ABSTRACT
The high-purity eicosapentaenoic acid (EPA) prescription fish oil–derived omega-3 fatty acid (omega-3), icosapent ethyl (IPE), was recently approved by the United States Food and Drug Administration (FDA) for cardiovascular disease (CVD) prevention in high-risk patients. This approval is based on the 25% CVD event risk reduction observed with IPE in the pre-specified primary composite endpoint (cardiovascular [CV] death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina) in the landmark Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT). Notably, this reduction in CVD event risk with IPE was an incremental benefit to well-controlled low-density lipoprotein cholesterol; patients in REDUCE-IT had elevated triglyceride (TG) levels (135–499 mg/dL) and either had a history of atherosclerotic CVD or diabetes with additional CV risk factors. Given the CVD event risk reduction in REDUCE-IT, within a year following trial results, several global medical societies added IPE to their clinical guidelines. IPE is a stable, highly purified, FDA-approved prescription EPA ethyl ester. In contrast, mixed omega-3 products (docosahexaenoic acid + EPA combinations) have limited or no evidence for CVD event risk reduction, and nonprescription fish oil dietary supplements are not regulated as medicine by the FDA. We offer our perspective and rationale for why this evidence-based EPA-only formulation, IPE, should be added to the ‘E’ in the ABCDEF methodology for CV prevention. We provide multiple lines of evidence regarding an unmet need for CVD prevention beyond statin therapy, IPE clinical trials, IPE cost-effectiveness analyses, and proposed pleiotropic (non-lipid) mechanisms of action of EPA, as well as other relevant clinical considerations.
Introduction
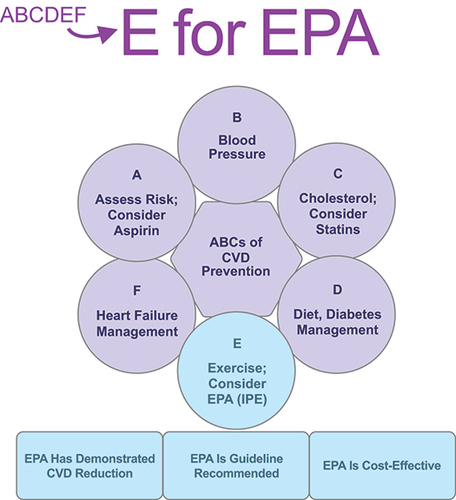
The current ‘ABCDEF’ strategy for cardiovascular disease (CVD) prevention includes the following approach: A for assessment of risk, antiplatelet therapy such as aspirin, and atrial fibrillation management; B for blood pressure management; C for cholesterol management and cessation of cigarette smoking; D for diet/lifestyle modification and diabetes management; E for exercise; and F for heart failure management [Citation1–3]. While statins are a mainstay for CVD prevention, there remains an approximate 65% residual risk of cardiovascular (CV) events that still may be present even in individuals who have well-controlled low-density lipoprotein cholesterol (LDL-C) [Citation4–7]. This residual risk for CV events represents a large unmet clinical need [Citation8].
Recently, the prescription fish oil–derived omega-3 fatty acid (omega-3), a highly purified, stable ethyl ester preparation of eicosapentaenoic acid (EPA), named icosapent ethyl (IPE; Vascepa®, Amarin Pharma, Inc., Bedminster, NJ) as an add-on to statins demonstrated a 25% reduction in atherosclerotic CVD events in the landmark Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT) [Citation9]. The strength of evidence of IPE in REDUCE-IT prompted several major medical societies to update their guidelines, practice standards, and scientific advisories within a year of announcement of the trial’s results [Citation10–13].
Here we offer evidence supporting the concept that prescription EPA (IPE) could be considered under ‘E’ in the traditional ABCDEF strategy for CVD prevention. We build our rationale for the inclusion of EPA by providing evidence of 1) unmet needs for CVD prevention beyond statin therapy with associated clinical and economic costs; 2) strong and consistent trial results in REDUCE-IT; 3) possible pleiotropic mechanisms of action of EPA; 4) cost-effectiveness analyses; 5) adoption of IPE by medical society guidelines, practice standards, and scientific statements; and 6) other relevant clinical considerations.
Unmet clinical needs in CVD prevention: a focus on hypertriglyceridemia
CVD is the leading cause of death in the United States (US), responsible for 1 in every 3 deaths [Citation14]. This translates to over 800,000 deaths annually, or 1 death every 38 seconds. Overall, 92.1 million American adults are living with some form of CVD or the after-effects of stroke. In 2017, the total (direct and indirect) costs of CVD were estimated to be 330 USD billion, and are forecasted to reach 749 USD billion by 2035 [Citation15].
An analysis from the National Health and Nutrition Examination Survey (NHANES) 2007–2014 estimates that over 56 million US adults have triglyceride (TG) levels >150 mg/dL [Citation16] and over 70 million US adults have TG levels >135 mg/dL [Citation17]. Of statin-treated adults, 15 million have TG levels >135 mg/dL. The threshold at which TG-associated CVD risk begins to increase has been reported to range from TG levels >135 mg/dL to TG levels as low as >50 mg/dL [Citation18–20]. Genetic data (mutational analyses, genome-wide association analyses, and Mendelian randomization studies), epidemiological data from general population studies, an analysis from a prospective clinical trial, and administrative claims data have suggested that TG levels and/or TG-rich lipoproteins may increase the risk for CV events [Citation21–24]. A recent article on real-world risk of CV outcomes showed that approximately 25% of patients with atherosclerotic CVD with well-controlled LDL-C (41–100 mg/dL) have elevated TG levels (135–499 mg/dL) ()), and the rate of adverse CV events increases when elevated TG levels remain untreated ()) [Citation20].
Figure 1. (a) Proportion of patients with atherosclerotic cardiovascular disease (ASCVD) in the Ontario, Canada population with hypertriglyceridemia and controlled low-density lipoprotein cholesterol (LDL-C). (b) The adjusted association between triglyceride level and cardiovascular events among individuals with ASCVD in the Ontario, Canada population. Adapted with permission from Lawler PR, Kotrri G, Koh M, et al. Real-world risk of cardiovascular outcomes associated with hypertriglyceridaemia among individuals with atherosclerotic cardiovascular disease and potential eligibility for emerging therapies. Eur Heart J. 2020;41(1):86–94, by permission of Oxford University Press [Citation20].
![Figure 1. (a) Proportion of patients with atherosclerotic cardiovascular disease (ASCVD) in the Ontario, Canada population with hypertriglyceridemia and controlled low-density lipoprotein cholesterol (LDL-C). (b) The adjusted association between triglyceride level and cardiovascular events among individuals with ASCVD in the Ontario, Canada population. Adapted with permission from Lawler PR, Kotrri G, Koh M, et al. Real-world risk of cardiovascular outcomes associated with hypertriglyceridaemia among individuals with atherosclerotic cardiovascular disease and potential eligibility for emerging therapies. Eur Heart J. 2020;41(1):86–94, by permission of Oxford University Press [Citation20].](/cms/asset/7e4478e7-ccd2-4f74-a71c-e727fb7e152d/ipgm_a_1783937_f0001_c.jpg)
Residual risk for CV events persists in cholesterol-treated patients even with very low LDL-C, indicating additional factors beyond LDL-C are critically important to address [Citation8]. Indeed, the results from a clinical trial of LDL-C–lowering therapy, evolocumab, evaluated as add-on therapy to statins, demonstrated that in spite of achieved median LDL-C as low as 30 mg/dL, significant residual risk still remained [Citation25].
