ABSTRACT
Background: Among patients with chronic pain using long-term opioid therapy, the incidence of opioid abuse, addiction, overdose, and associated death are not well quantified. The range of estimates for these adverse outcomes varies drastically and may depend on how they are measured (i.e. study definitions of outcomes) and on patient characteristics and opioid-use factors (e.g. regimen, daily dose).
Methods: Based on a review of the literature, the US Food and Drug Administration (FDA) required companies that manufacture and sell extended-release/long-acting (ER/LA) opioids conduct as a postmarketing requirement (PMR) a series of observational studies to estimate the rates of treatment-emergent misuse, abuse, addiction, overdose, and death using validated measures. The companies formed a consortium, the Opioid PMR Consortium (OPC), to conduct the studies.
Results: The FDA initially requested four observational studies (a cohort study, a questionnaire validation study, a code validation study, and a doctor-shopping validation study), but in order to achieve the FDA’s goals of the 4 studies, OPC and FDA agreed to 10 observational studies (a prospective cohort study, a retrospective database cohort study, three questionnaire validation studies, two code validation studies, and three doctor-shopping validation studies). The studies are continuing through 2020.
Conclusions: A series of 10 observational studies was or are being conducted in response to the FDA’s postmarketing requirement. All studies have been feasible to conduct, although a validated algorithm for measuring abuse and addiction in databases was not successful.
Introduction
Many Americans are affected by chronic pain, with 25 million reporting daily chronic pain and an additional 23 million reporting severe pain [Citation1,Citation2]. Opioid analgesics are one treatment option for chronic, severe pain under the appropriate circumstances [Citation2]. However, addiction, abuse, and overdose deaths associated with opioid analgesics and illicit opioids are a serious public health problem. Among patients with chronic pain using long-term opioid therapy, the incidence of opioid abuse, addiction, overdose, and associated death are not well quantified. The range of estimates for these adverse outcomes varies drastically and may depend on how they are measured (i.e. study methodology) [Citation3]. Extended-release/long-acting (ER/LA) opioids are a subgroup of opioid analgesics intended for treatment of chronic, persistent pain and for which alternative treatment options are inadequate. Extended-release/long-acting opioids comprised 9.6% of opioid analgesic prescriptions in the US as of July 2018, according to the IQVIA LRx prescription database, and often include higher dosage forms than immediate-release (IR) and short-acting opioids, which comprised the other 90% of opioid prescriptions.
Based on a review of the literature, the US Food and Drug Administration (FDA) concluded that more data are needed regarding the serious risks of misuse, abuse, addiction, overdose, and death associated with the long-term use of ER/LA opioids for the treatment of chronic pain. Thus, in September 2013, the FDA required that companies with New Drug Application (NDA) approvals to manufacture and sell ER/LA opioids should conduct as a postmarketing requirement (PMR) a series of observational studies to estimate the rates of treatment-emergent misuse, abuse, addiction, overdose, and death using validated measures [Citation4]. In addition, the FDA required ER/LA opioid NDA holders to conduct a postmarketing randomized, controlled clinical trial to assess the incidence of hyperalgesia, and risk relative to analgesic efficacy, among chronic pain patients. Study 11 (PMR 3033–11), the randomized controlled trial, is not covered in detail in this description of the observational study program. A description of the original Study 11 design can be found on www.clinicaltrials.gov but changes to the study design are currently being considered to meet patient enrollment challenges [Citation5]. The companies were strongly advised by FDA to collaborate, and formed a consortium, the Opioid PMR Consortium (OPC).
The goal of this article is to describe the series of 10 observational studies being conducted in response to the FDA’s PMR to estimate the incidence of serious risks of misuse, abuse, addiction, overdose, and death associated with long-term use of opioids to treat chronic pain. In addition, we discuss how the consortium of companies has collaborated with each other, the FDA, academic institutions, the National Institute of Drug Addiction and healthcare research groups to implement the studies.
