ABSTRACT
In patients with type 2 diabetes (T2D), microvascular changes in the kidney often result in diabetic kidney disease (DKD), the progression of which is associated with an increased risk of cardiovascular (CV) and all-cause mortality. Sodium-glucose cotransporter-2 inhibitors (SGLT-2is) are a newer class of oral glucose-lowering therapies that were associated with significant reductions in the risk of major adverse CV events, CV death, and hospitalization for heart failure compared with placebo in CV outcomes trials (CVOTs) of patients with T2D and established CV disease or varying levels of CV risk. In addition, SGLT-2is reduced the risks of clinically relevant renal outcomes in these large randomized studies, indicating the potential for renoprotective effects in patients with T2D and DKD. This review discussed the non-glycemic effects of SGLT-2is in patients with T2D and renal impairment, including reductions in systolic and diastolic blood pressure, decreases in albuminuria and plasma uric acid, changes in estimated glomerular filtration rate, and minimal changes in electrolytes. Potential mechanisms for the renoprotective effects of SGLT-2is observed in CVOTs were considered, including the likely incremental benefits of SGLT-2is when added to renin-aldosterone-angiotensin system inhibitors (RAASis). The possibility of extending the use of SGLT-2is to patients with non-DKD was also discussed. Although the exact mechanisms by which SGLT-2is improve renal outcomes are not fully understood, they are likely to be multifactorial and additive when these drugs are used in combination with RAASis in patients with DKD.
1. Introduction
In patients with type 2 diabetes (T2D), chronic hyperglycemia is associated with macrovascular (including cardiovascular [CV]) and microvascular (i.e. nephropathy, retinopathy, and neuropathy) complications [Citation1]. Microvascular changes in the kidney often lead to diabetic kidney disease (DKD), during which the activation of the renin-angiotensin-aldosterone system (RAAS) results in efferent arteriolar vasoconstriction and glomerular hyperfiltration [Citation2,Citation3]. Progression of DKD is associated with an increased risk of CV disease (CVD), with reductions in glomerular filtration rate (GFR) and increases in albuminuria both being independently and additively associated with increases in CV and all-cause mortality [Citation4]. Indeed, the prevalence of end-stage renal disease (ESRD) among patients with DKD may be limited by the competing risk of CV death [Citation5]. In an analysis of data from the Third National Health and Nutrition Examination Survey, cumulative 10-year all-cause mortality increased from approximately 12% among patients with T2D without DKD to 31% in those with DKD, and 10-year CV mortality increased from 7% among patients with T2D without DKD to 20% in those with DKD [Citation6]. Therefore, prevention of DKD progression is vital for decreasing CV mortality and avoiding ESRD development in patients with T2D [Citation2].
Two large studies of patients with T2D and nephropathy previously showed renoprotective effects with RAAS inhibitors (RAASis) [Citation7,Citation8]. In the Reduction of Endpoints in Non-Insulin Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan (RENAAL) study, the risk of doubling of serum creatinine was significantly reduced by 25% and ESRD by 28% with losartan (an angiotensin II receptor blocker [ARB]) compared with placebo [Citation7]. Similarly, the Irbesartan Diabetic Nephropathy Trial (IDNT) showed significant reductions in the risk of doubling of serum creatinine (by 33% to 37%) and ESRD (by 23%) with irbesartan (an ARB) compared with placebo or amlodipine (a calcium channel blocker) [Citation8]. Both studies also showed a reduction in proteinuria from baseline of 33% to 35% with RAASi therapy [Citation7,Citation8]. In addition to the improvements in renal outcomes with RAASis in these studies, both losartan and irbesartan were associated with a significant reduction in the rate of first hospitalization for heart failure (HF), although the risks of CV or all-cause mortality were not reduced [Citation7–Citation9]. This may indicate that RAASi therapy provides clinical benefits with regard to some CV events. Larger, longer-term studies of RAASi therapies may be required to demonstrate an association between their renoprotective effects and CVD morbidity and mortality benefits in patients with DKD.
As ARBs and angiotensin-converting enzyme inhibitors (ACEis) target different components of the RAAS pathway, it was hypothesized that dual RAASi therapy could provide a more complete RAAS blockade accompanied by increased renoprotective outcomes in patients with T2D. However, a study comparing combination therapy with irbesartan plus lisinopril (an ACEi) versus either treatment alone found combination therapy provided no additional benefit against the risk of nephropathy in patients with T2D [Citation10]. Furthermore, a post hoc analysis of the Olmesartan Reducing Incidence of End-stage Renal Disease in Diabetic Nephropathy Trial with Hypertension (ORIENT-Hypertension) showed no additional renal benefit of adding the ARB olmesartan to ACEi treatment in patients with T2D with overt nephropathy and hypertension, while dual RAASi therapy caused greater hyperkalemia than monotherapy [Citation11].