Omega-3s: IPE, a stable, highly purified EPA ethyl ester
EPA is a long-chain polyunsaturated fatty acid found in fish oil [Citation26]. IPE consists of >96% pure EPA as an ethyl ester. The FDA originally approved IPE 4 g/day as an adjunct to diet to reduce TG levels in adult patients with severe hypertriglyceridemia (TG ≥500 mg/dL) based on the results of the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension (MARINE) [Citation27]. In the MARINE study, 229 diet-stable patients with very high TG levels were randomized to IPE 4 g/day, IPE 2 g/day, or placebo. IPE 4 g/day, compared with placebo, decreased TG levels by 33.1% (P = 0.0001). MARINE was followed by the ANCHOR study, a placebo-controlled, randomized, double-blind, 12-week clinical trial which compared IPE 4 g/day, IPE 2 g/day, and placebo in high-risk statin-treated patients with residually elevated TG levels (≥200–499 mg/dL) [Citation28]. Compared with placebo, IPE 4 g/day reduced TG levels by 21.5% (P < 0.0001) [Citation27,Citation28]. In both of these studies, LDL-C levels were not increased. Subsequently, based on the results of REDUCE-IT, the FDA approved an indication for IPE as an adjunct to maximally tolerated statin therapy to reduce the risk of myocardial infarction (MI), stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated TG levels (≥150 mg/dL) and established CVD or diabetes mellitus and 2 or more additional risk factors for CVD [Citation29].
CV outcomes trials (CVOTs) of EPA
JELIS
Prior to REDUCE-IT, the open-label Japan EPA Lipid Intervention Study (JELIS [NCT00231738]; N = 18,645), using 1.8 g/day of a purified EPA ethyl ester formulation available only in Japan and nearly identical to IPE reported that EPA + statin significantly lowered major coronary events by 19% compared with the statin-only control (hazard ratio [HR], 0.81; 95% CI, 0.69–0.95; P = 0.011) [Citation30]. In JELIS, men aged 45 to 75 years and postmenopausal women aged up to 75 years with or without coronary artery disease and with a total cholesterol level ≥251.35 mg/dL on statin therapy were randomized to either 1.8 g/day of EPA versus statin-only control. Following then-current Japanese treatment guidelines, patients were treated with 10 mg of pravastatin or 5 mg of simvastatin once daily as first-line treatment (the pravastatin and simvastatin doses could be increased to 20 mg and 10 mg, respectively, if hypercholesterolemia was considered ‘uncontrolled’). Baseline total cholesterol, LDL-C, high-density lipoprotein cholesterol (HDL-C), and TG levels were 275 mg/dL, 181 mg/dL, 59 mg/dL, and 153 mg/dL, respectively, in the EPA group. There were no significant differences in the change in HDL-C or LDL-C between the EPA and statin-only groups. However, there was a small but statistically significant difference noted in TG level reductions between both groups; TG levels were lowered by 9% in the EPA group versus 4% in the statin-only group (P < 0.001). Therefore, the benefits of EPA in JELIS were not attributable to TG lowering alone; rather, other ‘pleiotropic,’ or non-lipid EPA mechanisms of action likely contributed to the benefits, some of which are summarized in [Citation9,Citation31–41]. A subanalysis of primary prevention cases (n = 14,981) in JELIS found that, among patients with high TG levels (>150 mg/dL) and low HDL-C levels (<40 mg/dL), EPA + statin significantly reduced the risk of major coronary events versus statin alone by 53% (HR, 0.47; 95% CI, 0.23–0.98; P = 0.043) [Citation42]. In JELIS, EPA was generally well tolerated, with most drug-related adverse events (AEs) characterized as mild [Citation30]. AEs were slightly higher in the EPA + statin group (25.3% vs 21.7%; P < 0.0001), including low rates of gastrointestinal disorders (3.8% vs 1.7%) and low rates of bleeding (1.1% vs 0.6%).
Table 1. Select Pleiotropic Effects of EPA.
REDUCE-IT
The results of REDUCE-IT, a randomized, double-blind, placebo-controlled, multi-center, international trial, were published online in November 2018 in the New England Journal of Medicine [Citation9] and simultaneously presented at the American Heart Association (AHA) annual scientific sessions. REDUCE-IT investigated the FDA-approved prescription, high-purity, stable EPA, IPE, at 4 g/day (given as 2 g twice daily) versus placebo as an add-on to statin therapy in 8179 patients with TG levels above 135 mg/dL. The trial initially had a TG entry criterion of 150 mg/dL, which included a 10% lower TG level (135 mg/dL) due to intra-individual variability. However, the first protocol amendment in May 2013 changed the lower limit of TG entry criterion to 200 mg/dL. The trial enrolled adults ≥45 years of age with established CVD or ≥50 years of age with diabetes and at least one additional CV risk factor. In addition, patients were required to have baseline LDL-C levels of 41 to 100 mg/dL and be on stable statin treatment for at least 4 weeks. These patients were well treated, with 93% receiving moderate- to high-intensity statin therapy. This was reflected in their baseline median LDL-C levels of 74.0 mg and 76.0 mg in the IPE and control groups, respectively.
After a median follow-up of 4.9 years, patients randomized to IPE experienced a statistically significant 25% relative risk reduction (HR, 0.75: 95% CI, 0.68–0.83; P < 0.001) and an absolute risk reduction of 4.8% (95% CI, 3.1–6.5) in the primary 5-point composite endpoint of time to first event for CV death, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina (17.2% of patients in the IPE group vs 22% in the placebo group; P < 0.001; ) [Citation9,Citation72]. The number needed to treat (NNT) to prevent one primary endpoint was 21 (95% CI, 15–33) over 4.9 years. The relative risk of MI was reduced by 31%, stroke by 28%, CV death by 20%, urgent or emergent revascularization by 35%, and hospitalization for unstable angina by 32%. In addition, the key 3-point composite secondary endpoint, which consisted of CV death, nonfatal MI, or nonfatal stroke, was reduced by 26% (11.2% of patients in the IPE group vs 14.8% of patients in the placebo group; HR, 0.74; 95% CI, 0.65–0.83; P < 0.001). This translated to an NNT of 28 (95% CI, 20–47) over 4.9 years [Citation9].
Figure 2. REDUCE-IT primary endpoint: cardiovascular death, myocardial infarction, stroke, coronary revascularization, and unstable angina. The relative risk reduction was 24.8%, the absolute risk reduction was 4.8%, and the number-needed-to-treat was 21. CI, confidence interval; REDUCE-IT, Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial. From N Engl J Med, Bhatt DL, Steg G, Miller M, et al, Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia, 380(1):11–22, ©2019 Massachusetts Medical Society. Adapted with permission from Massachusetts Medical Society [Citation9,Citation72].
![Figure 2. REDUCE-IT primary endpoint: cardiovascular death, myocardial infarction, stroke, coronary revascularization, and unstable angina. The relative risk reduction was 24.8%, the absolute risk reduction was 4.8%, and the number-needed-to-treat was 21. CI, confidence interval; REDUCE-IT, Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial. From N Engl J Med, Bhatt DL, Steg G, Miller M, et al, Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia, 380(1):11–22, ©2019 Massachusetts Medical Society. Adapted with permission from Massachusetts Medical Society [Citation9,Citation72].](/cms/asset/d362eeb1-0b63-4ae7-a5bb-4d1029da14ff/ipgm_a_1783937_f0002_c.jpg)
As noted above, IPE as add-on therapy to statins achieved a 25% risk reduction in the primary composite CVD endpoint incremental to that provided by statins, resulting in an NNT of 21 over 4.9 years () [Citation72]. In a subgroup of patients with TG levels ≥200 mg/dL and HDL-C levels ≤35 mg/dL, IPE reduced the primary composite endpoint by 38% compared with placebo (P value for interaction, 0.04; NNT 11).