Rationale for the study program
The magnitude of the incidence of misuse, abuse, addiction, overdose and death among patients who are treated with opioid analgesics for chronic pain has been unclear, as has been the extent to which the incidence of opioid misuse, abuse, addiction, overdose, and death vary with comorbidity with other substance use and psychiatric disorders or other risk factors. It is important to understand the risk magnitude and predictors of these serious adverse events associated with opioid use to develop effective prevention strategies. The estimates of abuse and addiction associated with long-term opioid use among chronic pain patients vary, with 41% of patients reported to have ‘any’ prescription opioid-use disorder in one study [Citation6], a Cochran review reporting 0.5% median incidence of dependence [Citation7] and rates of addiction averaging between 8% and 12% in a systematic review [Citation8]. Part of the discrepancy involves the definitions used to measure misuse, abuse, addiction, overdose, and death.
For our postmarketing initiative, definitions of misuse, abuse, and addiction to prescription opioids were used from an academic-FDA-industry workgroup that developed classifications and definitions of abuse-related events [Citation9]. Abuse was defined as the intentional use of drugs for nontherapeutic purposes of achieving positive psychological or physical effects. Misuse was defined as the intentional inappropriate use of drugs for therapeutic purposes outside of directions in labels, prescriptions, or directions by health-care practitioners.
Opioid addiction can be considered to fall on the severe end of the spectrum of the opioid-use disorder (OUD) diagnosis in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [Citation10]. The OUD diagnosis was formulated, however, without consideration of patients with chronic pain. In these patients, accurately diagnosing addiction may require assessing extra information about positively endorsed criteria (e.g. whether the behavior had therapeutic or non-therapeutic intent) and adjusting the diagnosis accordingly.
The most widely available information about patient care and conditions is that contained in medical claims data. If such data can be used to develop a model for identifying patients experiencing prescription opioid abuse/addiction, it could be widely applied to patient populations throughout the US. Methods are needed that can accurately identify patients experiencing opioid abuse/addiction based on these widely available claims data. Therefore, a goal of this study program was to develop and validate classification algorithms to detect opioid overdose and abuse/addiction combined (since it was thought that codes in claims would not have the specificity to distinguish between the two) and to use these to study the incidence of these events in large insurance claims or EHR databases.
Visiting many physicians and pharmacies to get opioid prescriptions, referred to as ‘doctor/pharmacy shopping’, is a behavior thought to be associated with opioid abuse and/or diversion [Citation11,Citation12]. Diversion, which describes any intentional act that results in transferring a prescription medication from lawful to unlawful distribution or possession, was used as a measure of demand for abuse. The criteria for defining doctor/pharmacy shopping as a measure of opioid abuse are unclear, and the validity of different thresholds have only partially been validated [Citation13,Citation14]. Three complementary observational studies were conducted to provide a comprehensive understanding of this behavior by defining and assessing the validity of ‘doctor/pharmacy-shopping’ behavior as an outcome suggestive of misuse, abuse, and addiction. The three studies utilized three different perspectives: 1) medical diagnosis of abuse/addiction in claims databases, 2) patient self-report of misuse and abuse, and 3) information described in EMRs.
Development of the study plan
The postmarketing program began with a multi-stakeholder meeting convened by the FDA, organized by the Duke Clinical Trials Transformation Initiative and chaired by Robert Califf in August 2013. Following that meeting, the FDA sent a letter to all companies with approved NDAs for ER/LA opioid analgesics, instructing them to conduct five PMR studies, either as individual companies or as a consortium sharing the studies, with final study protocols due to the FDA in October 2014 [Citation4]. The companies formed the OPC to pool resources for the FDA-required studies. In May 2014, the FDA held an open public meeting at FDA headquarters with a panel of 32 relevant experts selected by FDA to review the study proposals developed by the OPC [Citation15]. In addition, the FDA scientists have played, and continue to play, a very large role in designing and monitoring the progress of the studies. The FDA intensively reviewed the proposals submitted by the OPC and provided written comments and teleconference discussions on the study designs, analytical plans, and generalizability. The OPC recommended conducting 11 PMR studies instead of the five requested in the original letter from the FDA, in order to achieve the FDA’s goals, and the FDA accepted this recommendation. The FDA reissued the letter to all companies that held approved NDAs for ER/LA opioid analgesics, requiring them to conduct the 11 new PMR studies, 10 observational studies and 1 clinical trial [Citation16]. The FDA required that the final study protocols be approved by FDA reviewers before the studies could start and also set tight timelines for completion of the studies and final study report.