Sodium-glucose cotransporter-2 inhibitors (SGLT-2is) are a newer class of oral glucose-lowering therapies that decrease plasma glucose levels through inhibition of renal glucose reabsorption and the promotion of urinary glucose excretion [Citation12]. In CV outcomes trials (CVOTs) of SGLT-2is, empagliflozin and canagliflozin were associated with a significant reduction in the risk of major adverse CV events (MACE) [Citation13,Citation14]. In addition, there were significant reductions in the risk of the composite outcome of CV death or hospitalization for HF, as well as reductions in the risks of clinically relevant renal outcomes, with dapagliflozin, empagliflozin, and canagliflozin compared with placebo () [Citation13–Citation17]. Most patients included in these trials were also receiving RAASi treatment, which suggests additional incremental benefits of SGLT-2is. This indicates that SGLT-2is may provide renoprotective effects in patients with T2D and DKD, potentially through different mechanisms from RAASis.
Table 1. Major renal outcomes with sodium-glucose cotransporter-2 inhibitors in cardiovascular outcomes trials of patients with type 2 diabetes.
This review discussed the non-glycemic effects of SGLT-2is in patients with T2D and renal impairment and considered the potential mechanisms for their renoprotective effects in these patients in CVOTs.
2. Non-glycemic effects of SGLT-2is
Four SGLT-2is (empagliflozin, canagliflozin, dapagliflozin, and ertugliflozin) are approved for the treatment of adults with T2D in the United States, Europe, and other countries worldwide [Citation18–Citation21].
In clinical studies, SGLT-2is were associated with significant improvements in glycemic control and body weight in patients with T2D [Citation22–Citation25]. SGLT-2is were also associated with several non-glycemic effects, including reductions in systolic and diastolic blood pressure (SBP and DBP), decreased albuminuria, changes in estimated GFR (eGFR), reduced plasma uric acid levels, and minimal changes in electrolytes.
Within the first 2 weeks of initiating SGLT-2i therapy, initial decreases in SBP and DBP (~4 mm Hg and ~2 mm Hg, respectively) compared with placebo have been observed [Citation26]. The proposed mechanisms for the reductions in SBP and DBP include body weight loss and natriuresis [Citation27,Citation28]. As glucose and sodium are both reabsorbed by SGLT-2 in the renal proximal tubule, SGLT-2i therapy increases sodium excretion and osmotic diuresis, which leads to a decrease in plasma volume of ~7% [Citation29,Citation30]. This plasma volume reduction potentially explains the initial decreases in SBP and DBP that were observed [Citation29]. However, SBP and DBP reductions were also observed with SGLT-2i therapy in patients with renal impairment, without further increased glucosuria [Citation31,Citation32]. This suggests that other factors may contribute to the sustained BP reductions over 6–12 months of therapy, including reductions in body weight, plasma uric acid levels, and albuminuria, and changes in the RAAS [Citation29].
SGLT-2is were also associated with decreases in albuminuria, including after adjusting analyses for changes in glycated hemoglobin (A1C), body weight, BP, and eGFR, suggesting that reductions in albuminuria were largely independent of these factors [Citation33–Citation36]. Microalbuminuria is thought to be caused by microvascular permeability as a result of glomerular endothelial dysfunction in the kidneys and is recognized as a marker of systemic endothelial dysfunction and a risk factor for CV mortality [Citation2,Citation37]. The presence of albuminuria is closely associated with hypertension, which is a key predictor of both renal and CV complications in patients with T2D [Citation38]. The reduction in albuminuria during SGLT-2i therapy may be associated with decreased intraglomerular pressure [Citation33]. In patients with T2D, greater filtered glucose load, along with increased SGLT-2 expression and activity, lead to increased sodium reabsorption in the proximal tubule and reduced sodium chloride concentrations at the macula densa, which deactivates tubuloglomerular feedback and promotes afferent arteriolar vasodilation () [Citation2,Citation29]. Combined with concurrent renin secretion, which induces efferent arteriolar vasoconstriction, these changes potentially cause increases in single-nephron GFR, glomerular hyperfiltration, and glomerular hypertension [Citation2]. SGLT-2i therapy reduces sodium and chloride reabsorption in the proximal tubule, restoring solute delivery to the macula densa and reactivating tubuloglomerular feedback, which reverses afferent arteriolar vasodilation and normalizes glomerular hypertension [Citation27,Citation39,Citation40]. Treatment-related decreases in albuminuria were associated with a reduced risk of ESRD [Citation41], suggesting that decreases in albuminuria with SGLT-2is provide renoprotective effects.
Figure 1. Effect of T2D and SGLT-2is on afferent and efferent arteriolar modulation, GFR, and sodium excretion in the renal tubule. (A) In individuals without diabetes, all of the filtered glucose is reabsorbed (along with Na+) by SGLT-2 and SGLT-1, and TGF is maintained; (B) in patients with T2D, glucose reabsorption is increased by SGLT-2 and SGLT-1; and (C) in patients with T2D receiving SGLT-2is, reabsorption of glucose and Na+ is increased and glucose is excreted in the urine. Reproduced with permission from DeFronzo et al. Nat Rev Nephrol. 2017;13:11–26 [Citation29]. GFR, glomerular filtration rate; JGA, juxtaglomerular apparatus; Na+, sodium; SGLT, sodium–glucose cotransporter; SGLT-2is, SGLT-2 inhibitors; T2D, type 2 diabetes; TGF, tubuloglomerular feedback.