Another clinically relevant analysis from REDUCE-IT demonstrated that first and subsequent CVD events, in a total events analysis, were reduced by 30% (95% CI, 0.62–0.78; P < 0.0001) [Citation73]. Reductions of 25% occurred for the first CVD event, and reductions of 32%, 31%, and 48% occurred for successive second, third, and fourth CV events, respectively () [Citation73]. This is especially important given that patients who experience a first CVD event are at higher risk of subsequent events. The impact of total CVD event reduction in REDUCE-IT is substantial: for every 1000 patients treated with IPE for 5 years, approximately 159 total primary endpoints could be prevented, including 12 CV deaths, 42 MIs, 14 strokes, 76 coronary revascularizations, and 16 episodes of hospitalization for unstable angina [Citation74].
Figure 3. REDUCE-IT: reduction in first and subsequent ischemic events. The Wei-Lin-Weissfeld (WLW) method was used for 1st events, 2nd events, and 3rd events categories; a negative binomial model was used for ≥4 events and overall treatment comparison. CI, confidence interval; HR, hazard ratio; REDUCE-IT, Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial; RR, relative risk. Adapted with permission from Bhatt et al [Citation73].
![Figure 3. REDUCE-IT: reduction in first and subsequent ischemic events. The Wei-Lin-Weissfeld (WLW) method was used for 1st events, 2nd events, and 3rd events categories; a negative binomial model was used for ≥4 events and overall treatment comparison. CI, confidence interval; HR, hazard ratio; REDUCE-IT, Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial; RR, relative risk. Adapted with permission from Bhatt et al [Citation73].](/cms/asset/09028224-9451-4b41-9a37-3543c844d61c/ipgm_a_1783937_f0003_c.jpg)
Results from a pre-specified analysis of the 3146 REDUCE-IT patients from the USA subgroup who received IPE as add-on to statins demonstrated a 31% relative risk reduction (HR, 0.69; 95% CI, 0.59–0.80; P = 0.000001) in CVD events (NNT, 15) [Citation17]. Here again, consistency of results was evident with reduction in CV death of 34% (HR, 0.66; 95% CI, 0.49–0.90; P = 0.007), MI by 28% (HR, 0.72; 95% CI, 0.56–0.93; P = 0.01), and stroke by 37% (HR, 0.63; 95% CI, 0.43–0.93; P = 0.02). Unlike in the full study cohort, there was statistically significant 30% reduction in all-cause mortality in the USA subgroup (HR, 0.70; 95% CI, 0.55–0.90; P = 0.004) (NNT, 39; ). A limitation of this study is that REDUCE-IT was not powered to evaluate individual subgroups, including the pre-specified REDUCE-IT USA subgroup. Safety and tolerability findings from the USA subgroup were consistent with the full study cohort.
Figure 4. REDUCE-IT USA: all-cause mortality from 3146 patients randomized in the United States, Kaplan-Meier time-course. Importantly, all-cause mortality was not significantly reduced in the total REDUCE-IT study population; however, a statistically significant 30% reduction in all-cause mortality was observed in the USA subgroup. The relative risk reduction was 30%, the absolute risk reduction was 2.6%, and the number-needed-to-treat was 39. P for interaction = 0.02. CI, confidence interval; REDUCE-IT, Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial; Reproduced with permission from Bhatt DL, Miller M, Brinton EA, et al. REDUCE-IT USA: results from the 3,146 patients randomized in the United States. Circulation. 2020;141(5):367–375 [Citation17,Citation72].
![Figure 4. REDUCE-IT USA: all-cause mortality from 3146 patients randomized in the United States, Kaplan-Meier time-course. Importantly, all-cause mortality was not significantly reduced in the total REDUCE-IT study population; however, a statistically significant 30% reduction in all-cause mortality was observed in the USA subgroup. The relative risk reduction was 30%, the absolute risk reduction was 2.6%, and the number-needed-to-treat was 39. P for interaction = 0.02. CI, confidence interval; REDUCE-IT, Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial; Reproduced with permission from Bhatt DL, Miller M, Brinton EA, et al. REDUCE-IT USA: results from the 3,146 patients randomized in the United States. Circulation. 2020;141(5):367–375 [Citation17,Citation72].](/cms/asset/a6db0f63-7946-4b9b-8b8f-e1fd362a25d9/ipgm_a_1783937_f0004_c.jpg)
REDUCE-IT was not a TG-lowering study; like JELIS, it was designed as a CVOT, although baseline TG level was an inclusion criterion. In the IPE and control groups, baseline TG levels were 216.5 mg/dL and 216.0 mg/dL, respectively, and baseline LDL-C levels were 85.8 mg/dL and 86.7 mg/dL (Hopkins), respectively. There was a significant reduction from baseline to last visit in TG levels in both groups (21.6% in the IPE group, P < 0.001; 6.5% in the control group, P < 0.001); compared with the control group, IPE lowered TG levels by 14.1% (P < 0.001). There was a small, non-significant decrease in LDL-C levels in the IPE group (−1.2%, P = 0.14) and a significant increase in LDL-C levels in the control group (6.5%, P < 0.001). Compared with the control group, IPE lowered LDL-C levels by 7.4% (P < 0.001). Non-HDL-C levels decreased by 8.6% in the EPA group compared with the control group (P < 0.001). These IPE-induced reductions in TG, LDL-C, and non-HDL-C levels were modest and do not appear to account for any substantial portion of the CV risk reduction seen in REDUCE-IT, as shown in REDUCE-IT EPA [Citation75]. The 14 mg/dL median decrease in non-HDL-C levels from baseline would be expected to generate only a 6% to 8% reduction in CV events [Citation76]. In regards to TG lowering, primary and key secondary CVD event endpoint reductions were similar for IPE irrespective of baseline TG levels (≥150 vs <150 mg/dL or ≥200 vs <200 mg/dL) or achieved TG levels at 1 year (≥150 vs <150 mg/dL) [Citation9].
A greater percentage of patients in the IPE group than in the control group were hospitalized for atrial fibrillation or flutter, although the rates were low (3.1% vs 2.1%, respectively; P = 0.004) and the incidence was greater in patients with a previous history of atrial fibrillation or flutter [Citation77]. Adverse cardiac events typically associated with atrial fibrillation (such as heart attack, stroke, cardiac arrest, and sudden death) were each reduced by >25% in the IPE group [Citation9]. The overall rates of serious adverse bleeding events were 2.7% in the IPE group and 2.1% in the control group (P = 0.06), and there were no fatal bleeding events in either group. There were no significant differences between the IPE and control groups in rates of adjudicated hemorrhagic stroke, serious central nervous system bleeding, or gastrointestinal bleeding. As with any therapy, IPE’s noted AEs should be weighed against its efficacy in reducing CV events as part of a shared decision-making model with patients. This includes consideration of IPE’s atrial fibrillation/flutter and bleeding profile. Higher rates of peripheral edema and constipation were reported with IPE versus control (6.5% vs 5.0%, P = 0.002; 5.4% vs 3.6%, P < 0.001, respectively); however, there was no significant difference in heart failure between groups. Interestingly, there was a lower rate of anemia with IPE versus control (4.7% vs 5.8%; P = 0.03). The rates of AEs and serious AEs leading to discontinuation were similar in the two groups. There were no significant differences in total treatment-emergent AEs between the two groups.
