some features already published in 1999 consensus report that would help to differentiate chronic versus aggressive periodontitis. However, the clinician should base diagnostic decisions based on the patient history, clinical and radiographic signs (see ref 8). Regarding generalized chronic periodontic, AAP task force preferred the percentage of 30% affected teeth, instead of affected sites as an extent descriptor [Citation8]. Nevertheless, as mentioned above, a comprehensive update is planned for the current year (2017).
Chronic inflammation and cytokines in chronic periodontitis
Chronic inflammation is a complex biological process that occurs in response to infection and/or other triggers and leads to tissue injury [Citation9]. Chronic periodontitis is a periodontal disease characterized by both dysbiosis of oral microbiota and proinflammatory events involving both cells and mediators from innate and adaptive immunity [Citation10]. These events lead to chronic inflammation of periodontal soft and hard tissues sharing many features with other chronic inflammatory diseases [Citation11,Citation12]. Chronic inflammation is driven by various mediators, of which an important part is attributed to the interactions within cytokine networks. While proinflammatory cytokines, including IL-1α, IL-1β, TNF-α, IL-6, and IL-17 contribute to acute and chronic inflammation and tissue injury, a second group with antagonist effects is formed by cytokines such as IL-10 [Citation13].
Chronic periodontitis is characterized by deregulated inflammatory interactions, involving both innate and adaptive responses, that lead to a chronic inflammation in periodontal tissues [Citation14]. As other mucosal surfaces, the periodontal epithelium is located at the interface between outside body environment and inside underlying connective tissue (). From outside, it is firmly established that the principal trigger of chronic periodontitis is the presence of dysbiotic microbial communities with potential for destructive inflammation. However, in addition to periodontal microbiome influence, smoke is also an important factor underlying the development of periodontitis [Citation15]. Other environmental stressors, either chemical or mechanical, that cause or promote oral inflammation, could have a role in the development of chronic periodontitis. This is still an unexplored research area and future investigation is needed to identify other relevant etiological factors. In the inside of the epithelial barrier, host genetic factors may predispose to or protect from disease. Finally, systemic health status and environmental factors that modify the host response have also been implicated in chronic periodontitis [Citation15]. Overall, both environmental and host proinflammatory events favor a chronic state of inflammation in the periodontal tissues, that lead to injury and ultimately to bone resorption and tooth loss.
Figure 1. A conceptual framework for chronic inflammation in periodontal tissues.
Environmental stressors, (a), and host responses, (b), contribute to periodontal chronic inflammation. Several cellular and molecular factors are responsible for an imbalance towards a proinflammatory rather than anti-inflammatory immune response, that culminates into tissue injury. A bidirectional link with other systemic inflammatory diseases may operate and need to be further investigated.
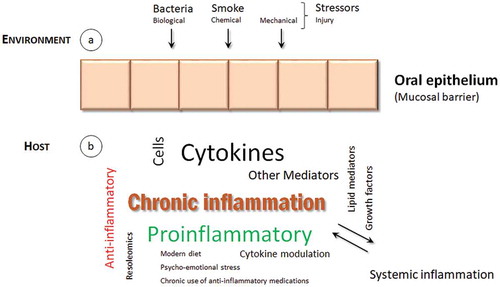
Extensive and detailed reviews on molecular and cellular aspects of cytokines in periodontitis have been published [Citation13,Citation14,Citation16]. Cytokines might be divided into proinflammatory or anti-inflammatory groups and imbalances between their relative concentrations might lead to tissue destruction in chronic periodontitis [Citation13,Citation14]. Other mediators such as lipid mediators, growth factors, or chemokines have also an important role in the perpetuation of inflammation [Citation17,Citation18].
Thus, chronic periodontitis is characterized by a persistent inflammation of the periodontal tissues and is now considered a chronic noncommunicable disease that shares epidemiological associations and underlying inflammatory mechanisms with other systemic chronic inflammatory diseases [Citation18–Citation20]. Several potential therapies, ranging from diet to cytokines, capable of suppressing inflammation warrant to for further investigations () [Citation17,Citation21,Citation22]. Cytokine-based treatment strategies have potential to improve both chronic periodontitis and systemic health [Citation23–Citation26]. However, given the complexity of cytokine networks, we believe that it is fundamental to investigate further their role in the chronic periodontitis disease process. The understanding of the equilibrium between inflammatory (proinflammatory) and suppressor (anti-inflammatory) responses, integrated with both internal and external environmental factors, could bring improved therapeutic interventions to modulate host responses in chronic periodontitis and other chronic inflammatory diseases.