![Figure 1. Effect of T2D and SGLT-2is on afferent and efferent arteriolar modulation, GFR, and sodium excretion in the renal tubule. (A) In individuals without diabetes, all of the filtered glucose is reabsorbed (along with Na+) by SGLT-2 and SGLT-1, and TGF is maintained; (B) in patients with T2D, glucose reabsorption is increased by SGLT-2 and SGLT-1; and (C) in patients with T2D receiving SGLT-2is, reabsorption of glucose and Na+ is increased and glucose is excreted in the urine. Reproduced with permission from DeFronzo et al. Nat Rev Nephrol. 2017;13:11–26 [Citation29]. GFR, glomerular filtration rate; JGA, juxtaglomerular apparatus; Na+, sodium; SGLT, sodium–glucose cotransporter; SGLT-2is, SGLT-2 inhibitors; T2D, type 2 diabetes; TGF, tubuloglomerular feedback.](/cms/asset/6a954f02-df15-477e-9758-3006b053472e/ipgm_a_1624582_f0001_c.jpg)
SGLT-2is were also associated with an initial dose-dependent decrease in eGFR of ~5 mL/min/1.73 m2 over 3–4 weeks, after which eGFR subsequently trended toward baseline and stabilized for the duration of the studies (26–192 weeks) [Citation15,Citation28,Citation31,Citation32,Citation42,Citation43]. This initial decline in eGFR with SGLT-2is was observed after several weeks of treatment in patients with or without DKD, as well as those with established CVD, and was reversed within 2 weeks of drug discontinuation [Citation15,Citation31]. The decrease in eGFR observed with SGLT-2is is potentially due to afferent arteriolar vasoconstriction through reactivation of tubuloglomerular feedback [Citation42]. However, the study showing this effect was conducted in patients with type 1 diabetes and hyperfiltration [Citation44], and these changes in renal hemodynamics are yet to be demonstrated in patients with T2D. The transient reduction in eGFR seen with SGLT-2is was less than that observed with RAASis, and yet may be associated with renoprotective benefits [Citation38,Citation45]. Since the transient reduction in eGFR with SGLT-2is was more modest than that observed with RAASis, SGLT-2is, which are used largely for the purpose of improving glycemic control, may prove to be safer to use in patients with a lower eGFR. However, the use of SGLT-2is is currently contraindicated in patients with eGFR <30 mL/min/1.73 m2 [Citation18–Citation21], until more experience in clinical trials of patients with lower eGFR have been completed. Furthermore, ertugliflozin is not recommended for patients with eGFR persistently between ≥30 and <60 mL/min/1.73 m2, and the use of dapagliflozin, empagliflozin, and canagliflozin is not recommended for patients with eGFR persistently <45 mL/min/1.73 m2.
Elevated plasma uric acid levels have been associated with an increased risk of hypertension, stroke, and HF, as well as activation of the RAAS in the kidneys [Citation42,Citation46]. SGLT-2is were associated with reductions in plasma uric acid levels of up to 13% compared with placebo in patients with T2D [Citation14,Citation32,Citation47]. Although the exact mechanism for the decrease in plasma uric acid associated with SGLT-2is is unclear, it is likely due to the promotion of uric acid excretion in the urine through the GLUT9 transporter and possible inhibition of reabsorption of uric acid in the collecting ducts [Citation48].
Small changes in electrolyte levels were observed with SGLT-2is and may be the result of osmotic diuresis-associated reductions in plasma volume [Citation38]. Across 13 pooled placebo-controlled studies, dapagliflozin was associated with small increases in serum phosphorus compared with placebo over 24 weeks (mean change +0.13 vs. −0.04 mg/dL, respectively) [Citation21]. In two pooled analyses of four placebo-controlled studies each, canagliflozin was associated with small mean percent increases in serum magnesium (8% to 13%) and phosphate (3% to 5%) after 18–26 weeks, although the proportion of patients with elevations in magnesium or phosphate that met outlier criteria was low [Citation49]. However, in studies of up to 78 weeks in patients with T2D, empagliflozin was not associated with changes in serum electrolytes, including sodium, potassium, magnesium, calcium, or phosphate [Citation50–Citation52].