Proposed non-lipid mechanisms of EPA
As mentioned previously, the IPE-induced reductions in TG, non-HDL-C, and LDL-C levels in REDUCE-IT cannot fully explain the magnitude of the CVD risk reduction. Thus, the non-lipid pleiotropic mechanisms of EPA may have contributed to the CVD reduction [Citation78].
Inflammation is one of the key components of atherosclerosis, and high-sensitivity C-reactive protein (hsCRP) is a nonspecific marker of inflammatory disease that has been shown to be predictive of CVD events independent of cholesterol level [Citation79,Citation80]. In a meta-analysis of population-based prospective cohort studies, hsCRP was shown to be predictive of CVD in both primary and secondary prevention [Citation81–83]. Notably, in REDUCE-IT, IPE lowered hsCRP levels by 39.9% at year 2 (P < 0.001) [Citation9]. The inflammatory hypothesis of atherothrombosis targeting a cytokine upstream to hsCRP was tested in the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS), which evaluated whether the fully human monoclonal antibody targeting interleukin-1b (canakinumab) could prevent recurrent vascular events [Citation84]. The primary endpoint of the first occurrence of nonfatal MI, nonfatal stroke, or CV death in patients taking 150 mg was reduced by 15% (HR, 0.85; 95% CI 0.74–0.98; P = 0.021) compared with placebo. Furthermore, hsCRP levels were significantly reduced by 37% in the 150 mg group compared with placebo (P < 0.001). In a secondary analysis of CANTOS, patients receiving canakinumab who achieved hsCRP levels of <2.0 mg/L had a 25% decrease in the trial’s primary endpoint compared with placebo (multivariable-adjusted HR [HRadj], 0.75; 95% CI, 0.66–0.85; P < 0.0001), whereas there was no significant between-group difference among those with on-treatment hsCRP levels ≥2.0 mg/L (HRadj, 0.90; 95% CI, 0.79–1.02; P = 0.11) [Citation85]. Likewise, in REDUCE-IT, anti-inflammatory effects of EPA, suggested by favorable changes in hsCRP, may have contributed to CVD event risk reduction.
Sudden cardiac death is frequently preceded by ventricular fibrillation (VF) or ventricular tachycardia (VT). Epidemiologic studies show lower levels of omega-3s are associated with increased risk of primary cardiac arrest [Citation86] and sudden death [Citation87]. Low EPA serum levels have been associated with increased risk of ventricular arrhythmias (VT and VF); in a study of 200 patients undergoing percutaneous coronary intervention (PCI) within 12 hours of MI and followed for 30 days, low EPA levels (<2.94% of total fatty acids), compared with high EPA levels (≥2.94%) were associated with a significantly higher incidence of ventricular arrhythmias (19.5% vs 6.2%; P = 0.009) [Citation88]. In a retrospective study in patients with Brugada syndrome, an autosomal-dominant disorder associated with characteristic ECG changes and sudden cardiac death secondary to VF, low levels of EPA independently predicted risk of cardiogenic syncope [Citation89]. The effect of EPA on arrhythmia was evaluated in 115 patients with an acute MI who underwent PCI and were randomized within 24 hours to 1.8 g/day of EPA versus control and followed for 30 days [Citation90]. EPA significantly reduced the composite endpoint of death, re-infarction, stroke, VF/flutter/paroxysmal atrial fibrillation compared with control (10.5% vs 29.3%; P = 0.01) [Citation90]. In a time-course analysis of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) Prevenzione trial, a supplement of EPA + DHA (1 g/day) significantly lowered total mortality after only 3 months of treatment by 41% (P = 0.037), with 42% of total mortality at 3 months comprising sudden cardiac death [Citation91]. In JELIS, no conclusions regarding EPA’s effect on sudden cardiac death could be made, as there were only 35 total cases, 17 (0.2%) in the control group and 18 (0.2%) in the EPA group [Citation30]. In REDUCE-IT, adjudicated sudden cardiac death, a pre-specified tertiary endpoint, was reduced by 31% in the EPA group compared with the placebo group (1.5% vs 2.1%; HR, 0.69; 95% CI 0.50–0.96) [Citation9]. However, it is appreciated that sudden cardiac death, as a pre-specified tertiary endpoint, was not adjusted for multiple comparisons and, compared with the primary and key secondary endpoints, represents a much smaller number of events.
Additional, multifaceted pleiotropic effects have been reported with EPA and are summarized in [Citation9,Citation31–41]. Some of these proposed mechanisms include anti-inflammatory, anti-oxidation, anti-arrhythmic, anti-thrombotic, anti-platelet, and cell membrane stability/signaling effects; improvement of endothelial function; reduction of cholesterol crystal domains; and plaque stabilization and/or regression [Citation9,Citation31–41,Citation92,Citation93].
Relationship between EPA levels and CVD events
JELIS
JELIS was the first CVOT to evaluate whether pure EPA (1.8 g/day) would reduce the risk of coronary artery disease; as noted previously, at a mean follow-up of 4.6 years, EPA reduced the risk of major coronary events by 19% compared with statin-only control (P < 0.011) [Citation30]. The EPA level increased from 97 mg/L to 169 mg/L in the EPA group, while it was unchanged at 93 mg/L in the control group [Citation30]. In further evaluation of JELIS, a higher plasma EPA level was inversely associated with the risk of major coronary events (HR, 0.71; P = 0.018 in the EPA intervention group) [Citation94]. Patients with on-treatment plasma EPA levels ≥150 mg/L had a 20% reduction in major coronary events versus those with plasma EPA levels <87 mg/L (adjusted HR, 0.80; P = 0.042). There was a 22% reduction in major coronary events in patients with on-treatment serum EPA levels ≥200 mg/L versus those with EPA levels <200 mg/L (HR, 0.78; 95% CI, 0.62–0.99; P = 0.043).
REDUCE-IT
The REDUCE-IT study investigators recently reported the evaluation of EPA levels and CV outcomes [Citation75]. Baseline median serum EPA level was 26.1 mg/L in both the IPE and the control group. At 5 years, the median serum EPA level and median % change from baseline in the IPE group were 158 mg/L and 463.6%, respectively; as expected, the serum EPA level and median % change from baseline in the control group was only 25.3 mg/L and −2%, respectively. The between-group difference for the median % change from baseline was 448.1% (P < 0.0001). The EPA levels achieved with IPE treatment correlated strongly (P < 0.001) with the primary endpoint and the key secondary endpoints, including CV death, MI, stroke, coronary revascularization, unstable angina, sudden cardiac death, cardiac arrest, new heart failure, and all-cause mortality. Serum EPA levels appeared to be associated with most of the relative risk reduction achieved by IPE in REDUCE-IT, with only minimal contribution by TG, LDL-C, HDL-C, non-HDL-C, apolipoprotein B, hsCRP, and remnant lipoprotein cholesterol [Citation75]. EPA’s myriad non-lipid beneficial pleiotropic effects likely contribute to its impact on reducing residual CVD event risk [Citation95]. Further investigation is warranted to elucidate these mechanisms of EPA and their possible relationship to CV event risk reduction.
Cost-effectiveness of IPE
Multiple analyses of IPE have demonstrated favorable cost-effectiveness. In a cost-effectiveness model, the ethyl ester preparation of pure EPA used in JELIS (same active moiety as IPE) led to cost savings and improved utility [Citation96]. In October 2019, the Institute for Clinical and Economic Review (ICER) published its cost-effectiveness review of IPE based on REDUCE-IT [Citation97]. The ICER report found that IPE 4 g/day as studied in REDUCE-IT resulted in 18,000 USD per quality-adjusted life-year (QALY) gained, and 16,000 USD per QALY when revascularization and unstable angina were included. For background, the AHA’s ‘high value’ standard is <$50,000 per QALY gained [Citation98]. Therefore, ICER deemed IPE ‘high long-term value for money.’ A preliminary analysis presented at the 2019 AHA annual scientific sessions found that use of IPE was projected to not only be cost-effective but also to reduce long-term healthcare costs; this analysis demonstrated that IPE led to exceptional benefit in terms of CVD event reduction as well as cost-savings in-trial and over patients’ lifetime in the majority of simulations undertaken [Citation99]. This analysis offers the rare finding of better outcomes at lower healthcare costs (dominant strategy).
