ABSTRACT
Excessive daytime sleepiness (EDS) affects approximately half of patients with obstructive sleep apnea (OSA) and can persist in some despite normalization of breathing, oxygenation, and sleep quality with primary OSA therapy, such as continuous positive airway pressure (CPAP). EDS is often overlooked and under discussed in the primary care setting and in the follow-up of CPAP-treated patients due to difficult assessment of such a multi-dimensional symptom. This review aims to provide suggestions for procedures that can be implemented into routine clinical practice to identify, evaluate, and manage EDS in patients treated for OSA, including how to appropriately use various self-report and objective assessments along the clinical pathway and options for pharmacotherapy. In addition, examples of when it is appropriate to refer a patient to a sleep specialist for evaluation are discussed.
1. Introduction
1.1. Prevalence of excessive daytime sleepiness in obstructive sleep apnea
The prevalence of obstructive sleep apnea (OSA) continues to increase, likely due, in part, to the growing obesity epidemic and heightened awareness by the public and healthcare providers. Recent estimates suggest that nearly 1 billion adults worldwide have OSA [Citation1]. Excessive daytime sleepiness (EDS) is a key symptom of OSA, but estimations of its prevalence have been inconsistent [Citation2]. Some reports approximate that 50 to 80% of patients with OSA report EDS prior to initiating therapy [Citation3,Citation4], whereas others claim that up to 60% do not report EDS [Citation5]. These discrepancies could be due to a number of reasons, such as under-recognition in the clinic due to the multidimensional nature of EDS and consequential ambiguity in patient reporting of symptoms, prevalence overlap with other comorbid etiologies of EDS, insufficient screening questionnaires, and low agreement among various EDS assessment tools [Citation6]. Indeed, there is an increasing recognition that OSA is a heterogeneous disorder with different clinical presentation patterns [Citation7].
Among those who do report EDS, studies have shown that the severity of EDS does not correlate with the severity of OSA (as determined by apnea hypopnea index [AHI]), suggesting that factors other than respiratory events and arousal (eg, hypoxia- and/or sleep fragmentation-related brain injury [Citation8]) contribute to EDS in this population [Citation9-11]. Indeed, EDS has been shown to persist in some patients despite the normalization of breathing, oxygenation, and sleep quality with continuous positive airway pressure (CPAP) (the gold standard treatment for OSA), known as residual EDS [Citation12,Citation13]. Population-based studies have estimated that 9 to 22% of CPAP-treated patients experience residual EDS, even when other potential causes of sleepiness (eg, depression, other sleep disorders, medications, comorbidities, and inadequate sleep duration) are controlled [Citation14,Citation15]. Notably, there is a dose-response relationship between objectively-measured CPAP adherence and residual EDS, such that the prevalence of residual EDS is significantly lower among those who use CPAP for >6 hours per night compared with those who use CPAP for ≤5 hours per night [Citation14,Citation16,Citation17].
To complicate matters, prevalence rates of EDS based on self-report measures are often inconsistent with rates based on objective measures. Prospective studies have suggested that 22 to 34% of patients self-report EDS after CPAP use; however, objective measures, such as the Maintenance of Wakefulness Test (MWT), which measures the ability to maintain alertness, or Multiple Sleep Latency Test (MSLT), which is a test of sleep propensity, indicate that, within those populations, up to 65% of patients may experience residual EDS [Citation16-18]. This discrepancy suggests that some patients may not be aware of their sleepiness and/or may reflect that these assessments measure different aspects of EDS.
1.2. Consequences associated with EDS in OSA
The adverse effects of EDS are multifactorial and extend beyond the individual patient, also impacting the patient’s family, workplace, and society. At the individual level, EDS diminishes mood, quality of life, and cognitive functioning, and can negatively affect marital satisfaction [Citation19-22]. In the workplace, EDS debilitates daily functioning, productivity, and social interactions, impairing time management, work quantity and quality, and on-the-job social interactions [Citation19,Citation23]. At the societal level, EDS is associated with an increased risk of motor vehicle and occupational accidents, thereby posing a safety risk to patients and the community [Citation24-27]. This is particularly concerning because some patients may be unaware of their sleepiness or related impairments [Citation16-18,Citation28]. In addition, patients with OSA and EDS have greater healthcare resource utilization, including more healthcare provider interactions and emergency department visits, than those without EDS [Citation29].
1.3. Unmet need and objective
Residual EDS is often overlooked and under discussed in clinical consultations [Citation30]. Given the potential deleterious consequences that EDS may have on patient and public safety, it is critical for physicians to recognize residual EDS so patients have the opportunity to receive a diagnosis and treatment. Furthermore, the high prevalence of OSA may exceed the capacity of tertiary sleep centers. With advances in assessment and treatment technologies, it is being advocated that primary care manage simpler cases of follow-up with CPAP-treated patients, reserving referrals to sleep specialists for more complex cases [Citation31]. Thus, it is vital that clinicians be informed on how to manage residual EDS along the clinical pathway, including how to appropriately assess for and diagnose residual EDS, when to intervene to optimize primary OSA treatment, when to use pharmacotherapy, how to choose a suitable agent, and when to refer to a sleep specialist.