2.1. Effects of SGLT-2is on CV and renal outcomes
Randomized, placebo-controlled CVOTs of empagliflozin (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; EMPA-REG OUTCOME; N = 7020), canagliflozin (Canagliflozin Cardiovascular Assessment Study; CANVAS; N = 10,142), and dapagliflozin (Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58; DECLARE-TIMI 58; N = 17,160) investigated CV and renal outcomes in patients with T2D and established CVD or varying levels of CV risk. Empagliflozin (10 mg and 25 mg) and canagliflozin (100 mg and 300 mg) significantly reduced the risk of three-point MACE (CV death, non-fatal myocardial infarction, or non-fatal stroke) by 14% compared with placebo in patients with T2D and established CVD in EMPA-REG OUTCOME or with T2D and established CVD or multiple CVD risk factors in CANVAS [Citation13,Citation14]. In EMPA-REG OUTCOME, empagliflozin also significantly reduced the risk of CV death (by 38%) and all-cause mortality (by 32%) versus placebo [Citation14]. The DECLARE-TIMI 58 study of dapagliflozin included a larger proportion of patients with multiple CV risk factors without established CVD (~60%) and a smaller proportion of patients with established CVD (~40%) compared with the other CVOTs [Citation17]. In DECLARE-TIMI 58, dapagliflozin 10 mg demonstrated non-inferiority versus placebo with respect to MACE and significantly reduced the risk of the composite end point of CV death or hospitalization for HF by 17% compared with placebo. This result was driven by a reduction in the risk of hospitalization for HF with dapagliflozin (by 27%). The risk of hospitalization for HF was also significantly reduced with empagliflozin (by 35%) and canagliflozin (by 33%) compared with placebo. The mechanisms underlying the cardioprotective effects of SGLT-2is have not been fully elucidated, but likely involve a number of factors, including improvements in ventricular loading and cardiac efficiency and reductions in cardiac glucotoxicity and BP [Citation42]. Additional mechanisms proposed for SGLT-2is, including mechanisms underlying renoprotective effects, are discussed further below.
In addition to CV outcomes, assessment of the prespecified renal outcomes indicated that SGLT-2is were associated with a significantly lower risk of kidney disease progression () [Citation15–Citation17]. In EMPA-REG OUTCOME, empagliflozin was associated with a significantly lower risk compared with placebo for incident or worsening nephropathy (relative risk reduction [RRR]: 39%), progression to macroalbuminuria (RRR: 38%), doubling of serum creatinine (RRR: 44%), and initiation of renal replacement therapy (RRR: 55%), with a consistent benefit across the two doses of empagliflozin (10 mg and 25 mg) [Citation15]. Similarly in CANVAS, canagliflozin was associated with a 47% reduction in risk of the composite outcome of sustained doubling of serum creatinine, ESRD, or death from renal causes compared with placebo [Citation16]. There were also reductions with canagliflozin versus placebo in the risk of new-onset microalbuminuria (by 20%); new-onset macroalbuminuria (by 42%); and the composite of 40% eGFR reduction, ESRD, or death from renal causes (by 40%) [Citation16]. Dose analyses suggested that the 100-mg and 300-mg doses of canagliflozin had similar effects on renal outcomes. In DECLARE-TIMI 58, dapagliflozin was associated with a reduction in the risk of prespecified renal outcomes versus placebo, including a 24% reduction in the risk of the composite outcome of ≥40% decrease in eGFR to <60 mL/min/1.73 m2, ESRD, or death from renal or CV causes [Citation17]. A larger RRR with dapagliflozin (47%) was observed for a similar composite outcome that excluded death from CV causes (i.e. composite of ≥40% decrease in eGFR to <60 mL/min/1.73 m2, ESRD, or death from renal causes) [Citation17]. A meta-analysis of these CVOTs found that SGLT-2is significantly reduced the risk of a composite renal outcome of worsening renal function, ESRD, or renal death by 45% compared with placebo (hazard ratio [HR], 0.55 [95% confidence interval 0.48–0.64]; p < 0.0001) [Citation53]. The benefit was similar in patients with or without atherosclerotic CVD at baseline. While all patient subgroups stratified by baseline eGFR had reductions in the risk of renal disease progression, those patients with greater renal function at baseline had greater reductions in risk. A separate meta-analysis of 25 placebo-controlled trials of SGLT-2is, including the three CVOTs, evaluated the effect of SGLT-2i dose on the preservation of renal function. The meta-regression analysis detected a significant association between preservation of eGFR and drug dosage (larger dose preferred; coefficient, −2.007; p = 0.015) [Citation54]. Together, these results suggest that SGLT-2is play an important role in providing renal protection in patients with T2D.
In these studies, most patients (~80%) were receiving concomitant RAASi therapy [Citation15–Citation17], which is recommended in patients with T2D and DKD [Citation55]. This indicates that SGLT-2is potentially provided incremental renoprotective effects, in addition to customary doses of RAASis. However, the dosage of RAASi therapy in these studies was unknown and may have been below proven renoprotective doses. The mechanisms underlying the synergistic effects of SGLT-2is and RAASis are not fully understood but may be due to differing methods of intraglomerular pressure reduction. RAASis reduce intraglomerular pressure through vasodilation of the efferent arteriole, while SGLT-2is likely act through BP-independent afferent arteriolar vasoconstriction [Citation56] or other as yet unknown effects. In addition, a shift to the non-classical RAAS pathway has been proposed as a mechanism underlying renoprotective effects in the presence of SGLT-2is plus RAASis. In this explanation, SGLT-2i–induced natriuresis and volume depletion activates the RAAS system, which in the presence of ACEi favors the non-classical RAAS system and downstream activation of Mas receptors, thereby leading to systemic arteriolar vasodilation, natriuresis, diuresis, reduced oxidative stress, and antiproliferative activity [Citation57].
