ABSTRACT
While A1C is the standard diagnostic test for evaluating long-term glucose management, additional glucose data, either from fingerstick blood glucose testing, or more recently, continuous glucose monitoring (CGM), is necessary for safe and effective management of diabetes, especially for individuals treated with insulin. CGM technology and retrospective pattern-based management using various CGM reports have the potential to improve glycemic management beyond what is possible with fingerstick blood glucose monitoring. CGM software can provide valuable retrospective data on Time-in-Ranges (above, below, within) metrics, the Ambulatory Glucose Profile (AGP), overlay reports, and daily views for persons with diabetes and their healthcare providers. This data can aid in glycemic pattern identification and evaluation of the impact of lifestyle factors on these patterns. Time-in-Ranges data provide an easy-to-define metric that can facilitate goal setting discussions between clinicians and persons with diabetes to improve glycemic management and can empower persons with diabetes in self-management between clinic consultation visits. Here we discuss multiple real-life scenarios from a primary care clinic for the application of CGM in persons with diabetes. Optimizing the use of the reports generated by CGM software, with attention to time in range, time below range, and postprandial glucose-induced time above range, can improve the safety and efficacy of ongoing glucose management.
© 2021 ELI LILLY AND COMPANY. Published with license by Taylor & Francis Francis Group, LLC.
Introduction
Evolution of glycemic metrics
It is estimated that the global prevalence of diabetes has nearly doubled in the adult population since the 1980s, making it a significant contributor to both morbidity and mortality worldwide [Citation1,Citation2]. The U.S. has been substantially impacted by this public health epidemic, with the CDC estimating that over 10% of the U.S. population has diabetes as of 2018, the vast majority (90–95%) of which is type 2 diabetes (T2D) [Citation3].
Promising therapies for the management of diabetes have emerged in the last 20 years, yet improved outcomes have been slow to follow [Citation4,Citation5]. Many factors have impacted the ability to optimize diabetes management, including issues of cost of care and access to care globally and nationally, and suboptimal therapeutic intensification or titration on an individual level. In fact, nationally we are seeing a resurgence in diabetes-related complications [Citation6]. Continuous glucose monitoring (CGM) technology as well as retrospective pattern-based management using CGM reporting tools such as the Ambulatory Glucose Profile (AGP) have the potential to dramatically improve glycemic management. Strategies to optimally use CGM data to improve the care of persons with diabetes (PwD) will be discussed.
A1C
The A1C test, which correlates to average of blood glucose (BG) values over a 2–3-month period, remains the standard diagnostic test for evaluating long-term glucose management. Studies have shown that higher A1C values are associated with an increased risk of developing complications [Citation7–10]. Importantly, A1C can also be inaccurate in certain physiological conditions such as chronic kidney disease, liver disease, anemia, and hemoglobinopathies and can be impacted by some medications, blood loss or transfusions, and pregnancy [Citation11].
Patient-specific A1C targets are currently recommended to improve health outcomes, with an A1C target of <7.0% recommended for many nonpregnant adults [Citation12]. Less intensive targets are sometimes used based on specific patient conditions and needs (e.g. frailty, elderly, history of severe hypoglycemia, and comorbidities). Both fasting blood glucose (FBG) and postprandial glucose (PPG) control are important determinants of A1C [Citation13–19].
While it is clear from multiple randomized control trials that achieving A1C targets is important in managing diabetes and preventing complications, reaching these glycemic goals remains a challenge for many PwD . Only slightly over 50% of adults with diabetes attained an A1C of <7.0% in the U.S. [Citation17–19], and the percentage of PwD attaining this A1C target has not improved significantly since 2005 [Citation4,Citation5].
Although A1C is a well-validated metric, it is a suboptimal tool for managing individual patients on a day-to-day basis. First, A1C may not reflect the quality of glycemic control. Since it represents an overall average, a given A1C level fails to capture the day-to-day variability that may exist [Citation20]. Second, the accuracy of A1C can be affected by a range of physiologic factors [Citation21]. Third, because of the length of time being measured (and time between measurements), A1C provides little in the way of actionable insights [Citation22,Citation23]. Glucose variability is correlated with an increased incidence of hypoglycemia among insulin users and may have a direct impact on safety, short-term health outcomes, and an increased risk for developing long-term complications [Citation24]. In addition, glucose excursions have been reported to have acute negative effects on quality-of-life metrics such as mental acuity, physical performance, and emotional stability [Citation25,Citation26].
Two viable alternatives to the A1C test, glycated albumin and fructosamine tests, measure the level of glycated serum proteins [Citation27]. While glycated albumin measures the percentage of serum albumin which is attached to a glucose molecule, fructosamine measures all ketoamine linkages resulting from serum protein glycation. The shorter half-life of these glycated biomarkers relative to A1C means that these tests reflect the average glycemia of the previous 2–3 weeks rather than 3 months. The 1,5-anhydroglucitol (1,5 AG), commercially available as the GlycoMark test, is another tool used to evaluate glucose control, is primarily used for assessing PPG excursions [Citation28]. When used in conjunction with A1C, the GlycoMark test provides a valuable metric, particularly for those patients who cannot (or will not) utilize CGM.