Organization of company opioid postmarketing consortium (OPC)
Initially, nine companies formed the OPC to design and implement the study program. This increased to 13 companies (as of June 2018) as new products were approved by the FDA. The OPC has a Steering Committee, as well as at least nine subteams, including observational study design and implementation, clinical trial design, clinical trial operations, legal, regulatory, vendor, finance, contracting, and quality control subteams. The program is administered by a Program Management Organization (PMO) maintained by a management consulting organization, which also contracts on behalf of the OPC with research partners. In addition, each of the studies has its own study team consisting of OPC company members, study investigators, and members of the PMO.
Overview of study plan
Based on the letter from the FDA to each of the individual sponsors, four core areas were identified where more information on ER/LA opioids was required to better understand the benefits and risks of these products and to inform patients and their health-care providers about the level of the associated risks, including:
Risks of abuse, addiction, overdose, death, and misuse among patients with chronic pain;
Predictors of the risks of abuse, addiction, overdose, death, and misuse among patients with chronic pain;
Validated proxies/surrogates for opioid abuse and addiction, such as doctor/pharmacy shopping measures; these proxies can also be used to measure diversion of opioid analgesics; and
The risk of hyperalgesia, and risk relative to analgesic efficacy, following long-term use of ER/LA opioid analgesics to treat chronic pain to be studied in a clinical trial, as a potential pathophysiological mechanism by which efficacy of opioids may wane.
An additional component of the study program is that validated measures to estimate the serious risks described in #1 above should be developed and their validity assessed.
This led to the development of 10 observational and 1 clinical trial studies to inform the four areas listed above. provides a list of the 11 studies and their goals. A figure showing how the 11 studies in the ER/LA Opioid Postmarketing Study program relate to the four core areas listed above from the FDA letter are shown in .
Figure 1. Conceptual Framework of the Observational Studies and Randomized Clinical Trial in the ER/LA Opioid Postmarketing Study Program.
DPS, doctor/pharmacy shopping; ER/LA, extended-release/long-acting; OUD, opioid-use disorder; PRISM, Psychiatric Research Interview for Substance and Mental Disorders, Fifth Edition, Opioid Study Version.
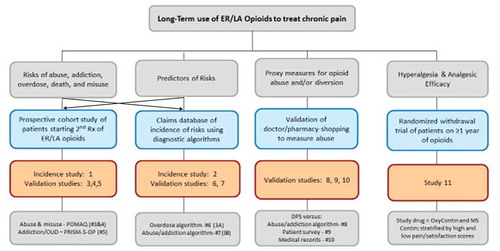
Table 1. List of Studies in the ER/LA Opioid Postmarketing Study Program, their Goals and ClinicalTrials.gov Number.
The goal of the randomized clinical trial is to evaluate the occurrence of opioid-induced hyperalgesia, and indirectly the effectiveness of long-term opioid treatment for chronic pain but is not the focus of this paper on the observational studies.