Chronic periodontitis and systemic diseases
The evolution of healthcare in the last century has led to improvement in the treatment of several diseases, leading to an increased quality and life expectancy of patients. Dental care and oral health is also a key factor involved in the improvement of patients’ health. Indeed, in the recent Tokyo Declaration on dental care and oral health for healthy longevity [Citation27], it was recognized that maintenance of oral and dental health through life is a fundamental factor for improved quality of life, helping to protect against noncommunicable diseases and contributing toward preventing the aggravation of such diseases. Dental associations and other health professionals around the world are encouraged to facilitate and enhance coordination of activities to increase global awareness of and contribute to the implementation of WHO’s Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020 [Citation27]. Periodontal and systemic diseases share many features of inflammaging and seem to be intertwined diseases [Citation28,Citation29].
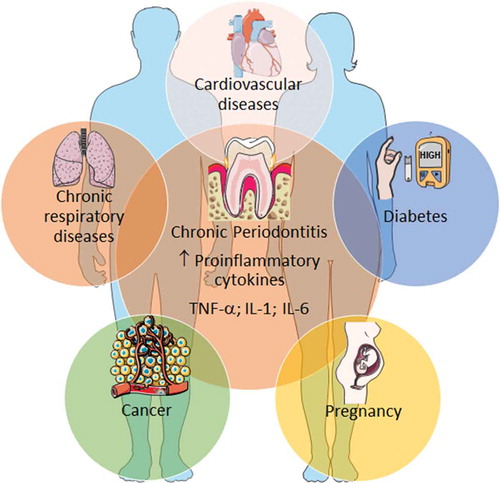
Hence, the association between chronic periodontitis and systemic illnesses may be bidirectional. One mechanism that has been proposed for chronic periodontitis as a risk factor for other systemic diseases, comprises systemic dissemination of oral bacteria and inflammatory mediators, that are capable of initiation and maintaining mechanisms associated with the development of chronic systemic diseases [Citation15]. Although several systemic diseases (e.g. rheumatoid arthritis, renal diseases, neurodegenerative diseases) show associations with chronic periodontitis, in this review we will focus on the most prevalent noncommunicable diseases (cardiovascular, diabetes, cancer, and respiratory disease) and on pregnancy complications. Moreover, we will also give emphases to the proinflammatory common systemic mediators such as TNF-α, IL-1 and/or IL-6. Interestingly, we have shown that these cytokines were increased in gingival crevicular fluid in patients with chronic periodontitis who did not have chronic systemic diseases (, chronic periodontitis at the center) [Citation30].
Figure 2. Chronic periodontitis and some common systemic diseases.
Chronic periodontitis shares several associations with numerous chronic inflammatory diseases. Proinflammatory cytokines are, among other factors, important immune-related mediators involved in the disease pathogenesis. Only the most common noncommunicable diseases and pregnancy complications were exemplified, although others (rheumatoid arthritis, neurodegenerative disorders, etc.) exhibit also associations with chronic periodontitis. Illustration adapted medical elements from Servier Medical Art http://smart.servier.com/, with permission to reproduce under the Creative Commons Attribution 3.0 Unported License https://creativecommons.org/licenses/by/3.0/
Chronic periodontitis and cardiovascular disease
In a recent report of the Joint EFP/AAP workshop on periodontitis and systemic diseases, it was concluded that there is a consistent and strong epidemiologic evidence that periodontitis imparts increased risk for future cardiovascular disease [Citation31]. It was also concluded that although in vitro, animal and clinical studies do support interaction and biological mechanisms, still, well-design intervention trials to investigate the impact of periodontal treatment on prevention of atherosclerotic cardiovascular disease, are a needed.