Monitoring of glucose values daily at various times is an important component of diabetes management in insulin-treated individuals and those on therapy predisposing to hypoglycemia (typically insulin, sulfonylurea, or meglitinide therapy). The methods currently employed include self-monitoring of BG (SMBG, or fingerstick BG monitoring) and CGM.
SMBG
SMBG captures point-in-time BG levels to inform decisions on adjusting insulin dose and more effectively manage ones’ diabetes; the frequency of glucose monitoring is dependent on a variety of factors relating to type of therapy, intensity of management, and hypoglycemia risk [Citation29]. Evidence has shown that among people with T1D, those utilizing frequent SMBG testing had lower A1C levels [Citation30]. While SMBG shows BG levels at specific times of day, it can be difficult to appreciate overall patterns, especially at times of day when testing is infrequent [Citation31].
CGM
CGM is a method of glucose monitoring that tracks glucose levels at frequent intervals throughout the day and night [Citation31]. A CGM system consists of a sensor, which provides glucose data based on measurements from interstitial fluid every 1–15 minutes. A real-time CGM system wirelessly transmits these data to a receiver which can be a stand-alone device, a smartphone, a smartwatch, or an insulin pump. CGM provides information about the current glucose levels and trend as well as the rate of change, which allows patients to respond immediately to signs of impending hypoglycemia and hyperglycemia. Real-time CGM data, including real-time glucose levels, trend arrows, and use of alarms, have the potential to improve the ease and safety of day-to-day diabetes management. Real-time data provide immediate feedback regarding the impact of dietary choices, exercise, medication changes, and other life-variables. In contrast to the real-time CGM, Flash GM (FGM, also known as intermittently scanned CGM or isCGM) uses a sensor that must be scanned with a reader or smart device in order to retrieve the glucose data [Citation32]. These systems do not currently include alarms that alert the PwD to trends of hypo- or hyperglycemia, although this capability is planned for future versions. CGM devices are described in .
Table 1. CGM Devices
In addition to providing real-time glucose data, CGM collects a wealth of retrospective data. Individuals and clinicians who interpret and leverage this powerful retrospective data can gain significant insights that may be applied toward future glycemic management. CGM-based glycemic metrics allow for qualitative analysis that was not previously attainable with A1C and SMBG. However, optimal outcomes associated with CGM are dependent on the patients’ adherence to wearing the device [Citation33].
PPG
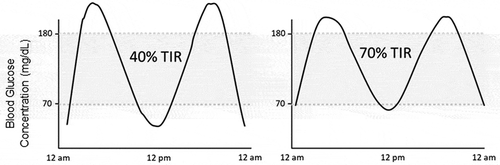
A given A1C can reflect a rather wide spectrum of glycemia when viewed as CGM-based metrics and glycemic profile (). While the two examples in reflect an A1C of ~7.0%, Times-in-Range vary considerably. CGM-based reports can provide a more accurate picture of true glycemic management. The PPG concentration, for instance, is a valuable indicator of acute glycemic control [Citation34] but can be under-reflected in an A1C measurement in spite of well-established association between PPG levels and longer-term A1C values [Citation15,Citation35]. Based on Monnier’s study, the relative impact of PPG was high (~70%) in patients with an A1C of <7.3% [Citation15]. The contribution of FBG increased in patients with worsening glycemic control (A1C >7.3%). Elevated PPG has also been associated with macrovascular dysfunction [Citation36]. Elevated PPG leads to endothelial activation and dysfunction mainly driven by oxidative stress. These data indicate that improving PPG excursions may be beneficial to long-term glycemic control and cardiovascular risk [Citation12,Citation16,Citation37].
Figure 1. Identical A1C values, but dramatically different amounts time spent in hypoglycemia and hyperglycemia. Two representative glucose profiles with the same A1C of ~7.0%. The TIR for the representative figures are 40% and 70%. Data from https://diatribe.org/time-range
CGM-based glycemic metrics
As the use of CGM has evolved, there has been an increasing need for CGM-based therapeutic targets to help guide therapy. In 2019 a panel of experts defined targets for CGM-based glycemic metrics [Citation38]; these metrics have since become accepted as standardized targets for management (). ‘Time-in-Ranges’ goals, representing the amount of time spent above, below, and within specified ranges over a 10-to-14-day period, were defined by this consensus group () [Citation38]. TIR goals should be individualized based on risk of hypoglycemia, presence of comorbidities, age, and pregnancy. Patient and provider goals were also defined by the consensus group.