A description of the 10 observational studies designed to estimate the serious risks of long-term opioid use among people with chronic pain, to identify predictors of serious risks and to develop validated measures to assess the risks is provided below is shown in . The goals, measurement tools of serious risks, design, data sources, and preparatory validation studies are shown in a conceptual framework in . The goals of each of the individual 11 studies and the numbers to locate their study protocols on Clintrials.gov are listed in . The elements of the 10 observational studies are summarized in . Study 1 is a prospective cohort study enrolling patients to assess misuse, abuse, and addiction rates, Study 2 is a retrospective cohort study using databases to assess overdose and death rates, Study 3, 4, and 5 are prospective validation studies enrolling patients to validate instruments that measure misuse, abuse, and addiction, Study 6 and 7 are retrospective validation studies of algorithms to identify abuse/addiction and overdose using health-care databases, Study 8 and 10 are retrospective database studies to assess the association between doctor shopping behavior and opioid abuse/addiction as measured algorithms or medical chart review, and Study 9 is a combination of a retrospective database study combined with a prospective survey of respondents identified from the database to identify self-reported abuse ()
Figure 2. Conceptual Framework for ER/LA Opioid Observational Studies Linking Goals to Measurement Tools, Design, Study Sites/Databases and Validation of Measurement Tools.
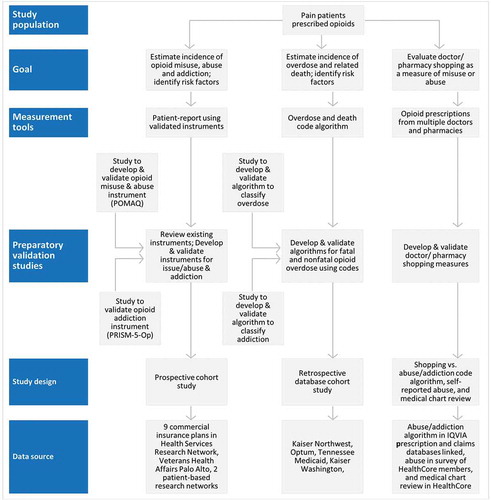
Table 2. Summary of Study Design, Data Sources, Population and Outcome Measures for 10 Postmarketing Safety Requirement Studies.
Discussion
The appropriate role of prescription opioids to treat severe chronic pain is the subject of much debate. Specifically, in light of limited or conflicting data, experts, researchers, clinicians, and lawmakers have differing opinions on the long-term efficacy and the magnitude of risk of opioid addiction, overdose, and death associated with long-term use. There are also limited data assessing the validity of using doctor/pharmacy shopping behavior as a surrogate of opioid abuse, addiction, misuse or diversion. In addition, with the evolving technological capacity for predictive analytics and machine learning, there are questions whether these techniques can be used to better identify individuals who develop opioid abuse, addiction or overdose than the diagnostic codes for these events and to predict who are at greater risk for abuse, addiction or overdose. Critical evidence gaps, therefore, exist in understanding the benefit–risk balance of long-term use of ER/LA opioids to treat chronic pain. The ER/LA opioid PMR program was designed to address these evidence gaps using the best available scientific information and the OPC was created to implement the 11 studies in the program.
The goal of the 11th study has been to conduct a randomized clinical trial to evaluate the occurrence of opioid-induced hyperalgesia, and indirectly the effectiveness of long-term opioid treatment for chronic pain, but the study has been discontinued due to logistical challenges and its redesign has been under discussion with the FDA. This paper is focused on the evaluation of the serious risks.
Differing study methodologies have been used to measure the serious risks associated with long-term opioid treatment, leading to a wide range of estimates of the incidence that complicate clinical decision-making. There is, therefore, a need for standardizing methodology to a consensus standard that a standard-setting organization like the FDA can lead. However, standard methods need to be validated to reduce inaccuracies due to misclassification. For these reasons, the FDA mandated that the OPC conduct studies and the OPC created partnerships with several leading scientific experts and regulatory authorities to develop validated instruments and diagnostic algorithms to measure the risks of opioid use in patients prescribed ER/LA opioids. The POMAQ and algorithms developed and validated will be made publicly available for other researchers to use, and the PRISM-5-OP will be available from the principal investigator, once the studies have been published.