Impact of REDUCE-IT findings on clinical practice recommendations
REDUCE-IT results have important implications for the role of IPE in the treatment paradigm for CVD. Indeed, after the trial results were published, several global medical societies adopted IPE into their guidelines, practice standards, or advisories. provides a summary of the recommendations from the American Diabetes Association (ADA), the AHA, the European Society of Cardiology (ESC), the European Atherosclerosis Society (EAS), and the National Lipid Association (NLA) [Citation10–13,Citation100,Citation101,Citation102].
Table 2. Statements From Medical Societies and the US FDA Regarding IPE (pure EPA) Post-REDUCE-IT.
The guideline recommendations regarding patients for whom IPE treatment would be appropriate are closely based on REDUCE-IT entry criteria and most predate the label expansion. The wording of the new FDA-approved indication () has several notable aspects that are distinct from the REDUCE-IT entry criteria and warrant further discussion. Among these is that, in contrast to REDUCE-IT entry criteria, which required TG levels to be ≥135 to <500 mg/dL during screening, the new FDA-approved indication does not specify a maximal TG level and the minimal level was set at 150 mg/dL. Another notable difference is that the new FDA indication states that patients should be on ‘maximally tolerated statin therapy,’ which was not an element of the REDUCE-IT design or any of the REDUCE-IT–related guideline updates. Maximally tolerated statin therapy is a component of the 2018 Multi-Society Blood Cholesterol Guidelines [Citation103]. Statin doses, however, were not maximized for REDUCE-IT. Instead, LDL-C was controlled, generally on moderate- to high-intensity statin therapy. The degree of LDL-C control, rather than the statin dosage, is the primary focus of cholesterol guidelines from the American Association of Clinical Endocrinologists [Citation104], EAS, and ESC [Citation13]. In contrast, maximally tolerated statin therapy is defined as the highest tolerated intensity and frequency of a statin, which can be zero in patients who are statin-intolerant [Citation105]. Statin intolerance has been defined as experiencing unacceptable AEs/significant biomarker abnormalities while on statin treatment that resolve with statin discontinuation and recur with re-challenge with ≥2 different statins (including 1 at the lowest approved dose) [Citation105–107]. Also, the FDA removed age from the indication; in REDUCE-IT, all patients had to be 45 years of age or older. Finally, the qualifying number of CV risk factors in patients with diabetes differs between the approved label and the REDUCE-IT criteria: while the study included patients 50 years of age or older with diabetes and ‘at least one additional risk factor,’ the FDA-approved indication includes patients with diabetes and ‘2 or more additional risk factors.’
Additional considerations
We explore a few additional considerations by answering some common questions.
What is the historic evidence for the use of mixed Omega-3s (EPA + DHA combinations) in CVD prevention?
Recently, two large CVOTs of mixed omega-3 fatty acids (EPA + DHA combinations) – the Vitamin D and Omega-3 Trial (VITAL) and A Study of Cardiovascular Events in Diabetes (ASCEND) – reported results. The mixed omega-3 fatty acid (EPA + DHA) combinations failed to lower primary endpoints in these CV outcome trials [Citation108,Citation109]. In addition, a recent systematic review and meta-analysis of these trials found that each 1-g/day increase in EPA intake was associated with a significant 7% relative risk reduction in major vascular events (P < 0.0001) while each 1-g/day increase in DHA intake was not (P = 0.27) [Citation110]. A limitation of this meta-analysis is that studies included were quite heterogeneous, which may have resulted in skewed data.
What is the evidence for the use of fish oil dietary supplements in CVD Prevention?
A recent review concluded that dietary fish oil supplements should not be considered as an effective substitute for prescription omega-3 fatty acids [Citation111]. Considering the totality of evidence supporting this claim, practice guidelines have advised as follows: the ADA standards of care states that other, non-prescription formulations of omega-3 lack compelling evidence for CVD event risk reduction and should not be used for prevention [Citation10]. The AHA noted in a science advisory that fish oil dietary supplements are neither reviewed nor approved by the FDA and that fish oil dietary supplements are not indicated for TG level lowering for patients with any degree of TG level elevation [Citation11]. The AHA science advisory also noted that products containing DHA may increase LDL-C levels in patients with very high TG levels [Citation11]. Preclinical and clinical studies have demonstrated that EPA and DHA have distinct tissue distributions, as well as disparate effects on membrane structure and lipid dynamics, rates of lipid oxidation, and cholesterol domains [Citation32,Citation40,Citation112,Citation113]. In laboratory studies, EPA inhibited oxidation of HDL and apoB–containing lipoproteins over a greater period of time than DHA [Citation114,Citation115]. In a membrane laboratory model, DHA, unlike EPA, altered the membrane by increasing cholesterol domain formation and membrane fluidity [Citation116]. How these membrane-stabilizing and anti-oxidant effects of EPA possibly translate to CVD risk reduction is unknown [Citation112,Citation113]. Unless and until mixed omega-3s (EPA + DHA combinations) show compelling evidence of CV benefit, REDUCE-IT findings should not be extrapolated to other omega-3 fatty acid products, including other prescription products.
What are some considerations regarding the use of fibrates or niacin in CVD prevention?
Niacin and fibrates in large outcome studies, as add-on therapy to statins, failed to meet their primary CV endpoints. In the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study, the addition of niacin to a statin did not show CV benefit compared with statin alone. In the Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), the niacin group not only failed to achieve its primary CV endpoint, but had an increased risk of serious AEs [Citation117,Citation118]. Similarly, studies of fibrates, including the Action to Control Cardiovascular Risk in Diabetes (ACCORD Lipid) and Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) failed to achieve their primary CV endpoints, although fibrates may potentially benefit patients with high TG and low HDL levels [Citation119–121]. Given the lack of positive CVOT data associated with the combined use of statin with fibrates or niacin to date, the 2019 ADA Standards of Care states that combinations of statins with either fibrates or with niacin are generally not recommended for CV event prevention (Level A evidence; ) [Citation100]. The FDA also removed the indications for the use of fibrates and niacin with statins in 2016 due to a lack of benefit in CVOTs with these combinations. An ongoing CVOT utilizing a newer fibrate (pemafibrate) in patients with type 2 diabetes, elevated TG levels, and on statin therapy (PROMINENT; NCT03071692) may be informative.
What CVOTs of other EPA or mixed omega-3 combinations are ongoing or recently terminated?
A few CV outcomes trials of other omega-3s of particular interest are ongoing* or recently terminated** [Citation32]:
RESPECT-EPA* (UMIN000012069) [Citation122]: The Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy–Statin and Eicosapentaenoic Acid is currently following approximately 3900 patients with established atherosclerotic CVD, on a statin, randomized to 1.8 g/day EPA versus no EPA to explore benefit on CV outcomes.
STRENGTH** (NCT02104817) [Citation123]: The Statin Residual Risk Reduction With Epanova in High CV Risk Patients With Hypertriglyceridemia trial was aimed at evaluating the impact of prescription mixed EPA + DHA omega-3 carboxylic acids on CV outcomes. However, following the recommendation from an independent Data Monitoring Committee, the trial was closed due to its low likelihood of demonstrating CV benefit.