Table 2. Consensus CGM Metrics for Clinical Care
TIR is becoming a focus of clinical management in persons with T1D or T2D. Evidence supports an association between increasing TIR and decreasing risk of diabetes-related microvascular complications [Citation42,Citation43]. Recent analyses evaluating the relationship between TIR and A1C showed an inverse relationship between the two: increased TIR was associated with A1C reductions and vice versa [Citation44,Citation45]. Analysis of datasets from four randomized trials showed that a 10% increase in TIR corresponded with an A1C decrease of approximately 0.5% (5.0 mmol/mol) [Citation44]. Similar associations were seen in an analysis of 18 randomized controlled trials encompassing over 2,500 individuals with T1D or T2D over a range of ages and A1C levels [Citation45]. Optimizing TIR while reducing TBR allows PwD to focus on factors which allow real-time modifications to improve long-term outcomes. To that end, it has been shown that every 5% increase in TIR is clinically meaningful [Citation44]. TIR is heavily influenced by TAR; therefore, improvement in TAR improves TIR, and vice versa, as long as improvements in TAR and TIR values are not done at the cost of increasing time in hypoglycemia.
Composite metrics
Attempts to develop a single composite metric for CGM have been made [Citation46]. Ideally, a composite metric would summarize the features of CGM data with a number, allowing the physician to quickly determine the patient’s glycemic control. One such new metric, the personal glycemic state, used four features of CGM data to assess glycemic control [Citation47]. The utility of a single composite CGM metric in clinical practice remains to be defined.
CGM reports
The AGP, first developed in 1987 and optimized with the evolution of CGM technology and refinement of CGM-based metrics, was developed to evaluate glycemic control [Citation48]. AGP is a standardized visualization format for CGM and SMBG data which simplifies and optimizes the analysis and interpretation of the data by combining data from defined periods of time into a single report, highlighting a patient’s glucose patterns, Time-in-Ranges, and glucose variability [Citation34,Citation49]. A retrospective AGP analysis rapidly identifies glucose patterns, which facilitate adjustments to therapeutic management and lifestyle behaviors.
The effective use of AGP by patients and healthcare providers can potentially minimize TAR and TBR, optimize TIR, and reduce PPG excursions [Citation34]. AGP has been adopted by many device manufactures and is endorsed by international consensus panels [Citation38,Citation50]. Most recently, ADA 2020 Standards of Medical Care in Diabetes has endorsed the use of ‘standardized reports with visual cues such as the “Ambulatory Glucose Profile”’ [Citation51].
Other views of CGM-based data, such as the sensor overlay view (‘spaghetti graph’ view) or individual daily views, are available and can be useful in clarifying and identifying specific factors related to glycemic management. These reports are particularly useful for patients whose daily meal and activity schedules vary considerably, as diurnal patterns may not be apparent on AGP reports for these individuals. Overlay and daily formats can reveal the timing and magnitude of PPG peaks as well as the impact of physical activity, bolus insulin, and conditions that precede and follow both hypoglycemia and hyperglycemia.
The AGP can be divided into three sections: Glucose Statistics, Targets and Time-in-Ranges; AGP; and Daily Glucose Profiles ().
Figure 2. Example Ambulatory Glucose Profile Report [Citation38]
![Figure 2. Example Ambulatory Glucose Profile Report [Citation38]](/cms/asset/ecd130ea-e244-4c08-becb-9f397005b9bb/ipgm_a_1851946_f0002_c.jpg)
Glucose statistics, targets and time-in-ranges
At the top of the page, Time-in-Ranges metrics can be rapidly evaluated with the ‘stacked bar graph.’ To the left of the stacked bar graph is an overview of Time-in-Range targets, along with an average glucose value and ‘glucose management indicator’ (GMI). GMI is an averaged representation of glycemic control presented as a value in a format similar to that of A1C. Importantly, A1C and GMI are inherently different measures and some degree of divergence between these values is common. Percent time that the CGM is active is also indicated, allowing for the evaluation of data adequacy. Fourteen days of data are considered optimal for interpretation. The upper portion of the AGP report is useful in rapidly determining ‘Do I need to take action?’, i.e., ‘Are TIR and TBR measures acceptable and safe?’.
AGP graph
The middle portion of the report contains the modal day AGP view. This is a summary view of glucose values over the time the CGM was active. The AGP is presented as averaged data as if it occurred in a single day, with lines indicating median and outlying values. The AGP view can allow rapid assessment of glycemic patterns throughout the day, including patterns of hypoglycemia or rapid glycemic change during specific times of the day.
Daily glucose profiles
Finally, the bottom 3rd of the report contains daily glucose profiles, allowing identification of patterns of PPG or hyperglycemia, hypoglycemia, and rapid change in glycemia throughout individual days. This view allows the patient and clinician to review the impact of lifestyle and medication administration on daily glycemia, which can often vary throughout the week with changes in activity, exercise, sleep/wake patterns, and eating patterns. As previously noted, when schedules vary from day to day, daily and sensor overlay views can be used to isolate cause-and-effect relationships and further fine-tune glycemic management.