Vascular diseases are nowadays recognized to have a strong local and systemic inflammatory pathogenic contribution. In atherosclerosis, along with the accumulation of cholesterol debris on the artery wall, it is well established that immune reactions and the participation cells and mediators such as cytokines are implicated in the immunopathogenesis of vascular diseases [Citation32]. Among several factors, inflammatory cytokines such as TNF-α, IL-1, and IL-6, have been shown to be secreted by infiltrating leukocytes or by foam cells [Citation33]. Parallelly, several investigations have demonstrated the importance of chronic infections in the atherosclerotic process, namely by inducing a systemic inflammatory state and of autoimmunity [Citation33–Citation36]. Some microbes can cause persistent infection in the vessel wall and promote a proinflammatory environment. Alternatively, the infection may also induce autoimmunity against vascular cells and lead to atherosclerotic process [Citation37]. In this context, chronic periodontitis and its chronic inflammatory nature are associated with an increased risk for cardiovascular disease [Citation38]. Bacteremia and an inflammatory systemic state associated with chronic periodontitis are important factors in the initiation of the endothelial lesion as well as in the potentiation of the vascular wall inflammatory process [Citation39–Citation41]. The inflammatory process is also modulated by proinflammatory cytokines such as TNF-α, IL-1, and IL-6 both in chronic periodontitis and in cardiovascular diseases () [Citation42]. Finally, some studies demonstrate a decrease on systemic inflammatory biomarkers following periodontal treatment and consequent possible benefit in the reduction of cardiovascular risk [Citation43–Citation46]. However, while there is evidence suggesting that periodontal therapy reduces systemic inflammation and improves endothelial function, evidence on its effects on cardiovascular events in long term is still limited [Citation43] and further studies are needed. Accordingly, a recent publication of American Heart Association (AHA) stated that although periodontal interventions result in a reduction in systemic inflammation and endothelial dysfunction in short-term studies, there is no evidence that they prevent atherosclerotic vascular disease or modify its outcomes [Citation47].
Chronic periodontitis and diabetes
Association between chronic periodontitis and diabetes is known for a long time, and it has a bidirectional association. On one hand, diabetes is a modifier factor of chronic periodontitis, and, on the other hand, periodontitis is a complication of diabetes [Citation42,Citation48,Citation49]. Several studies showed a higher prevalence of periodontitis in diabetic patients, as well as a higher periodontal disease severity in diabetic patients [Citation50–Citation52]. The pathophysiological mechanisms involved appear to be a result of chronic hyperglycemia resulting in decreased macrophage and neutrophil function, accumulation of advanced glycosylation, and inflammation [Citation53]. In contrast, there is also evidence that concomitant presence of periodontitis impairs glycemic control in diabetics, thereby increasing the risk of other complications in diabetes [Citation54–Citation56]. Proinflammatory mediators, such as TNF-α, IL-1, and IL-6 that are increased in both diseases, represent a fundamental linkage () [Citation52,Citation53,Citation57]. In addition, the interindividual variability in both diseases is likely to be influenced by genetic, epigenetic, and environmental factors [Citation52].
Periodontal treatment has been shown to be associated with an improved glycemic control [Citation58–Citation60]. However, in spite of a modest reduction in HbA1c observed as a result of periodontal therapy in subjects with type 2 diabetes, there is limited confidence in the conclusion due to a lack of multicenter trials of sufficient sample size [Citation60,Citation61].
Chronic periodontitis and obstetric complications
Maternal infections associated with systemic inflammatory states, where we may include chronic periodontitis, appear, according to the literature, to contribute to a higher risk of obstetric complications, such as premature births and low birth weight. During pregnancy, the hormonal changes increase vascular permeability in the gingival tissues that facilitate the diffusion of pathogenic microorganisms and their products into the circulation and to the placenta. Here, they may stimulate immune and inflammatory responses leading to a high secretion of proinflammatory cytokine levels in the fetal tissues and can lead to the premature rupture of membranes and lead to uterine contractions, which in turn can cause miscarriage or premature delivery [Citation62]. Several meta-analysis and systematic reviews reaffirm the association of periodontitis with this complication [Citation62–Citation66]. One of the mechanisms implicated may be mediated by elevated levels of proinflammatory cytokines () [Citation62,Citation67–Citation69].
In addition, some studies have also demonstrated the presence of periodontal pathogenic microorganisms in the amniotic fluid in women with periodontitis, and a correlation between the severity of periodontitis and the risk of preterm birth was found [Citation62,Citation70,Citation71].
Several studies have sought to determine if there is a benefit of periodontal treatment in women at risk for obstetric complications, and although there is no consensus among the various studies [Citation72], it seems to be good practice to apply preventive strategies in these women [Citation73–Citation75].