OMEMI* (NCT01841944) [Citation124]: The Omega-3 Fatty Acids in Elderly Patients With Acute Myocardial Infarction trial includes 1400 participants aged 70 to 82 years with acute MI who were discharged from the hospital. Patients are randomized to 1.8 g/day EPA + DHA to assess whether supplementation in addition to modern therapy versus corn oil placebo would reduce the combined CV endpoint of death, nonfatal MI, stroke, and revascularization by at least 30% over a planned 2-year follow-up period. The mixed omega 3 dietary supplement used in this study is Pikasol (3 capsules [3 g concentrated fish oil] needed to provide the 1.8 g of omega-3s [~930 mg EPA and ~660 mg DHA]).
If, in the future, a mixed omega-3 product shows a compelling CV outcome benefit, the ‘E’ in the ABCDEF CV prevention strategy may be revisited and expanded to also include such an EPA + DHA combination product. Until then, high-purity EPA as IPE holds the evidence.
Conclusions
Despite the use of statins and other cholesterol-lowering therapies, substantial residual CVD event risk poses a major healthcare and economic challenge, even with well-controlled LDL-C levels. The cost of CVD is substantial. In the landmark CV outcomes trial REDUCE-IT, IPE, a stable, highly purified prescription EPA at 4 g/day, decreased CVD events by 25% when added to statins. This represents the largest CVD event risk reduction of any therapy studied in addition to statins and led to the FDA expanding the approval of IPE to include CVD event prevention. The benefits in CVD event risk reduction with IPE are likely due to multifactorial pleiotropic mechanisms beyond TG level lowering. IPE has been deemed very cost-effective in multiple analyses and has been added to several global practice guidelines. IPE, a highly purified and stable ethyl ester preparation of EPA, holds evidence of CV benefit whereas mixed omega-3s (combinations of EPA + DHA) have failed to impress in recent CV outcome trials. Fish oil dietary supplements are not regulated as medicine by the FDA. Therefore, due to all of the evidence presented, we believe that evidence-based EPA as IPE should be considered as a new entry under ‘E’ in the ABCDEFs for CVD prevention as a new standard of care.
Declaration of interest
Kamini Trivedi is a former stock shareholder of Amarin Pharma, Inc.
Viet Le reports no conflicts of interest.
John R. Nelson is a former stock shareholder of and currently serves as a speaker’s bureau member, consultant, and advisor to Amarin Pharma Inc., from which he has received honorarium.
Declaration of interest
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
A reviewer on this manuscript has disclosed that they are a REDUCE-IT Steering Committee member. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgments
Editing, manuscript review, formatting, reference checks, obtaining permissions, figure/table design support, and submission preparation provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA, and funded by Amarin Pharma, Inc, Bedminster, NJ, USA.
References
- Arnett DK, Blumenthal RS, Albert MA, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;74(10):e177–e232.
- Alfaddagh A, Arps K, Blumenthal RS, et al. The ABCs of primary cardiovascular prevention: 2019 update. J Am Coll Cardiol. 2019. [updated 2019 Mar 21, cited 2019 Mar 21]. Available from: https://www.acc.org/latest-in-cardiology/articles/2019/03/21/14/39/abcs-of-primary-cv-prevention-2019-update-gl-prevention
- Arps K, Pallazola VA, Cardoso R, et al. Clinician’s guide to the updated ABCs of cardiovascular disease prevention: a review part 2. Am J Med. 2019;132(7):e599–e609. Epub 2019/ 02/05.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas coronary atherosclerosis prevention Study. JAMA. 1998;279(20):1615–1622.
- Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–1158.
- Ganda OP, Bhatt DL, Mason RP, et al. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018;72(3):330–343.
- Bhatt DL, Steg G, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22.
- American Diabetes Association. Living standards of medical care in diabetes. Arlington County, VA: American Diabetes Association; 2019 [updated 2019 Jul 31, cited 2019 Jul 31]. Available from: https://care.diabetesjournals.org/living-standards#2018
- Skulas-Ray AC, Wilson PWF, Harris WS, et al. Omega-3 fatty acids for the management of hypertriglyceridemia: A science advisory from the American Heart Association. Circulation. 2019;140(12):e673–e691.
- National Lipid Association. NLA position on the use of icosapent ethyl in high and very-high risk patients. Jacksonville, FL: National Lipid Association; 2019 [cited 2019 Sep 16]. Available from: https://www.lipid.org/nla/nla-position-use-icosapent-ethyl-high-and-very-high-risk-patients
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41(1):111–188.
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492.
- Heart disease and stroke statistics 2018 at-a-glance: American Heart Association. American Stroke Association; 2018 [cited 2018 Feb 5]. Available from: http://professional.heart.org/idc/groups/ahamah-public/@wcm/@sop/@smd/documents/downloadable/ucm_498848.pdf
- Fan W, Philip S, Granowitz C, et al. Hypertriglyceridemia in statin-treated US adults: the National Health and Nutrition examination survey. J Clin Lipidol. 2019;13:100–108.
- Bhatt DL, Miller M, Brinton EA, et al. REDUCE-IT USA: results from the 3,146 patients randomized in the United States. Circulation. 2020;141(5):367–375.
- Navar AM, Pagidipati N, Mulder H, et al. Triglycerides as a risk factor for coronary heart disease: what measure and what cutoff? [abstract]. J Am Coll Cardiol. 2019;73(9(suppl1)). DOI:10.1016/S0735-1097(19)32471-4.
- Pedersen SB, Langsted A, Nordestgaard BG. Nonfasting mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis. JAMA Intern Med. 2016;176(12):1834–1842.
- Lawler PR, Kotrri G, Koh M, et al. Real-world risk of cardiovascular outcomes associated with hypertriglyceridaemia among individuals with atherosclerotic cardiovascular disease and potential eligibility for emerging therapies. Eur Heart J. 2020;41(1):86–94.
- Miller M, Cannon CP, Murphy SA, et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51(7):724–730.
- Budoff M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol. 2016;118(1):138–145.
- Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118(4):547–563.
- Toth PP, Granowitz C, Hull M, et al. High triglycerides are associated with increased cardiovascular events, medical costs, and resource utilization: a real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J Am Heart Assoc. 2018;7(15):e008740.
- Nelson JR, True WS, Le V, et al. Can pleiotropic effects of eicosapentaenoic acid (EPA) impact residual cardiovascular risk? Postgrad Med. 2017;129(8):822–827.
- Jump DB, Depner CM, Tripathy S. Omega-3 fatty acid supplementation and cardiovascular disease. J Lipid Res. 2012;53(12):2525–2545.
- Bays HE, Ballantyne CM, Kastelein JJ, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol. 2011;108(5):682–690.
- Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012;110(7):984–992.
- Sperling LS, Nelson JR. History and future of omega-3 fatty acids in cardiovascular disease. Curr Med Res Opin. 2016;32(2):301–311.
- Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–1098.
- Brinton EA, Ballantyne CM, Bays HE, et al. Effects of icosapent ethyl on lipid and inflammatory parameters in patients with diabetes mellitus-2, residual elevated triglycerides (200–500 mg/dL), and on statin therapy at LDL-C goal: the ANCHOR study. Cardiovasc Diabetol. 2013;12(1):100.
- Mason RP. New insights into mechanisms of action for omega-3 fatty acids in atherothrombotic cardiovascular disease. Curr Atheroscler Rep. 2019;21(2).
- Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242(1):357–366.