Application of glycemic targets and AGP
A structured and systematic approach to reviewing retrospective data allows for identification of patterns throughout the day, including both fasting values and PPG patterns. Retrospective CGM analysis, using clinical targets based on guidance from the International Consensus on Time in Range [Citation38], has the potential to significantly improve patient care on an individual level, both in optimizing short-term glycemic management, and potentially, in decreasing long-term microvascular damage [Citation42,Citation43].
Body
Patient-provided insights into lifestyle, i.e. diet, exercise, stress, medications, and other events, add context to CGM-generated data, which can guide shared decision-making and medication changes at the time of clinical interactions. Furthermore, a collaborative review of the CGM data can enhance self-efficacy and empowerment for PwD managing their diabetes outside of clinical interactions. Insight-guided review helps to inform the question ‘Do I need to take action to improve glycemic management?’. Review of the AGP and associated daily views help to determine ‘What action do I need to take?’. A nine-step interpretation method previously described by Johnson et al. [Citation49] is outlined in . Referral to a certified diabetes care and education specialist (CDCES) may be beneficial to have detailed and meaningful discussions about CGM reports and interpretation.
Figure 3. A CGM Toolbox for Clinicians. A toolbox to guide clinicians through CGM data analysis. Specifically, (1) Patient-specific data that should be marked on the AGP, (2) Data adequacy, glycemic variability, and Time-in-Ranges targets, (3) nine-step CGM AGP Report Interpretation, and (4) Billing codes are described

To help illustrate the role of CGM in optimizing diabetes therapies, two insulin-managed individuals with T2D were considered. Both individuals had A1C values of 7.5% and completed 2 weeks of blinded (not real-time) CGM. Patient A is a 76-year-old African American female with T2D diagnosed 25 years prior. Her demographic data are presented in . She has been insulin-managed for many years; metformin was discontinued 6 years prior because of gastrointestinal intolerance. She walks daily for exercise.
Table 3. Patient A and B Demographic Data
Evaluation of CGM-generated AGP reports
Below the nine-step interpretation guide as seen in ) was used to evaluate Patient A. Data review starts with:
Evaluation of Data Adequacy: Patient Ahas an optimal amount of data.
Figure 4. Patient A and B AGP reports at the time of clinic visit. (a) Patient A’s AGP report, and (b) Patient B’s AGP report [Citation38]
![Figure 4. Patient A and B AGP reports at the time of clinic visit. (a) Patient A’s AGP report, and (b) Patient B’s AGP report [Citation38]](/cms/asset/379de20a-200d-45bf-b47b-2967c56c6b3a/ipgm_a_1851946_f0004_c.jpg)
The ‘stacked bar graph’ in the Time-in-Ranges view allows rapid review of the efficacy and safety of diabetes therapy, with quantification of TIR, TAR, and TBR. These metrics will answer the question ‘Do I need to take action?’. In this case, the ‘stacked bar graph’ shows a TIR well above the clinical goal of >70%, and time spent with a glucose between 54 and 69 mg/dL to be 1%, with no time spent below 54 mg/dL. TBR is well below the clinical goal of <4%.
AGP and daily glucose profiles
The more detailed AGP and daily views are needed to answer the question ‘What action do I need to take?’. In reviewing the AGP view, it is important to view this data in the context of patient-specific factors that can affect glycemic patterns such as
Age
Duration of diabetes
Weight and BMI
Renal function
Patient-specific comorbidities
Glycemic regimen doses and timing of insulin administration
Time of meals
Time and days of exercise
Varying work and sleep schedules
Snacking.
It can be very helpful to print the AGP and (2) mark these factors on the AGP report while discussing the results with the patient. The impact of these factors can help to inform clinicians and patients while evaluating glycemic patterns and can serve as teachable moments linking behavior(s) to the outcomes seen in the report.
(3) Ask the patient what they see.Asking open-ended questions while reviewing an AGP report can help uncover patient insights and facilitate shared decision-making.
(4) Review the AGP for patterns of low glucose (TBR).
Verify patients are educated on treatment of hypoglycemia and its potential for short- and long-term side effects.
Address hypoglycemia first, given the obvious safety implications of significant hypoglycemia.
If the 5% trend line dips below 54 mg/dL, take immediate action to prevent further hypoglycemia.
If the 5% trend line dips below 70 mg/dL, consider therapeutic modification to prevent hypoglycemia.
Although Patient A appears to have acceptable levels of hypoglycemia, it is important to ask about each event: how did she respond and what might she do differently next time to prevent it. In reviewing Patient A’s overall AGP, her levels of hypoglycemia indicate no need to change her current therapy.
(5) Review hyperglycemia (TAR).
Although Patient A’s time above 180 mg/dL is modest (14%), request her perspective on causality.
Assess the need for dietary guidance.
Be mindful that improvements in evening hyperglycemia may increase the risk for hypoglycemia 6 or 8 hours later.