Chronic periodontitis and cancer
An increased risk of various neoplasms has been associated with chronic periodontitis, namely oral cancers [Citation76] and squamous cell carcinoma [Citation77]. However, a deficient control of variables such as the history of tobacco and alcohol use, and oral human papillomavirus infection limits these results [Citation78]. There is also an increasing interest in the link between periodontal disease and overall cancer risk, with systemic inflammation serving as the focus for biological plausibility. In a recent meta-analysis provided support for a positive association between periodontal disease and risk of oral, lung, and pancreatic cancers. However, future studies should include sufficiently large sample sizes, improved measurements for periodontal disease, and thorough adjustment for smoking and other risk factors [Citation79]. Interestingly, currently, there is a large body of evidence demonstrating that inflammation profoundly affects all phases of cancer, from initiation at the single-cell level, to early growth, progression, and dissemination [Citation80,Citation81]. Another emerging concept is that cancer, like most diseases, is a systemic rather than a local disease. Systemic inflammation in a functional relationship with energy metabolism and genetic instability predisposes individuals to cancer and regulates the neoplastic disease [Citation80]. Cytokines that are known to be elevated in chronic periodontitis, such as TNF-α, IL-1, and IL-6, have been shown to promote breast cancer and other cancers () [Citation81,Citation82].
Periodontitis and chronic respiratory diseases
There is emerging evidence for an association between periodontal disease and pneumonia or chronic obstructive pulmonary disease (COPD). The oral cavity is a reservoir for pulmonary infection and there is some evidence that chronic periodontitis may be associated with risk for pneumonia [Citation83–Citation85]. It is likely that oral bacteria present in the dental plaque are shed into saliva and are then aspirated into the lower respiratory tract and the lungs to cause infection. Additionally, cytokines and enzymes induced from the periodontally inflamed tissues by the oral biofilm may also be transferred into the lungs where they may stimulate local inflammatory processes preceding colonization of pathogens [Citation86]. Several works demonstrated that application of strategies to reduce oral microbial burden are associated with a lower risk to develop pneumonia [Citation83,Citation87,Citation88]. COPD is a group of diseases characterized by the pathological limitation of airflow in the airway, including chronic bronchitis, and emphysema [Citation89]. COPD and chronic periodontitis share common risk factors such as cigarette smoke, age, and poor socioeconomic status [Citation90]. Moreover, some studies supported an association between chronic periodontitis and COPD although large-scale prospective epidemiological studies are needed [Citation78,Citation91]. COPD and chronic periodontitis share some proinflammatory mediators that may facilitate disease progression of both pathologies () [Citation90,Citation92]. Interestingly, in a pilot trial, it has been shown that periodontal therapy in COPD patients with chronic periodontitis may improve lung function and decrease the frequency of COPD exacerbation [Citation93].
Conclusion
Like to other human bodyborders, such as the gut, maintaining a healthy oral mucosa may also have a positive impact on the host health and might be preventive for other systemic inflammatory disorders. Thus, it is important to encourage further integrated studies to investigate the interrelationship between inflammation, oral mucosal, and systemic chronic diseases.
Declaration of interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Petersen PE, Ogawa H. Strengthening the prevention of periodontal disease: the WHO approach. J Periodontol. 2005;76(12):2187–2193.
- Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000. 2012;60(1):15–39.
- WHO 2015. Assessing national capacity for the prevention and control of Noncommunicable diseases: report of the 2015 global survey. WHO. ISBN 978 92 4 156536 3.
- Petersen PE, Baehni PC. Periodontal health and global public health. Periodontol 2000. 2012;60(1):7–14.
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4(1):1–6.
- Wiebe CB, Putnins EE. The periodontal disease classification system of the American academy of periodontology–an update. J Can Dent Assoc. 2000;66(11):594–597.
- Armitage GC, Cullinan MP, Seymour GJ. Comparative biology of chronic and aggressive periodontitis: introduction. Periodontol 2000. 2010;53:7–11.
- AAP, American Academy of Periodontology Task Force. American academy of periodontology task force report on the update to the 1999 classification of periodontal diseases and conditions. J Periodontol. 2015;86(7): 835–838. DOI:10.1902/jop.2015.157001
- Monti D, Ostan R, Borelli V, et al. Inflammaging and human longevity in the omics era. Mech Ageing Dev. 2017;165(Pt B):129–138.
- Yucel-Lindberg T, Båge T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev Mol Med. 2013;15:e7.