One serious opioid risk that has been most affected by the absence of validated definitions and widely differing incidence estimates is opioid addiction. Although DSM-5 criteria no longer use the term ‘addiction’ and instead use the term ‘opioid use disorder’, the FDA preferred to use the term ‘opioid addiction’ as it is more commonly used by the general public. Substantial debate exists around whether addiction in pain patients using long-term opioid treatment has similar diagnostic criteria to illicit drug addiction or whether a condition called ‘problematic opioid use’ better describes the disorder in pain patients. The PMR studies that involve patient-reported instruments and prospective cohort evaluation (Studies 1 and 5) use a definition of ‘addiction’ as defined by DSM-5 criteria, while those that evaluated whether a diagnostic algorithm could be used to retrospectively identify the condition in claims data used a definition of ‘problematic opioid use’ (Study 7).
The OPC provided funds to a variety of academic researchers and experts to partner in the development and conduct of the studies with researchers from OPC member companies and substantial design oversight by FDA researchers, an expert panel that FDA convened, and an Advisory Board of experts that OPC convened. Contractual agreements allow the funded researchers to publish their findings, either collaboratively with the OPC or independently, ensuring transparency and intellectual freedom in communicating the results.
An important contribution to the scientific understanding of abuse and addiction from the studies is the potential to identify genetic risk factors for these outcomes from a large prospective cohort study of people with chronic pain who used opioid analgesics for at least 90 days (Study 1). Research has shown that there is a strong genetic component to the risk for addiction [Citation27], in addition to psychosocial and behavioral risk factors [Citation28]. Study 1 is collecting genetic data from consenting subjects to further understand the role of genetics in opioid risks.
A key challenge amongst these studies is enrollment of the appropriate study subjects (i.e. patients with chronic non-cancer pain prescribed long-term opioid treatment). There has been a decline in the number of new patients starting long-term opioid treatment, particularly ER/LA opioids, due to changes in clinician prescribing practices in light of the opioid epidemic. These changes have made it increasingly difficult to recruit patients into the prospective cohort studies.
Another initiative to address the opioid public health problem is the Risk Evaluation and Mitigation Strategy (REMS). The OPC differs from the REMS participating companies (RPC) as the former consists of 13 companies that are license holders for branded ER/LA opioids, while the latter consists of companies that are license holders for branded and generic ER/LA opioids and IR opioids (approximately 81 companies). The legislative authority of FDA allows it to require the conduct of REMS, but not to require postmarketing studies, from license holders of generic products. Key components of the Opioid Analgesic REMS are to make education available to prescribers and the health-care team through accredited continuing education (CE), to provide patient educational materials, and assess the impact of such activities on health outcomes [Citation29–Citation31]. In addition to the class-wide opioid PMR studies, companies that are license holders of abuse deterrent opioids are required to conduct separate postmarketing studies to evaluate abuse deterrence in real-world use. Companies may also have additional requirements related to safety signals, postmarketing utilization patterns, or pediatric use.
Despite challenges, significant progress continues to be made on conducting the 10 observational studies. The 13-member companies of the OPC, who are usually competitors, have pooled financial resources to implement a much more extensive research program than any one company alone could support and pooled research expertise to have experienced study leaders guide the implementation of studies to success. The results from one study were published soon after study completion [Citation32] and results from the remainder studies will be published on completion. These studies will provide estimates of the incidence of misuse, abuse, addiction, overdose, and death in people with chronic pain and long-term opioid use; the FDA will have been involved in reviewing and approving the final study protocols and reports and in the development and validation of the instruments used to measure the study endpoints; the studies may contribute toward safer and more appropriate prescribing of opioids to patients with severe chronic pain. The description of the study program can assist other countries and regions to design postmarketing programs of the risks of opioid analgesics or other prescription medications at risk of abuse.
Author disclosures
Paul Coplan, M. Soledad Capeda and David M. Kern are employee’s of Johnson and Johnson.
Kenneth R. Petronis and Alexandra I. Barsdorf are employee’s of Pfizer Inc.