Discuss the contributions of diet and insulin dosing to improve PPG control.
Insights into the impact of lifestyle changes on PPG and hyperglycemia can facilitate productive conversations while improving patient empowerment and self-efficacy.
(6) Evaluate areas of increased glycemic variability. Although Patient A’s profile is relatively flat and glucose variability is a modest 28.7% (goal ≤36%), discussing how to address evening glycemic spikes related to PPG variability may be an opportunity to further improve the profile. The broad definition of glucose variability considers the intra-day glycemic excursions, including episodes of hyperglycemia and hypoglycemia. There is a significant association between glucose variability and the increased incidence of hypoglycemia [Citation52].
(7) If previous AGP reports are available,
Compare previous reports and reinforce positive changes.
(8 & 9) The clinician and PwD should collaborate on development of an action plan.
Document the AGP and agreed-upon plan in the medical record (the AGP can be ‘copied’ into a patient note or scanned into the electronic medical record)
In Patient A, TIR targets are being safely and effectively met. Counseling might focus on further optimizing intermittent evening hyperglycemia through dietary and lifestyle interventions.
Similar A1C, different clinical picture
Now, let us consider the case of Patient B, a 63-year-old male from Laos who was diagnosed with T2D 6 months ago (). At the time of diagnosis, Patient B’s A1C was >14% and a random BG was 329 mg/dL. He has a history of poor medication adherence and was not on metformin because of heavy alcohol use. His medical history includes hypertension, hyperlipidemia, Graves’ disease with ophthalmopathy, and hypothyroidism after radioiodine treatment. He rarely checks fingerstick glucose, and complains that when he does check BG, he is rarely able to get any blood.
The same structured approach to data adequacy, timing of medications and meals, and relevant lifestyle factors as was utilized in Patient A, was used with Patient B data. Review of the ‘Time-in-Ranges’ helps determine whether action needs to be taken. For Patient B, TIR is lower than optimal, but the TBR is striking ()). Hypoglycemia will need to be addressed aggressively. Although the average glucose looks reasonable, glucose variability is high at 52.6%. Because 22% of the CGM data are in the hypoglycemic range and 37% are in the hyperglycemic range, the average glucose is balanced. However, the reported average glucose does not accurately reflect Patient B’s glycemic control. Although the measured A1C and GMI meet population-based glycemic goals, the CGM data indicate that the current regimen is neither safe nor effective. Review of the AGP and daily views will provide further insights needed to address the significant hypoglycemia in this profile.
Patterns are clearly visible in reviewing the AGP and daily views; a pattern of consistent hyperglycemia with daytime dietary intake, and relatively consistent hypoglycemia during the evening and nighttime hours are seen. With 13% of the time spent below a glucose of 54 mg/dL, immediate action is needed. Beyond the obvious hypoglycemia, hyperglycemia and glucose variability are significant issues, and addressing postprandial hyperglycemia will be a necessary component of ongoing management. Hypoglycemia should be addressed first.
Patient B has both significant glycemic management issues and significant barriers to care. First, the combination of insulin and sulfonylurea therapy can be problematic and can increase the risk for hypoglycemia [Citation53]. Although several interventions could be considered, eliminating the sulfonylurea agent would likely resolve the hypoglycemia issues, leaving the more predictable, titratable, and stable action of the basal insulin to manage non-prandial glycemia. Additionally, ongoing CGM use may dramatically improve the safety of future glycemic management.
Once hypoglycemia has been addressed, issues of PPG and daytime hyperglycemia as well as the contributors to glycemic variability will need to be addressed. An obvious answer to Patient B’s PPG excursions would be addition of prandial insulin, which, with appropriate teaching, would be flexible and predictably effective while adding only a modest level of complexity to Patient B’s glycemic management. Non-insulin therapies could be considered, with less guarantee of success. Re-initiation of metformin would be appropriate if renal function is sufficient and excessive alcohol use is not an issue. Non-insulin therapies that do not predispose to hypoglycemia, such as SGLT 2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, or even TZD classes, could be considered as options. Patient B may benefit significantly from seeing a Registered Dietitian and CDCES for specific help with his high carbohydrate diet and postprandial hyperglycemia. Patient B would benefit from close follow-up (within 1–2 weeks) and a team approach to improve the safety and efficacy of his diabetes management. Once the AGP discussion has been documented in the medical record along with an agreed upon plan, a written plan of action and follow-up date should be provided to the patient along with their AGP report.
Diving deeper into the causes of hypoglycemia
One more case using CGM data to optimize glycemic therapy was also considered. Patient C, a 66-year-old male, has a preexisting comorbidity of atherosclerotic cardiovascular disease ().
Table 4. Patient C Demographic Data
Here, review of Patient C’s report shows high glycemic variability and percent TIR below goal (). Additionally, TBR is well above goal at 10%. Does action need to be taken? Yes: review of the AGP and daily views will help to indicate what actions should be taken.