- Kramer CD, Genco CA. Microbiota, immune subversion, and chronic inflammation. Front Immunol. 2017;8:255.
- Kornman KS, Giannobile WV, Duff GW. Quo vadis: what is the future of periodontics? How will we get there? Periodontol 2000. 2017;75(1):353–371.
- Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89(12):1349–1363.
- Liu YC, Lerner UH, Teng YT. Cytokine responses against periodontal infection: protective and destructive roles. Periodontol 2000. 2010;52:163–206.
- Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44.
- Preshaw PM, Taylor JJ. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J Clin Periodontol. 2011;38:60–84.
- Bosma-Den Boer MM, Van Wetten ML, Pruimboom L. Chronic inflammatory diseases are stimulated by current lifestyle: how diet, stress levels and medication prevent our body from recovering. Nutr Metab (Lond). 2012;9:32.
- Van Dyke TE, Van Winkelhoff AJ. Infection and inflammatory mechanisms. J Clin Periodontol. 2013;40:S1–7.
- Chapple I, Wilson N. Chronic non-communicable diseases. Br Dent J. 2014;216:487.
- Rettori E, De Laurentiis A, Dees WL, et al. Host neuro-immuno-endocrine responses in periodontal disease. Curr Pharm Des. 2014;20:4749–4759.
- Sculley DV. Periodontal disease: modulation of the inflammatory cascade by dietary n-3 polyunsaturated fatty acids. J Periodontal Res. 2014;49:277–281.
- Kinane DF, Preshaw PM, Loos BG. Working Group 2 of Seventh European Workshop on Periodontology. Host-response: understanding the cellular and molecular mechanisms of host-microbial interactions-consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38:44–48.
- Garlet GP, Sfeir CS, Little SR. Restoring host-microbe homeostasis via selective chemoattraction of Tregs. J Dent Res. 2014;93(9):834–839.
- Azodi S, Jacobson S. Cytokine therapies in neurological disease. Neurotherapeutics. 2016;13(3):555–561.
- Drutskaya MS, Efimov GA, Kruglov AA, et al. Can we design a better anti-cytokine therapy? J Leukoc Biol. 2017;102(3):783–790.
- Kirichenko TV, Sobenin IA, Nikolic D, et al. Anti-cytokine therapy for prevention of atherosclerosis. Phytomedicine. 2016;23(11):1198–1210.
- Tokyodeclaration_final. 2015. [cited 2017 Jun 05]. Available from: http://www.who.int/oral_health/tokyodeclaration_final.pdf?ua=1
- Ebersole JL, Graves CL, Gonzalez OA, et al. Aging, inflammation, immunity and periodontal disease. Periodontol 2000. 2016;72(1):54–75.
- Franceschi C, Garagnani P, Vitale G, et al. Inflammaging and ‘Garb-aging’. Trends Endocrinol Metab. 2017;28(3):199–212.
- Reis C, Da Costa AV, Guimarães JT, et al. Clinical improvement following therapy for periodontitis: association with a decrease in IL-1 and IL-6. Exp Ther Med. 2014;8(1):323–327.
- Tonetti MS, Van Dyke TE. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP workshop on periodontitis and systemic diseases. J Periodontol. 2013;84(4Suppl):S24–29.
- Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. 2015;116(2):307–311.
- Nguyen CM, Kim JW, Quan VH, et al. Periodontal associations in cardiovascular diseases: the latest evidence and understanding. J Oral Biol Craniofac Res. 2015;5(3):203–206.
- Mayr M, Kiechl S, Mendall MA, et al. Increased risk of atherosclerosis is confined to CagA-positive Helicobacter pylori strains: prospective results from the Bruneck study. Stroke. 2003;34(3):610–615.
- Kiechl S, Egger G, Mayr M, et al. Chronic infections and the risk of carotid atherosclerosis: prospective results from a large population study. Circulation. 2001;103(8):1064–1070.
- Matsuura E, Kobayashi K, Matsunami Y, et al. Autoimmunity, infectious immunity, and atherosclerosis. J Clin Immunol. 2009;29(6):714–721.
- Leinonen M, Saikku P. Evidence for infectious agents in cardiovascular disease and atherosclerosis. Lancet Infect Dis. 2002;2(1):11–17.