- Katoh A, Ikeda H. Daily intake of eicosapentaenoic acid inhibits the progression of carotid intimal-media thickness in patients with dyslipidemia [in Japanese]. Ther Res. 2011;32(6):863–868.
- Budoff M, Brent Muhlestein J, Le VT, et al. Effect of Vascepa (icosapent ethyl) on progression of coronary atherosclerosis in patients with elevated triglycerides (200-499 mg/dL) on statin therapy: rationale and design of the EVAPORATE study. Clin Cardiol. 2018;41(1):13–19.
- Watanabe T, Ando K, Daidoji H, et al. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol. 2017;70(6):537–544.
- Konishi T, Sunaga D, Funayama N, et al. Eicosapentaenoic acid therapy is associated with decreased coronary plaque instability assessed using optical frequency domain imaging. Clin Cardiol. 2019;42(6):618–628.
- Mita T, Watada H, Ogihara T, et al. Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2 diabetes. Atherosclerosis. 2007;191(1):162–167.
- Mason RP, Chowdhury S. Omega-3 products: what’s inside? HealthLine. 2016;2(1):6–7.
- Mason RP, Jacob RF, Shrivastava S, et al. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim Biophys Acta. 2016;1858(12):3131–3140.
- Mason RP, Dawoud H, Jacob RF, et al. Eicosapentaenoic acid improves endothelial function and nitric oxide bioavailability in a manner that is enhanced in combination with a statin. Biomed Pharmacother. 2018;103:1231–1237.
- Saito Y, Yokoyama M, Origasa H, et al. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis. 2008;200(1):135–140.
- Gryglewski RJ, Salmon JA, Ubatuba FB, et al. Effects of all cis-5,8,11,14,17 eicosapentaenoic acid and PGH3 on platelet aggregation. Prostaglandins. 1979;18(3):453–478.
- Li XL, Steiner M. Fish oil: a potent inhibitor of platelet adhesiveness. Blood. 1990;76(5):938–945.
- Kramer HJ, Stevens J, Grimminger F, et al. Fish oil fatty acids and human platelets: dose-dependent decrease in dienoic and increase in trienoic thromboxane generation. Biochem Pharmacol. 1996;52(8):1211–1217.
- Dyerberg J, Bang HO, Stoffersen E, et al. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;2(8081):117–119.
- Nishio R, Shinke T, Otake H, et al. Stabilizing effect of combined eicosapentaenoic acid and statin therapy on coronary thin-cap fibroatheroma. Atherosclerosis. 2014;234(1):114–119.
- Miller M, Ballantyne CM, Bays HE, et al. Effects of icosapent ethyl (eicosapentaenoic acid ethyl ester) on atherogenic lipid/lipoprotein, apolipoprotein, and inflammatory parameters in patients with elevated high-sensitivity C-reactive protein (from the ANCHOR study). Am J Cardiol. 2019;124(5):696–701.
- Mo Z, Tang C, Li H, et al. Eicosapentaenoic acid prevents inflammation induced by acute cerebral infarction through inhibition of NLRP3 inflammasome activation. Life Sci. 2020;242:117133.
- Lee KR, Midgette Y, Shah R. Fish oil derived omega 3 fatty acids suppress adipose NLRP3 inflammasome signaling in human obesity. J Endocr Soc. 2019;3(3):504–515.
- Albracht-Schulte K, Gonzalez S, Jackson A, et al. Eicosapentaenoic acid improves hepatic metabolism and reduces inflammation independent of obesity in high-fat-fed mice and in HepG2 cells. Nutrients. 2019;11(3):599.
- Allam-Ndoul B, Guenard F, Barbier O, et al. Effect of n-3 fatty acids on the expression of inflammatory genes in THP-1 macrophages. Lipids Health Dis. 2016;15:69.
- Kawashima A, Harada T, Imada K, et al. Eicosapentaenoic acid inhibits interleukin-6 production in interleukin-1beta-stimulated C6 glioma cells through peroxisome proliferator-activated receptor-gamma. Prostaglandins Leukot Essent Fatty Acids. 2008;79(1–2):59–65.
- Jaudszus A, Gruen M, Watzl B, et al. Evaluation of suppressive and pro-resolving effects of EPA and DHA in human primary monocytes and T-helper cells. J Lipid Res. 2013;54(4):923–935.
- Satoh-Asahara N, Shimatsu A, Sasaki Y, et al. Highly purified eicosapentaenoic acid increases interleukin-10 levels of peripheral blood monocytes in obese patients with dyslipidemia. Diabetes Care. 2012;35(12):2631–2639.
- Yamada H, Yoshida M, Nakano Y, et al. In vivo and in vitro inhibition of monocyte adhesion to endothelial cells and endothelial adhesion molecules by eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2008;28(12):2173–2179.
- Arita M, Ohira T, Sun YP, et al. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178(6):3912–3917.
- Oh SF, Dona M, Fredman G, et al. Resolvin E2 formation and impact in inflammation resolution. J Immunol. 2012;188(9):4527–4534.
- Isobe Y, Arita M, Matsueda S, et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J Biol Chem. 2012;287(13):10525–10534.
- Golzari MH, Hosseini S, Koohdani F, et al. The effect of eicosapentaenoic acid on the serum levels and enzymatic activity of paraoxonase 1 in the patients with type 2 diabetes mellitus. Acta Med Iran. 2017;55(8):486–495.
- Golzari MH, Javanbakht MH, Ghaedi E, et al. Effect of eicosapentaenoic acid supplementation on paraoxonase 2 gene expression in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Clin Nutr Res. 2019;8(1):17–27.
- Sherratt SCR, Juliano RA, Mason RP. Eicosapentaenoic acid (EPA) has optimal chain length and degree of unsaturation to inhibit oxidation of small dense LDL and membrane cholesterol domains as compared to related fatty acids in vitro. Biochimica Et Biophysica Acta Biomembranes. 2020;1862:183254. Epub ahead of print.
- Okuda Y, Kawashima K, Sawada T, et al. Eicosapentaenoic acid enhances nitric oxide production by cultured human endothelial cells. Biochem Biophys Res Commun. 1997;232(2):487–491.
- Fukumoto K, Takemoto Y, Yoshikawa J, et al. Increase in EPA/AA ratio predicts improvement in endothelial function in purified eicosapentaenoic acid-treated patients [abstract P6205]. Eur Heart J. 2019;40(suppl 1):3816.
- Yamakawa K, Shimabukuro M, Higa N, et al. Eicosapentaenoic acid supplementation changes fatty acid composition and corrects endothelial dysfunction in hyperlipidemic patients. Cardiol Res Pract. 2012;2012:754181.
- Sasaki J, Miwa T, Odawara M. Administration of highly purified eicosapentaenoic acid to statin-treated diabetic patients further improves vascular function. Endocr J. 2012;59(4):297–304.
- Toyama K, Nishioka T, Isshiki A, et al. Eicosapentaenoic acid combined with optimal statin therapy improves endothelial dysfunction in patients with coronary artery disease. Cardiovasc Drugs Ther. 2014;28(1):53–59.
- Tanaka N, Irino Y, Shinohara M, et al. Eicosapentaenoic acid-enriched high-density lipoproteins exhibit anti-atherogenic properties. Circ J. 2018;82(2):596–601.
- Itoh M, Suganami T, Satoh N, et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol. 2007;27(9):1918–1925.
- Xiao YF, Gomez AM, Morgan JP, et al. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc Natl Acad Sci USA. 1997;94(8):4182–4187.
- Xiao YF, Ke Q, Wang SY, et al. Single point mutations affect fatty acid block of human myocardial sodium channel alpha subunit Na+ channels. Proc Natl Acad Sci USA. 2001;98(6):3606–3611.