Figure 5. Patient C AGP reports at the time of clinic visit [Citation38]
![Figure 5. Patient C AGP reports at the time of clinic visit [Citation38]](/cms/asset/32088ad4-cb31-45a9-bcad-6b6c279b1b03/ipgm_a_1851946_f0005_c.jpg)
The AGP shows a distinctive pattern of increasing hyperglycemia throughout the day in a stair-step fashion which correlates with meals and impact of PPG. The increasing hyperglycemia is followed by a steep drop overnight with early morning hypoglycemia and an average glucose level nadiring around 7:00 a.m. This pattern is an exaggerated example of the very common pattern of daytime hyperglycemia followed by nocturnal over-correction so often seen in individuals treated for T2D. The nocturnal glucose decline can be impacted by physical activity, particularly when the exercise takes place in the evening.
A steep and excessive drop in glycemia overnight is often seen in individuals treated with too much basal insulin (‘over-basalization’). This often occurs when adequate mealtime and PPG control are absent. Basal insulin therapy addresses fasting and overnight glucose but has a limited impact on PPG. Once the limits of basal insulin in treating fasting and overnight glucose levels are reached, addition of basal insulin predisposes to nocturnal hypoglycemia with limited impact on postprandial values. Our patient weighs 87.5 kg and is on a basal insulin dose of 60 units per day, which is 0.69 units/kg/day of basal insulin. Once basal insulin dose increases beyond 0.5 units/kg/day, therapy to address the postprandial hyperglycemia is needed [Citation54].
Postprandial hyperglycemia is typically addressed with combination injectable therapy: basal insulin plus GLP-1 therapy or basal insulin plus mealtime insulin at 1, 2, or 3 meals per day. In appropriate individuals, a switch to premixed insulin could also be considered. As this patient is already on maximal dose GLP-1 therapy, addition of mealtime (prandial) insulin is the most appropriate option. Reduction in basal insulin needs to occur urgently given the significant time this individual is spending under a glucose level of 54 mg/dL. In conjunction with close glycemic monitoring, this glycemic pattern could be improved by dividing the total daily basal insulin dose 50:50 between basal insulin and prandial insulin. The prandial insulin is then split between meals. The insulin dose would then include 30 units of insulin glargine and 30 units of a rapid acting insulin split between meals. Use of CGM would inform the need for further basal or prandial dose adjustments to optimize the safe and effective use of this new insulin regimen.
Conclusion
The majority of PwD treated in the primary care setting check their glucose too infrequently, perhaps only checking FBG. It is important for PwD to understand that A1C is an average value and does not reflect glucose variability. CGM can assist both the clinician and PwD in better understanding day-to-day glycemic patterns. Viewing one’s own data can motivate PwD to improve medication adherence and make lifestyle choices that are conducive to better glycemic control. Patient education on the self-evaluation of real-time data may lead to a better understanding of the effects of their medical therapy and lifestyle choices. Identifying and understanding the cause-and-effect relationships is essential to honing optimal behaviors and outcomes. Ultimately, understanding CGM data can empower PwD to make real-time, effective treatment adjustments leading to improved patient outcomes [Citation33]. Furthermore, because of the usual 3-month (or more) cadence for clinical visits, team-based remote/virtual management using CGM data can facilitate more timely assessments and titration of therapy. Overall, real-time CGM use can improve dietary insights, A1C values, and decrease hypoglycemia in individuals with T2D using insulin [Citation55,Citation56] and non-insulin therapies [Citation57,Citation58]. Although patients need to be more proactive to retrieve glucose data and potentiate the mitigation of hypoglycemic events, FGM can provide similar improvements as real-time CGM [Citation59–61]. Despite the many benefits that CGM can provide, the cost of a CGM system can be limiting for some patients. Intermittent use of CGM using Professional or Personal CGM equipment or an FGM system, may allow significant insights with less burden and cost to the patient. Further study is warranted in this area. Regardless of the strategy chosen, structured analysis and interpretation of CGM data with ongoing titration and advancement of therapies is necessary to realize the full potential of this very promising technology.
In summary, CGM can provide valuable data, including Time-in-Ranges, the AGP, and overlay/daily views. This data can aid pattern identification for both FBG, premeal, overnight and PPG management. Time-in-Ranges represents an easy-to-define qualitative metric that can facilitate goal setting discussions between clinicians and PwD. Review of Time-in-Ranges, combined with patient-provided insight into lifestyle, can enhance shared decision-making during clinical visits and can empower PwD in self-management between clinic visits. Using AGP reports from CGM, with attention to TIR, TBR, and PPG-induced TAR, can optimize the safety and efficacy of clinical decisions.
Declaration of financial/other relationships
TM receives research support from Abbott Diabetes Care, Dexcom, Insulet, Medtronic, Novo-Nordisk, and Eli Lilly and Company. His employer, nonprofit IDC/Park Nicollet/HealthPartners Institute, contracts for his services and no personal income goes to him.