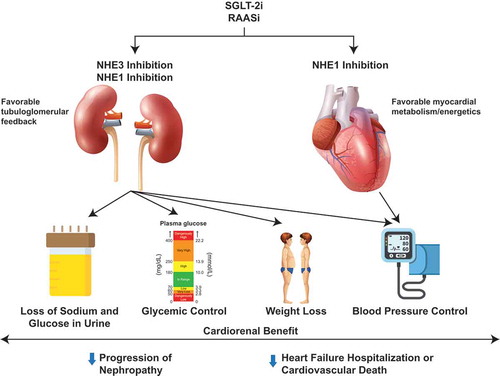
SGLT-2is and RAASis may also additively reduce the risk of nephropathy by simultaneously blocking the activity of sodium-hydrogen exchangers (NHEs) in the kidney () [Citation58]. Expression of the NHE3 isoform is limited to the kidney and intestine, while NHE1 is expressed ubiquitously and is the predominant isoform expressed by the heart and vasculature. NHE activity is stimulated by hyperglycemia, hyperinsulinemia, and adipokines, and may contribute to glomerular hyperfiltration and other features of diabetic nephropathy, as well as having an important role in the pathophysiology of HF. Both SGLT-2is and RAASis reduce intraglomerular pressure through renal NHE blockade, and RAASis may further reduce mesangial and tubular cell proliferation and fibrosis that occur in DKD. Furthermore, the reduced risk of HF with SGLT-2is in patients with T2D may be linked to NHE blockade in the heart and vasculature () [Citation58]. As the magnitude of cardiorenal protection with SGLT-2is appears greater than expected due to improvements in glycemic control, BP, and body weight, and occurs despite reduced renal function and less glycosuria in patients with T2D, inhibition of NHE may account for additive benefits [Citation59].
Figure 2. Synergistic effects of SGLT-2is and RAASis proposed for cardiorenal benefit. SGLT-2is and RAASis can inhibit the activity of NHE isoforms in the kidney, leading to favorable tubuloglomerular feedback and multiple cardiorenal benefits, and in the heart and vasculature, leading to favorable myocardial metabolism and energetics and mitigating cardiac hypertrophy, injury, and fibrosis. NHE, sodium-hydrogen exchanger; RAASi, renin-aldosterone-angiotensin system inhibitor; SGLT-2i, sodium-glucose cotransporter-2 inhibitor.
The randomized CVOTs of SGLT-2is demonstrated no renal safety issues in patients with T2D and established CVD or varying levels of CV risk [Citation15–Citation17]. In EMPA-REG OUTCOME, adverse events (AEs) consistent with acute renal failure, including acute kidney injury (AKI), and hyperkalemia occurred with lower incidence with empagliflozin than with placebo [Citation15]. Similarly, AKI occurred less frequently with dapagliflozin versus placebo in DECLARE-TIMI 58 [Citation17]. The rates of renal-associated serious AEs in CANVAS were not significantly different in the canagliflozin and placebo groups, and the incidence of AKI and hyperkalemia also did not differ between groups [Citation16]. Despite initial decreases in mean eGFR reported with SGLT-2is in EMPA-REG OUTCOME and CANVAS, eGFR subsequently stabilized with empagliflozin [Citation15] or canagliflozin [Citation16], while steady decreases in eGFR were observed with placebo in both studies. Postmarketing reports of AKI in some patients receiving SGLT-2is have raised questions regarding a possible increase in the risk of AKI with SGLT-2is [Citation18,Citation19,Citation21]. Mechanisms of SGLT-2is that may potentially be associated with AKI include dehydration and altered glomerular hemodynamics [Citation60]. However, a real-world study of patients with T2D who newly initiated SGLT-2is or dipeptidyl peptidase-4 inhibitors (DPP-4is) indicated that the risks of AKI, hospitalization, or all-cause mortality over 24 weeks were lower with SGLT-2is than with DPP-4is [Citation61].
In the DECLARE-TIMI 58 study, a creatinine clearance of <60 mL/min (based on the Cockcroft-Gault equation) was an exclusion criterion; however, the study population included 7% of the patients with eGFR <60 mL/min/1.73 m2 (based on the Modification of Diet in Renal Disease formula) [Citation17]. In the EMPA-REG OUTCOME and CANVAS studies, the proportion of patients with an eGFR of 30–60 mL/min/1.73 m2 at baseline was 20% to 26% [Citation15,Citation16]. Across all three CVOTs of SGLT-2is, microalbuminuria was present in 23% to 29% of the patients, and 7% to 11% had macroalbuminuria at baseline [Citation15,Citation16,Citation62].