- Bhatt DL, Steg G, Miller M, et al. Reduction of cardiovascular events with icosapent ethyl–intervention trial [oral presentation]. Chicago (IL); 2018 Nov 10–12.
- Bhatt DL, Steg G, Miller M, et al. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;380:2791–2802.
- Bhatt DL, Steg PG, Miller M, et al. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73(22):2791–2802.
- Bhatt DL, Miller M, Steg G, et al. EPA levels and cardiovascular outcomes in the reduction of cardiovascular events with icosapent ethyl-intervention trial [oral presentation]. Chicago (IL); 2020 Mar 28-30;380:2791–2802.
- Kastelein JJP, Stroes ESG. FISHing for the miracle of eicosapentaenoic acid. N Engl J Med. 2019;380(1):89–90.
- Vascepa [package insert]. Bridgewater (NJ): Amarin Pharma Inc.; 2019.
- Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2020;40:1135–1147. Epub ahead of print.
- Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–979.
- Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843.
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387–1397.
- Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321(7255):199–204.
- Singh TP, Morris DR, Smith S, et al. Systematic review and meta-analysis of the association between c-reactive protein and major cardiovascular events in patients with peripheral artery disease. Eur J Vasc Endovasc Surg. 2017;54(2):220–233.
- Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131.
- Ridker PM, MacFadyen JG, Everett BM, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319–328.
- Siscovick DS, Raghunathan TE, King I, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274(17):1363–1367.
- Albert CM, Campos H, Stampfer MJ, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346(15):1113–1118.
- Endo T, Tomita H, Higuma T, et al. Low serum eicosapentaenoic acid level is a risk for ventricular arrhythmia in patients with acute myocardial infarction: a possible link to J-waves. Heart Vessels. 2014;29(6):847–854.
- Yagi S, Soeki T, Aihara KI, et al. Low serum levels of eicosapentaenoic acid and docosahexaenoic acid are risk factors for cardiogenic syncope in patients with Brugada syndrome. Int Heart J. 2017;58(5):720–723.
- Doi M, Nosaka K, Miyoshi T, et al. Early eicosapentaenoic acid treatment after percutaneous coronary intervention reduces acute inflammatory responses and ventricular arrhythmias in patients with acute myocardial infarction: a randomized, controlled study. Int J Cardiol. 2014;176:577–582.
- Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105(16):1897–1903.
- Den Ruijter HM, Berecki G, Verkerk AO, et al. Acute administration of fish oil inhibits triggered activity in isolated myocytes from rabbits and patients with heart failure. Circulation. 2008;117(4):536–544.
- Leaf A. The electrophysiological basis for the antiarrhythmic actions of polyunsaturated fatty acids. Eur Heart J Suppl. 2001;3(supplD):D98–D105.
- Itakura H, Yokoyama M, Matsuzaki M, et al. Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb. 2011;18(2):99–107.
- Le V, Nelson JR. Eicosapentaenoic acid: pleiotrope extraordinaire? LipidSpin. 2017;15(1):12–14 35.
- Philip S, Chowdhury S, Nelson JR, et al. A novel cost-effectiveness model of prescription eicosapentaenoic acid extrapolated to secondary prevention of cardiovascular diseases in the United States. J Med Econ. 2016;19(10):1003–1010.
- Ollendorf D, McQueen R, Fazioli K, et al. Additive therapies for cardiovascular disease: effectiveness and value. Institute for Clinical and Economic Review; 2019 Nov 11 [cited 2020 Jun 25]. Available from: https://icer-review.org/material/cvd-final-evidence-report/
- Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association task force on performance measures and task force on practice guidelines. Circulation. 2014;129(22):2329–2345.
- Weintraub WS, Bhatt DL, Zhang Z, et al. Cost-effectiveness of icosapent ethyl in REDUCE-IT [abstract]. Presentation at the American Heart Association meeting. Philadelphia (PA); 2019 Nov 16–18.
- American Diabetes Association. Standards of medical care in diabetes - 2019. Diabetes Care. 2019;41(suppl 1):S1–S193.
- Arnold SV, Bhatt DL, Barsness GW, et al. Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2020;141(19):e779–e806.
- Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111–s134.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350.
- Jellinger PS, Handelsman Y, Rosenblit PD, et al. American association of clinical endocrinologists and American college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease: executive summary. Endocr Pract. 2017;23(4):479–497.
- Baum SJ, Toth PP, Underberg JA, et al. PCSK9 inhibitor access barriers-issues and recommendations: improving the access process for patients, clinicians and payers. Clin Cardiol. 2017;40(4):243–254.
- Banach M, Rizzo M, Toth PP, et al. Statin intolerance - an attempt at a unified definition. Position paper from an international lipid expert panel. Arch Med Sci. 2015;11(1):1–23.
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68(1):92–125.
- Manson JE, Cook NR, Lee IM, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23–32.
- ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379(16):1540–1550.
- Marston NA, Giugliano RP, Im K, et al. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: A systematic review and meta-regression analysis of randomized controlled trials. Circulation. 2019;140(16):1308–1317.
- Sherratt SCR, Lero M, Mason RP. Are dietary fish oil supplements appropriate for dyslipidemia management? A review of the evidence. Curr Opin Lipidol. 2020;31(2):94–100.
- Nelson JR, Wani O, May HT, et al. Potential benefits of eicosapentaenoic acid on atherosclerotic plaques. Vascul Pharmacol. 2017;91:1–9.
- Sato T, Horikawa M, Takei S, et al. Preferential incorporation of administered eicosapentaenoic acid into thin-cap atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2019;39(9):1802–1816.
- Sherratt SCR, Mason RP. Eicosapentaenoic acid inhibits oxidation of high density lipoprotein particles in a manner distinct from docosahexaenoic acid. Biochem Biophys Res Commun. 2018;496(2):335–338.
- Mason RP, Sherratt SCR, Jacob RF. Eicosapentaenoic acid inhibits oxidation of apoB-containing lipoprotein particles of different size in vitro when administered alone or in combination with atorvastatin active metabolite compared with other triglyceride-lowering agents. J Cardiovasc Pharmacol. 2016;68(1):33–40.
- Mason RP, Sherratt SCR. Omega-3 fatty acid fish oil dietary supplements contain saturated fats and oxidized lipids that may interfere with their intended biological benefits. Biochem Biophys Res Commun. 2017;483(1):425–429.
- The AIM-HIGH Investigators, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–2267.
- The HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203–212.
- The ACCORD Study Group, Ginsberg HN, Elam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–1574.
- The FIELD Study Investigators, Keech A, Simes RJ, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849–1861.
- Fruchart JC, Sacks FM, Hermans MP. Implications of the ACCORD lipid study: perspective from the Residual Risk Reduction Initiative (R(3)i). Curr Med Res Opin. 2010;26(8):1793–1797.
- Randomized trial for evaluation in secondary prevention efficacy of combination therapy - statin and eicosapentaenoic acid UMIN000012069. UMIN Clinical Trials Registry; 2016 [updated 2017 Jan 9]. Available from: https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000014051&type=summary&language=E
- Nicholls SJ, Lincoff AM, Bash D, et al. Assessment of omega-3 carboxylic acids in statin treated patients with high levels of triglycerides and low levels of high density lipoprotein cholesterol: rationale and design of the STRENGTH trial. Clin Cardiol. 2018;41(10):1281–1288.
- Laake K, Myhre P, Nordby LM, et al. Effects of omega3 supplementation in elderly patients with acute myocardial infarction: design of a prospective randomized placebo controlled study. BMC Geriatr. 2014;14:74.