RMB received research support from, consulted for, or has been on a scientific advisory board for Abbott Diabetes Care, Ascensia, Dexcom, Eli Lilly and Company, Hygieia, Johnson & Johnson, Medtronic, Novo-Nordisk, Onduo, Roche, Sanofi and United Healthcare. His employer, non-profit IDC/Park Nicollet/HealthPartners Institute, contracts for his services, and no personal income goes to him.
GS serves as a paid speaker and consultant for Dexcom and Senseonics.
TP serves as an advisor for Eli Lilly and Company and is a faculty member of Lifescan Diabetes Institute.
ALC receives research support from and/or consulting for Abbott Diabetes Care, Dexcom, Eli Lilly and Company, Medtronic, Novo-Nordisk, Sanofi, and Sensionics. His employer, nonprofit IDC/Park Nicollet/HealthPartners Institute, contracts for his services and no personal income goes to him.
CC is an advisor to Boehringer Ingelheim, Eli Lilly and Company, Janssen, Novo-Nordisk, and Sanofi Aventis.
BL is an employee of Eli Lilly and Company.
KS is an employee of Eli Lilly and Company.
RDP is an employee of Eli Lilly and Company.
A reviewer on this manuscript has disclosed that they are receiving research support from DEXCOM for the conduction of inpatient clinical trials. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Declaration of interest
No potential conflict of interest was reported by the authors.
Acknowledgments
None stated.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- The Emerging Risk Factors Collaboration. . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222.
- World Health Organization. Diabetes Fact Sheet. 2018. cited 2020 Mar 10. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes.
- Centers for Disease Control and Prevention. National Diabetes Statistics Report 2020: Estimates of Diabetes and Its Burden in the United States. 2020.
- Edelman SV and Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care. 2017;40(11):1425–1432.
- Kazemian P, Shebl FM, McCann N, et al. Evaluation of the cascade of diabetes care in the United States, 2005-2016. JAMA Intern Med. 2019; 179(10):1376-1385.
- Gregg EW, Hora I, and Benoit SR. Resurgence in diabetes-related complications. JAMA. 2019;321(19):1867–1868.
- UK prospective diabetes study (UKPDS) group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853.
- Lind M, Odén A, Fahlén M,et al. The true value of HbA1c as a predictor of diabetic complications: simulations of HbA1c variables. PLoS One. 2009;4(2):e4412.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986.
- Virk SA, Donaghue KC, Cho YH, et al. Association between HbA1c variability and risk of microvascular complications in adolescents with type 1 diabetes. J Clin Endocrinol Metab. 2016;101(9):3257–3263.
- Centers for Disease Control and Prevention. All About Your A1C. 2018.
- American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S66–S76.
- Kang X, Wang C, Chen D, et al. Contributions of basal glucose and postprandial glucose concentrations to hemoglobin A1c in the newly diagnosed patients with type 2 diabetes–the preliminary study. Diabetes Technol Ther. 2015;17(7):445–448.
- Ketema EB and Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch Public Health. 2015;73:43.
- Monnier L and Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract. 2006;12(Suppl 1):42–46.
- Monnier L, Colette C, and Owens D. Postprandial and basal glucose in type 2 diabetes: assessment and respective impacts. Diabetes Technol Ther. 2011;13(Suppl 1):S25–32.
- Carls G, Huynh J, Tuttle E, et al. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Therapy. 2017;8(4):863–873.
- Casagrande SS, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36(8):2271–2279.
- Foster NC, Beck RW, Miller KM,et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66–72.
- Beck RW, Connor CG, Mullen DM, et al. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(8):994–999.
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388–394.
- Qu Y, Jacober SJ, Zhang Q,et al. Rate of hypoglycemia in insulin-treated patients with type 2 diabetes can be predicted from glycemic variability data. Diabetes Technol Ther. 2012;14(11):1008–1012.
- Cox DJ, Kovatchev BP, Julian DM,et al. Frequency of severe hypoglycemia in insulin-dependent diabetes mellitus can be predicted from self-monitoring blood glucose data. J Clin Endocrinol Metab. 1994;79(6):1659–1662.
- Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2019;7(3):221–230.
- Omladič JS, Ozimič AS, Vovk A, et al. Acute hyperglycemia and spatial working memory in adolescents with type 1 diabetes. Diabetes Care. 2020;43(8):1941–1944.
- Martin DD, Davis EA, and Jones TW. Acute effects of hyperglycaemia in children with type 1 diabetes mellitus: the patient’s perspective. J Pediatr Endocrinol Metab. 2006;19:927–936.
- Zelnick LR, Batacchi ZO, Ahmad I, et al. Continuous glucose monitoring and use of alternative markers to assess glycemia in chronic kidney disease. Diabetes Care. 2020;43(10):2379–2387.