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study (N = 4401) assessed whether canagliflozin 100 mg reduced the risk of renal failure and CV events in patients with T2D and markers of established kidney disease (eGFR of ≥30 to <90 mL/min/1.73 m2 and macroalbuminuria [urine albumin-to-creatinine ratio (UACR) of >300 to ≤5000 mg/g]) who were receiving RAASis [Citation63]. The trial was stopped early after demonstrating a 30% reduction in the relative risk of the primary composite outcome of ESRD, doubling of serum creatinine, or death from renal or CV causes with canagliflozin versus placebo (p = 0.00001). Canagliflozin also reduced the relative risk of secondary renal-specific outcomes compared with placebo, including the composite of ESRD, doubling of serum creatinine, or death from renal causes (by 34%; p < 0.001) and ESRD (by 32%; p = 0.002). In addition, canagliflozin reduced the relative risk of the composite outcome of CV death, myocardial infarction, or stroke by 20% (p = 0.01) and hospitalization for HF by 39% (p < 0.001). There were no safety concerns regarding the use of canagliflozin in patients with more advanced kidney disease.
The DELIGHT study (N = 448) assessed the albuminuria-lowering effects of dapagliflozin (with or without the DPP-4i saxagliptin) in patients with T2D and moderate-to-severe chronic kidney disease (CKD) (UACR of 30–3500 mg/g and eGFR of 25–75 mL/min/1.73 m2) who were receiving RAASis [Citation64]. Patients treated with dapagliflozin or with dapagliflozin plus saxagliptin had significant reductions in the UACR over the course of the study, while the UACR remained relatively stable in the placebo group. At week 24, the mean percentage change in the UACR from baseline versus placebo was −21.0% (p = 0.011) in the dapagliflozin group and −38.0% (p < 0.0001) in the dapagliflozin plus saxagliptin group.
The exact mechanisms underlying the renoprotective effects of SGLT-2is are not fully understood but are likely to be multifactorial. Modest reductions in BP and body weight, thought to be caused in part by SGLT-2i−associated natriuresis, may contribute to improvements in renal outcomes [Citation40]. As discussed above, reduced sodium reabsorption in the proximal tubule with SGLT-2is leads to increased sodium chloride delivery at the macula densa; this reactivates tubuloglomerular feedback and afferent arteriolar vasoconstriction () [Citation27,Citation29,Citation40]. SGLT-2is have also been associated with reductions in atrial natriuretic peptide secretion, which may have an important role in decreasing intraglomerular pressure [Citation35]. Reductions in arterial stiffness and vascular resistance with SGLT-2is are thought to contribute to the observed improvements in renal and CV outcomes [Citation15,Citation65,Citation66].
SGLT-2i therapy may be associated with changes in systemic and renal neurohormonal factors, including an increase in circulating RAAS mediators (e.g. aldosterone and angiotensin II), perhaps due to natriuresis and volume depletion [Citation30,Citation44], with a potential shift to the non-classical RAAS pathway in the presences of RAASis as discussed above. Empagliflozin has been associated with increased blood glucagon levels in patients with T2D [Citation67], which may influence fasting-state natriuresis, protein-induced hyperfiltration, and nitrogen end-product excretion (e.g. urea and ammonia) [Citation40,Citation68].
Furthermore, SGLT-2is may also provide renoprotective effects through activation of hypoxia-inducible factor 1 (HIF1), which is involved in the regulation of erythropoiesis [Citation40,Citation69]. In a mouse model of renal ischemic injury, dapagliflozin increased HIF1 expression and reduced apoptotic cell death [Citation69].
2.2. Potential for beneficial effects of SGLT-2is in non-dkd
As the renoprotective effects of SGLT-2is are likely mediated by their non-glycemic properties, it is possible that their use may be extended to patients with non-DKDs, particularly those that are also characterized by albuminuria, hyperfiltration, and glomerular hypertension [Citation70]. In patients with hypertensive nephrosclerosis, decreased afferent and efferent arteriolar autoregulation is associated with hyperfiltration and glomerular lesions [Citation71]. Obesity-related CKD is likely caused by similar mechanisms to DKD, including changes in tubuloglomerular feedback that lead to hyperfiltration [Citation72]. Patients with immunoglobulin A (IgA) nephropathy and early kidney disease have been shown to have increased arterial stiffness, increased circulating RAAS mediators, and decreased sensitivity to angiotensin II, and often present with albuminuria [Citation73]. While primary focal segmental glomerulosclerosis (FSGS) is characterized by podocyte injury due to circulating factors, adaptive FSGS arises following a period of glomerular hyperfiltration and glomerular hypertension associated with certain diseases, or can be caused by a reduction in renal mass due to prematurity, renal abnormalities, AKI, or reflux nephropathy [Citation74].
Although SGLT-2is have the potential to improve renal outcomes in patients with non-DKD, it is important to determine their safety in this patient population. Currently, clinical trial data of SGLT-2is in patients without T2D are limited. SGLT-2is were well tolerated in small studies of healthy adult volunteers, with no episodes of hypoglycemia reported with multiple-dose administration [Citation75–Citation77]. A small pilot study of patients with primary or adaptive FSGS (N = 10) found that 8 weeks’ treatment with dapagliflozin had no effect on renal hemodynamic function or proteinuria [Citation78]. Ongoing studies, including the Effects of Dapagliflozin in Non-diabetic Patients with Proteinuria (DIAMOND; NCT03190694), a Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and CV Mortality in Patients With CKD (DAPA-CKD; NCT03036150), and a Study of Heart and Kidney Protection With Empagliflozin (EMPA-KIDNEY; NCT03594110), are currently assessing the efficacy and safety of SGLT-2is in patients with non-DKD. Data from these studies are anticipated with interest.