- Spahr A, Klein E, Khuseyinova N, et al. Periodontal infections and coronary heart disease: role of periodontal bactéria and importance of total pathogen burden in the Coronary Event and Periodontal Disease (CORODONT) study. Arch Intern Med. 2006;166(5):554–559.
- Boillot A, Demmer RT, Mallat Z, et al. Periodontal microbiota and phospholipases: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Atherosclerosis. 2015;242(2):418–423.
- Mustapha IZ, Debrey S, Oladubu M, et al. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: a systematic review and meta-analysis. J Periodontol. 2007;78(12):2289–2302.
- Kebschull M, Demmer RT, Papapanou PN. “Gum bug, leave my heart alone!”–epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res. 2010;89(9):879–902.
- Cullinan MP, Seymour GJ. Periodontal disease and systemic illness: will the evidence ever be enough? Periodontol 2000. 2013;62(1):271–286.
- D’Aiuto F, Orlandi M, Gunsolley JC. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J Periodontol. 2013;84(4Suppl):S85–S105.
- Seinost G, Wimmer G, Skerget M, et al. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am Heart J. 2005;149(6):1050–1054.
- Elter JR, Hinderliter AL, Offenbacher S, et al. The effects of periodontal therapy on vascular endothelial function: a pilot trial. Am Heart J. 2006;151(1):47.
- Tonetti MS. Periodontitis and risk for atherosclerosis: an update on intervention trials. J Clin Periodontol. 2009;36(Suppl 10):15–19.
- Lockhart PB, Bolger AF, Papapanou PN, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012;125(20):2520–2544.
- Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7(12):738–748.
- Chapple IL, Genco R. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. Working group 2 of joint EFP/AAP workshop J Clin Periodontol. 2013;40Suppl 14:S106–112. DOI:10.1111/jcpe.12077
- Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim). 2017;11(2):72–80.
- Khader YS, Dauod AS, El-Qaderi SS, et al. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications. 2006;20(1):59–68.
- Taylor JJ, Preshaw PM, Lalla E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. 2013;40(Suppl 14):S113–134.
- Preshaw PM, Foster N, Taylor JJ. Cross-susceptibility between periodontal disease and type 2 diabetes mellitus: an immunobiological perspective. Periodontol. 2000;2007(45):138–157.
- Saremi A, Nelson RG, Tulloch-Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28(1):27–32.
- Shultis WA, Weil EJ, Looker HC, et al. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30(2):306–311.
- Borgnakke WS, Anderson PF, Shannon C, et al. Is there a relationship between oral health and diabetic neuropathy? Curr Diab Rep. 2015;15(11):93.
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107.
- Darré L, Vergnes JN, Gourdy P, et al. Efficacy of periodontal treatment on glycaemic control in diabetic patients: a meta-analysis of interventional studies. Diabetes Metab. 2008;34(5):497–506.
- Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):421–427.
- Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. Periodontol. 2013;84(4 Suppl):S153–69.
- Simpson TC, Weldon JC, Worthington HV, et al. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2015;(11):CD004714. DOI:10.1002/14651858.CD004714.pub3
- Madianos PN, Bobetsis YA, Offenbacher S. Adverse pregnancy outcomes (APOs) and periodontal disease: pathogenic mechanisms. J Periodontol. 2013;84(4 Suppl):S170–80.
- Vergnes JN, Sixou M. Preterm low birth weight and maternal periodontal status: a meta-analysis. Am J Obstet Gynecol. 2007;196(2):135.e1–7.
- Xiong X, Buekens P, Goldenberg RL, et al. Optimal timing of periodontal disease treatment for prevention of adverse pregnancy outcomes: before or during pregnancy? Am J Obstet Gynecol. 2011;205(2):111.e1–6.
- Chambrone L, Pannuti CM, Guglielmetti MR, et al. Evidence grade associating periodontitis with preterm birth and/or low birth weight: II: a systematic review of randomized trials evaluating the effects of periodontal treatment. J Clin Periodontol. 2011;38(10):902–914.
- Ide M, Papapanou PN. Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes–systematic review. J Periodontol. 2013;84(4Suppl):S181–94.
- Silver RM, Schwinzer B, McGregor JA. Interleukin-6 levels in amniotic fluid in normal and abnormal pregnancies: preeclampsia, small-for-gestational-age fetus, and premature labor. Am J Obstet Gynecol. 1993;169(5):1101–1105.