- Lee J-E. Alternative biomarkers for assessing glycemic control in diabetes: fructosamine, glycated albumin, and 1,5-anhydroglucitol. Ann Pediatr Endocrinol Metab. 2015;20:74-78.
- Kirk JK and Stegner J. Self-monitoring of blood glucose: practical aspects. J Diabetes Sci Technol. 2010;4(2):435–439.
- Miller KM, Beck RW, Bergenstal RM, et al. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36(7):2009–2014.
- Patton SR and Clements MA. Continuous glucose monitoring versus self-monitoring of blood glucose in children with type 1 diabetes- are there pros and cons for both? US Endocrinol. 2012;8(1):27–29.
- Ang E, Lee ZX, Moore S, et al. Flash glucose monitoring (FGM): A clinical review on glycaemic outcomes and impact on quality of life. J Diabetes Complications. 2020;34(6):107559.
- Klonoff DC. Improved outcomes from diabetes monitoring: the benefits of better adherence, therapy adjustments, patient education, and telemedicine support. J Diabetes Sci Technol. 2012;6(3):486–490.
- Carlson AL, Mullen DM, and Bergenstal RM. Clinical use of continuous glucose monitoring in adults with type 2 diabetes. Diabetes Technol Ther. 2017;19(S2):S4–S11.
- Monnier L, Lapinski H, and Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care. 2003;26(3):881-885.
- Ceriello A, Hanefeld M, Leiter L,et al. Postprandial glucose regulation and diabetic complications. Arch Intern Med. 2004;164:2090–2095.
- Færch K, Alssema M, Mela DJ, et al. Relative contributions of preprandial and postprandial glucose exposures, glycemic variability, and non-glycemic factors to HbA 1c in individuals with and without diabetes. Nutr Diabetes. 2018;8(1):38.
- Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603.
- Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2017;40(7):832–838.
- Rodbard D. Hypo- and hyperglycemia in relation to the mean, standard deviation, coefficient of variation, and nature of the glucose distribution. Diabetes Technol Ther. 2012;14(10):868–876.
- Chandran SR, Tay WL, Lye WK, et al. Beyond HbA1c: comparing glycemic variability and glycemic indices in predicting hypoglycemia in type 1 and type 2 diabetes. Diabetes Technol Ther. 2018;20(5):353–362.
- Lu J, Ma X, Zhou J,et al. Association of Time in Range, as Assessed by Continuous Glucose Monitoring, With Diabetic Retinopathy in Type 2 Diabetes. Diabetes Care. 2018;41(11):2370–2376.
- Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400–405.
- Beck RW, Bergenstal RM, Cheng P, et al. The Relationships Between Time in Range, Hyperglycemia Metrics, and HbA1c. J Diabetes Sci Technol. 2019;13(4):614–626.
- Vigersky RA and McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther. 2019;21(2):81–85.
- Nguyen M, Han J, Spanakis EK, et al. A Review of Continuous Glucose Monitoring-Based Composite Metrics for Glycemic Control. Diabetes Technol Ther. 2020;22(8):613–622.
- Hirsch IB, Balo AK, Sayer K, et al. A simple composite metric for the assessment of glycemic status from continuous glucose monitoring data: implications for clinical practice and the artificial pancreas. Diabetes Technol Ther. 2017;19(S3):S38–S48.
- Mazze RS, Lucido D, Langer O, et al. Ambulatory glucose profile: representation of verified self-monitored blood glucose data. Diabetes Care. 1987;10(1):111-117.
- Johnson ML, Martens TW, Criego AB, et al. Utilizing the ambulatory glucose profile to standardize and implement continuous glucose monitoring in clinical practice. Diabetes Technol Ther. 2019;21(S2):S217–S225.
- Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640.
- American Diabetes Association. 7. Diabetes technology: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S77–S88.
- DeVries JH. Glucose variability: where it is important and how to measure it. Diabetes. 2013;62(5):1405–1408.
- DeVries JH, Meneghini L, Barnett AH, et al. A patient-level analysis of efficacy and hypoglycaemia outcomes across treat-to-target trials with insulin glargine added to oral antidiabetes agents in people with type 2 diabetes. Eur Endocrinol. 2014;10(1):23–30.
- American Diabetes Association. 8. Pharmacologic Approaches to Glycemic Treatment. Diabetes Care. 2017;40(Suppl 1):S64–S74.
- Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32–38.
- Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365–374.
- Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82(1):73–79.
- Allen NA, Fain JA, Braun B, et al. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: A randomized clinical trial. Diabetes Res Clin Pract. 2008;80(3):371–379.
- Ajjan RA, Jackson N, Thomson SA. Reduction in HbA1c using professional flash glucose monitoring in insulin-treated type 2 diabetes patients managed in primary and secondary care settings: A pilot, multicentre, randomised controlled trial. Diab Vasc Dis Res. 2019;16(4):385–395.
- Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55–73.
- Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42(7):1178–1184.