3. Conclusions
Large CVOTs and renal studies of SGLT-2is demonstrated the potential of this class of glucose-lowering therapies for improving CV and renal outcomes in patients with T2D. Although the exact mechanisms by which SGLT-2is improve renal outcomes are not fully understood, they are likely to be multifactorial and associated with incremental improvements when these drugs are used in combination with RAASis in patients with DKD. SGLT-2is may also represent a more user-friendly option than RAASis to provide renal protection, given the lack of effect on serum potassium and a more modest initial effect on eGFR. The results of ongoing studies in patients with kidney disease, with and without T2D, may allow for confirmation of the renoprotective effects of SGLT-2is in patients with renal impairment.
Declaration of interest
M Weir has acted as a scientific advisor for AstraZeneca, Akebia Therapeutics, AbbVie Inc, Bayer, Boehringer Ingelheim, Merck Sharp & Dohme, Janssen, Relypsa Inc, Boston Scientific, and Vifor Pharma. M Weir has received NIH grant funding (R01 DK066013, U01 DK106102, U01 DK116095, R01 HL127422, R01 HL132732) for research unrelated to the development of this manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Acknowledgments
Sarah Greig, of inScience Communications, Springer Healthcare (Auckland, New Zealand), provided medical writing support funded by AstraZeneca.
Additional information
Funding
References
- Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82.
- Anders HJ, Huber TB, Isermann B, et al. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14:361–377.
- Toth-Manikowski S, Atta MG. Diabetic kidney disease: pathophysiology and therapeutic targets. J Diabetes Res. 2015;2015:697010.
- Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA consensus conference. Diabetes Care. 2014;37:2864–2883.
- Jiang Y, Fine JP, Mottl AK. Competing risk of death with end-stage renal disease in diabetic kidney disease. Adv Chronic Kidney Dis. 2018;25:133–140.
- Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–308.
- Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869.
- Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860.
- Berl T, Hunsicker LG, Lewis JB, et al. Cardiovascular outcomes in the irbesartan diabetic nephropathy trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med. 2003;138:542–549.
- Fernandez Juarez G, Luno J, Barrio V, et al. Effect of dual blockade of the renin-angiotensin system on the progression of type 2 diabetic nephropathy: a randomized trial. Am J Kidney Dis. 2013;61:211–218.
- Imai E, Haneda M, Yamasaki T, et al. Effects of dual blockade of the renin-angiotensin system on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy and hypertension in the ORIENT: a post-hoc analysis (ORIENT-Hypertension). Hypertens Res. 2013;36:1051–1059.
- Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes. 2013;62:3324–3328.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128.
- Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334.
- Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357.
- US Food and Drug Administration. Invokana® (canagliflozin) tablets, for oral use: prescribing information 2018 [cited 2019 Mar 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/204042s027lbl.pdf.
- US Food and Drug Administration. Jardiance® (empagliflozin) tablets, for oral use: prescribing information 2018 [cited 2019 Mar 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/204629s019lbl.pdf.
- US Food and Drug Administration. Steglatro™ (ertugliflozin) tablets, for oral use: prescribing information 2018 [cited 2019 Mar 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209803s001lbl.pdf.
- US Food and Drug Administration. Farxiga® (dapagliflozin) tablets, for oral use: prescribing information 2019 [cited 2019 Mar 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202293s015lbl.pdf.
- Markham A. Ertugliflozin: first global approval. Drugs. 2018;78:513–519.
- Plosker GL. Dapagliflozin: a review of its use in patients with type 2 diabetes. Drugs. 2014;74:2191–2209.
- Plosker GL. Canagliflozin: a review of its use in patients with type 2 diabetes mellitus. Drugs. 2014;74:807–824.
- Scott LJ. Empagliflozin: a review of its use in patients with type 2 diabetes mellitus. Drugs. 2014;74:1769–1784.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–274.
- Alicic RZ, Johnson EJ, Tuttle KR. SGLT2 inhibition for the prevention and treatment of diabetic kidney disease: a review. Am J Kidney Dis. 2018;72:267–277.
- Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463–473.
- DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13:11–26.
- Lambers Heerspink HJ, de Zeeuw D, Wie L, et al. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862.
- Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:369–384.
- Kohan DE, Fioretto P, Tang W, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–971.
- Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:610–621.
- Fioretto P, Stefansson BV, Johnsson E, et al. Dapagliflozin reduces albuminuria over 2 years in patients with type 2 diabetes mellitus and renal impairment. Diabetologia. 2016;59:2036–2039.
- Heerspink HJ, Desai M, Jardine M, et al. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017;28:368–375.
- Heerspink HJ, Johnsson E, Gause-Nilsson I, et al. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016;18:590–597.
- van Sloten TT, Henry RMA, Dekker JM, et al. Endothelial dysfunction plays a key role in increasing cardiovascular risk in type 2 diabetes: the Hoorn study. Hypertension. 2014;64:1299–1305.