- Heyborne KD, Witkin SS, McGregor JA. Tumor necrosis factor-alpha in midtrimester amniotic fluid is associated with impaired intrauterine fetal growth. Am J Obstet Gynecol. 1992;167(4 Pt 1):920–925.
- Cappelletti M, Della Bella S, Ferrazzi E, et al. Inflammation and preterm birth. J Leukoc Biol. 2016;99(1):67–78.
- Bearfield C, Davenport ES, Sivapathasundaram V, et al. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. Bjog. 2002;109(5):527–533.
- Lin D, Moss K, Beck JD, et al. Persistently high levels of periodontal pathogens associated with preterm pregnancy outcome. J Periodontol. 2007;78(5):833–841.
- Michalowicz BS, Gustafsson A, Thumbigere-Math V, et al. The effects of periodontal treatment on pregnancy outcomes. J Periodontol. 2013;84(4Suppl):S195–208.
- George A, Ajwani S, Bhole S, et al. Knowledge, attitude and practises of dentists towards oral health care during pregnancy: a cross sectional survey in New South Wales, Australia. Aust Dent J. 2017. DOI:10.1111/adj.12505
- Kim AJ, Lo AJ, Pullin DA, et al. Scaling and root planing treatment for periodontitis to reduce preterm birth and low birth weight: a systematic review and meta-analysis of randomized controlled trials. J Periodontol. 2012 Mar 1;83(12):1508–1519. DOI:10.1902/jop.2012.110636
- Sanz M, Kornman K. Periodontitis and adverse pregnancy outcomes: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. working group 3 of the joint EFP/AAP workshop J Periodontol. 2013;844 Suppl:S164–169. DOI:10.1902/jop.2013.1340016
- Tezal M, Sullivan MA, Reid ME, et al. Chronic periodontitis and the risk of tongue cancer. Arch Otolaryngol Head Neck Surg. 2007;133(5):450–454.
- Tezal M, Sullivan MA, Hyland A, et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18(9):2406–2412.
- Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Clin Periodontol. 2013;40(Suppl 14):S8–19.
- Michaud DS, Fu Z, Shi J, et al. Periodontal disease, tooth loss, and cancer risk. Epidemiol Rev. 2017;39(1):49–58.
- Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706.
- Inácio Pinto N, Carnier J, Oyama LM, et al. Cancer as a proinflammatory environment: metastasis and Cachexia. Mediators Inflamm. 2015;2015:791060.
- Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr Drug Targets. 2010;11(9):1133–1146.
- Scannapieco FA, Cantos A. Oral inflammation and infection, and chronic medical diseases: implications for the elderly. Periodontol 2000. 2016;72(1):153–175.
- De Melo Neto JP, Melo MS, Dos Santos-Pereira SA, et al. Periodontal infections and community-acquired pneumonia: a case-control study. Eur J Clin Microbiol Infect Dis. 2013;32(1):27–32.
- Terpenning MS, Taylor GW, Lopatin DE, et al. Aspiration pneumonia: dental and oral risk factors in an older veteran population. J Am Geriatr Soc. 2001;49(5):557–563.
- Paju S, Scannapieco FA. Oral biofilms, periodontitis, and pulmonary infections. Oral Dis. 2007;13(6):508–512.
- Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006;77(9):1465–1482.
- Sjögren P, Wårdh I, Zimmerman M, et al. Oral care and mortality in older adults with pneumonia in hospitals or nursing homes: systematic review and meta-analysis. J Am Geriatr Soc. 2016;64(10):2109–2115.
- Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8(3):183–192.
- Hobbins S, Chapple IL, Sapey E, et al. Is periodontitis a comorbidity of COPD or can associations be explained by shared risk factors/behaviors? Int J Chron Obstruct Pulmon Dis. 2017;12:1339–1349. eCollection 2017. DOI:10.2147/COPD.S127802
- Chung JH, Hwang HJ, Kim SH, et al. Associations between periodontitis and chronic obstructive pulmonary disease: the 2010 to 2012 Korean National Health and Nutrition Examination Survey. J Periodontol. 2016;87(8):864–871.
- Öztekin G, Baser U, Kucukcoskun M, et al. The association between periodontal disease and chronic obstructive pulmonar disease: a case control study. Copd. 2014;11(4):424–430.
- Zhou X, Han J, Liu Z, et al. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2-year pilot randomized controlled trial. J Clin Periodontol. 2014;41:564–572.