Angela DeVeaugh-Geiss and Richard Fanelli are employees of Purdue Pharma L.P.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
All authors are members of the Opioid PMR Consortium (OPC), and employees of the safety, epidemiology or patient sciences groups within pharmaceutical companies that market analgesic drug products including opioids. The OPC focusses on the class of opioid products and no individual pharmaceutical products have been studied or mentioned in this paper or in the OPC studies.
Acknowledgments
The authors would like to acknowledge the members of OPC who contributed to the design of these studies, especially Howard Chilcoat, as well as the Advisory Board of Experts.
Additional information
Funding
References
- Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academies Press; 2011.
- Nahin RL. Estimates of pain prevalence and severity in adults: united States, 2012. J Pain. 2015;16:769–780.
- Fishbain DA, Cole B, Lewis J, et al. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9(4):444–459.
- US Food and Drug Administration. Extended release (ER) and long-acting (LA) opioid analgesic Risk Evaluation and Mitigation Strategy (REMS). [ cited January 18, 2018]. Available from: https://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/UCM367697.pdf
- Opioid PMR Consortium. Structured discontinuation vs Continued therapy in suboptimal and optimal responders to high-dose long-term opioids for chronic pain. www.clinicaltrials.gov. Identifier: NCT02741076. [cited 2018 Jan 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT02741076
- Boscarino JA, Hoffman SN, Han JJ. Opioid-use disorder among patients on long-term opioid therapy: impact of final DSM-5 diagnostic criteria on prevalence and correlates. Subst Abuse Rehabil. 2015 Aug 19;6:83–91.
- Minozzi S, Amato L, Davoli M. Development of dependence following treatment with opioid analgesics for pain relief: a systematic review. Addiction. 2013 Apr;108(4):688–698.
- Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015 Apr;156(4):569–576.
- Smith SM, Dart RC, Katz NP, et al. Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. Pain. 2013;154:2287–2296.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, Fifth Edition (DSM-5). Arlington, VA: American Psychiatric Association; 2013.
- Cepeda MS, Fife D, Kihm MA, et al. Comparison of the risks of shopping behavior and opioid abuse between tapentadol and oxycodone and association of shopping behavior and opioid abuse. Clin J Pain. 2014;30(12):1051–1056.
- Katz N, Panas L, Kim M, et al. Usefulness of prescription monitoring programs for surveillance–analysis of Schedule II opioid prescription data in Massachusetts, 1996–2006. Pharmacoepidemiol Drug Saf. 2010;19(2):115–123.
- Chilcoat HD, Coplan PM, Harikrishnan V, et al. Decreased diversion by doctor-shopping for a reformulated extended release oxycodone product (OxyContin). Drug Alcohol Depend. 2016 Aug;1(165):221–228.
- Cepeda MS, Fife D, Chow W, et al. Assessing opioid shopping behaviour: a large cohort study from a medication dispensing database in the US. Drug Saf. 2012 Apr 1;35(4):325–334.