- Weir MR. The kidney and type 2 diabetes mellitus: therapeutic implications of SGLT2 inhibitors. Postgrad Med. 2016;128:290–298.
- Thomson SC, Rieg T, Miracle C, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302:R75–R83.
- Wanner C. EMPA-REG OUTCOME: the nephrologist’s point of view. Am J Cardiol. 2017;120:S59–S67.
- Heerspink HJ, Kröpelin TF, Hoekman J, et al. Drug-induced reduction in albuminuria is associated with subsequent renoprotection: a meta-analysis. J Am Soc Nephrol. 2015;26:2055–2064.
- Heerspink HJL, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772.
- Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–950.
- Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597.
- van Bommel EJ, Muskiet MH, Tonneijck L, et al. SGLT2 inhibition in the diabetic kidney—from mechanisms to clinical outcome. Clin J Am Soc Nephrol. 2017;12:700–710.
- Kanbay M, Segal M, Afsar B, et al. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart. 2013;99:759–766.
- Davies MJ, Trujillo A, Vijapurkar U, et al. Effect of canagliflozin on serum uric acid in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17:426–429.
- Lytvyn Y, Skrtic M, Yang GK, et al. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2015;308:F77–F83.
- Weir MR, Kline I, Xie J, et al. Effect of canagliflozin on serum electrolytes in patients with type 2 diabetes in relation to estimated glomerular filtration rate (eGFR). Curr Med Res Opin. 2014;30:1759–1768.
- Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36:4015–4021.
- Häring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396–3404.
- Heise T, Seman L, Macha S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther. 2013;4:331–345.
- Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39.
- Wang C, Zhou Y, Kong Z, et al. The renoprotective effects of sodium-glucose cotransporter 2 inhibitors versus placebo in patients with type 2 diabetes with or without prevalent kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab. 2019;21:1018–1026.
- KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:340–414.
- Satirapoj B. Sodium-glucose cotransporter 2 inhibitors with renoprotective effects. Kidney Dis (Basel). 2017;3:24–32.
- de Albuquerque Rocha N, Neeland IJ, McCullough PA, et al. Effects of sodium glucose co-transporter 2 inhibitors on the kidney. Diab Vasc Dis Res. 2018;15:375–386.
- Packer M. Activation and inhibition of sodium-hydrogen exchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation. 2017;136:1548–1559.
- McCullough PA, Kluger AY, Tecson KM, et al. Inhibition of the sodium-proton antiporter (exchanger) is a plausible mechanism of potential benefit and harm for drugs designed to block sodium glucose co-transporter 2. Rev Cardiovasc Med. 2018;19:51–63.
- Szalat A, Perlman A, Muszkat M, et al. Can SGLT2 inhibitors cause acute renal failure? Plausible role for altered glomerular hemodynamics and medullary hypoxia. Drug Saf. 2018;41:239–252.
- Cahn A, Melzer-Cohen C, Pollack R, et al. Acute renal outcomes with sodium-glucose co-transporter-2 inhibitors: real-world data analysis. Diabetes Obes Metab. 2019;21:340–348.
- Raz I, Mosenzon O, Bonaca MP, et al. DECLARE-TIMI 58: participants’ baseline characteristics. Diabetes Obes Metab. 2018;20:1102–1110.
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019. DOI:10.1056/NEJMoa1811744. [Epub ahead of print]
- Pollock C, Stefánsson B, Reyner D, et al. Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:429–441.
- Cherney DZ, Perkins BA, Soleymanlou N, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28.
- Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–1193.
- Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508.
- Bankir L, Roussel R, Bouby N. Protein- and diabetes-induced glomerular hyperfiltration: role of glucagon, vasopressin, and urea. Am J Physiol Renal Physiol. 2015;309:F2–F23.
- Chang YK, Choi H, Jeong JY, et al. Dapagliflozin, SGLT2 inhibitor, attenuates renal ischemia-reperfusion injury. PLoS One. 2016;11:e0158810.
- Dekkers CCJ, Gansevoort RT, Heerspink HJL. New diabetes therapies and diabetic kidney disease progression: the role of SGLT-2 inhibitors. Curr Diab Rep. 2018;18:27.
- Hill GS. Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens. 2008;17:266–270.
- Whaley-Connell A, Sowers JR. Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int. 2017;92:313–323.
- Abdi-Ali A, Mann MC, Hemmelgarn BR, et al. IgA nephropathy with early kidney disease is associated with increased arterial stiffness and renin-angiotensin system activity. J Renin Angiotensin Aldosterone Syst. 2015;16:521–528.
- Rosenberg AZ, Kopp JB. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12:502–517.
- Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85:520–526.
- Macha S, Brand T, Meinicke T, et al. Pharmacokinetics and pharmacodynamics of twice daily and once daily regimens of empagliflozin in healthy subjects. Clin Ther. 2015;37:1789–1796.
- Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13:669–672.
- Rajasekeran H, Reich HN, Hladunewich MA, et al. Dapagliflozin in focal segmental glomerulosclerosis: a combined human-rodent pilot study. Am J Physiol Renal Physiol. 2018;314:F412–F422.