- Postmarketing Requirements for the Class-Wide Extended-Release/Long-Acting Opioid Analgesics. [cited 2018 Jan 28]. Available from: http://wayback.archive-it.org/7993/20161022055348/http://www.fda.gov/Drugs/NewsEvents/ucm384489.htm
- Food and Drug Administration (FDA). Release from postmarketing requirement & new postmarketing requirement. [cited 2018 Jan 28]. Available from: https://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/UCM484415.pdf
- Opioid PMR Consortium. A prospective investigation of the risks of opioid misuse, abuse, and addiction among patients treated with extended-release/Long Acting (ER/LA) Opioids for the treatment of chronic pain www.clinicaltrials.gov. Identifier: NCT02751762. [cited 2018 Jul 13]. Available from: https://clinicaltrials.gov/ct2/show/NCT02751762?term=opioid+PMR&rank=5
- Opioid PMR Consortium. Incidence and predictors of opioid overdose and death in ER/LA opioid users as measured by diagnoses and death records. www.clinicaltrials.gov. Identifier: NCT02662153. [cited 2018 Jan 29]. Available from: https://clinicaltrials.gov/show/NCT02662153
- Opioid PMR Consortium. A quantitative study to assess the construct validity of the Prescription Opioid Misuse and Abuse Questionnaire (POMAQ). www.clinicaltrials.gov. Identifier: NCT02678507. [cited 2018 Jan 29]. Available from: https://clinicaltrials.gov/show/NCT02678507
- Opioid PMR Consortium. A qualitative study to assess the content validity of the Prescription Opioid Misuse and Abuse Questionnaire (POMAQ). www.clinicaltrials.gov. Identifier: NCT02660606. [cited 2018 Jan 29]. Available from: https://clinicaltrials.gov/show/NCT02660606
- Opioid PMR Consortium. Validation of PRISM-5-Op, measure of addiction to prescription opioid medication. www.clinicaltrials.gov. Identifier: NCT02660619. [cited 2018 Jan 29]. Available from: https://clinicaltrials.gov/show/NCT02660619
- Opioid PMR Consortium. Study to validate coded medical terms used to identify opioid-related overdose in databases used for PMR study 1B. www.clinicaltrials.gov. Identifier: NCT02667197. [cited 2018 Jan 29]. Available from: https://clinicaltrials.gov/show/NCT02667197
- Opioid PMR Consortium. An observational study to develop algorithms for identifying opioid abuse and addiction based on admin claims data. www.clinicaltrials.gov. Identifier: NCT02667262. [cited 2018 Jan 29]. Available from: https://clinicaltrials.gov/show/NCT02667262
- Opioid PMR Consortium. Cross-Sectional Study to Define and Validate “Doctor/Pharmacy Shopping” as Outcomes Suggestive of Abuse and/or Addiction. www.clinicaltrials.gov. Identifier: NCT02668549. [cited 2018 Jan 29]. Available from: https://clinicaltrials.gov/show/NCT02668549
- Opioid PMR Consortium. A survey to evaluate the relationship between Doctor/Pharmacy Shopping and outcomes suggestive of misuse, abuse and/or Diversion. www.clinicaltrials.gov. Identifier: NCT02667158. cited 2018 Jan 29. Available from: https://clinicaltrials.gov/show/NCT02667158
- Opioid PMR Consortium. Study to Evaluate the Relationship Between Doctor/Pharmacy Shopping & Outcomes of Misuse, Diversion, Abuse, Addiction by Medical Record Review. www.clinicaltrials.gov. Identifier: NCT02667210. cited 2019 Nov 2. Available from: https://clinicaltrials.gov/ct2/show/NCT02667210
- Windle M, Kogan SM, Lee S. Neighborhood serotonin transporter linked polymorphic region (5-HTTLPR) interactions for substance use from ages 10 to 24 years using a harmonized data set of African American children. Dev Psychopathol. 2016;28(2):415–431.
- Dasgupta N, Beletsky L, Ciccarone D. Opioid crisis: no easy fix to its social and economic determinants. Am J Public Health. 2018;108(2):182–186.
- Cepeda MS, Coplan PM, Kopper NW, et al. ER/LA opioid analgesics REMS: overview of ongoing assessments of its progress and its impact on health outcomes. Pain Med. 2017 Jan 1;18(1):78–85.
- Divino V, Cepeda MS, Coplan P, et al. Assessing the impact of the extended-release/long-acting opioid analgesics risk evaluation and mitigation strategies on opioid prescription volume. J Opioid Manag. 2017;13(3):157–168.
- Bucher Bartelson B, Le Lait MC, Green JL, et al. Changes in misuse and abuse of prescription opioids following implementation of extended-release and long-acting opioid analgesic risk evaluation and mitigation strategy. Pharmacoepidemiol Drug Saf. 2017 Sep;26(9):1061–1070.
- Walker AM, Weatherby LB, Cepeda MS, et al. Possible opioid shopping and its correlates. Clin J Pain. 2017;33(11):976–982.