Recent reviews have provided thorough reports of the definition, prevalence, pathophysiology, assessment tools, and treatment options for EDS associated with OSA [Citation32,Citation33]; however, there remains a need for step-by-step guidance for practice procedures to help clinicians who encounter patients with sleep disorders (eg, primary care physicians [PCPs], respiratory/sleep specialists, cardiologists, otolaryngologists, and nurse practitioners) appropriately evaluate and manage residual EDS. This review aims to provide suggestions for procedures that can be implemented into routine practice to assess for, identify, and manage residual EDS in patients treated for OSA. These strategies are summarized in and discussed in detail below.
2. Recognizing and assessing residual EDS
2.1. Risk factors for residual EDS in OSA
Risk factors for residual EDS in patients with OSA are not well defined, but some have been identified () [Citation4,Citation5,Citation18]. Those with severe EDS at diagnosis are more likely to experience residual EDS after CPAP treatment [Citation4,Citation14,Citation15,Citation18]. Other possible risk factors include younger age and depression [Citation14,Citation15]. Interestingly, baseline AHI and body mass index (BMI) are not risk factors for residual EDS in patients who are adherent to CPAP [Citation4,Citation14,Citation15,Citation18]. It is inconclusive whether comorbid diabetes and hypertension influence the risk of residual EDS, as some [Citation4], but not all [Citation14,Citation15,Citation18], studies have reported an association.
Table 1. Risk factors for EDS in patients with OSA based on a combination of available studies.a
2.2. Patient complaints of EDS
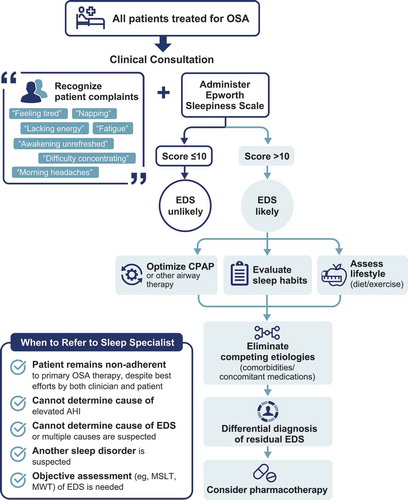
In theory, the easiest way for a clinician to identify whether a patient has residual EDS is through recognition of subjective symptoms. Unfortunately, patients’ complaints are not always straightforward and illuminating. A patient may use terms other than EDS to express their sleepiness or they may describe associated consequences, such as feeling tired, unrefreshed awakening, lack of energy, feeling fatigued, napping, morning headaches, irritability, or difficulty concentrating [Citation34,Citation35]. In fact, patients are more likely to report fatigue, tiredness, and lack of energy than sleepiness [Citation34]. Recent evidence has demonstrated that fatigue, anxiety, depression, and negative affect are dimensions associated with EDS that influence the perception and emotional experience of the symptom, suggesting that discrepancies in clinical presentation may reflect EDS’ multidimensional nature [Citation6]. As patient complaints may be inconsistent, initial assessment should begin with a clinical consultation, during which the clinician can obtain a past medical history and physical examination.
As previously discussed, some patients may simply be unaware of their sleepiness or may not perceive it to be troublesome [Citation16-18,Citation28]. In these cases, symptomatic indications of EDS may be even more subtle [Citation6]. To reduce ambiguity, the clinician should utilize self-report EDS assessments. All patients treated for OSA should be assessed for residual EDS, regardless of whether they complain of sleepiness.
2.3. Self-report assessments of EDS
Self-report questionnaires are brief, inexpensive tools, making them convenient for use in routine clinical practice () [Citation36,Citation37].
Table 2. Tools for evaluating EDS
The Epworth Sleepiness Scale (ESS) is the most commonly used self-administered assessment for EDS. The ESS assesses the propensity to fall asleep in real-world situations [Citation38]. Patients are asked to rate, on a scale of 0 to 3 (with higher numbers indicating more sleepiness), how likely they would be to doze off or fall asleep in 8 situations, such as while reading, watching television, or riding as a passenger in a car. Total scores range from 0 to 24, with higher scores representing greater levels of sleepiness. To identify a patient with EDS, scores ≤10 are considered within the normal range, whereas scores >10 indicate EDS [Citation38,Citation39].
Advantages of the ESS include its brevity, accessibility, and widespread use in clinical and research studies. It takes ~2 to 3 min to complete and has been translated, tested, and validated in many languages and versions (e.g. a pictorial version for patients with diminished literacy skills), making it accessible worldwide. In addition, the ESS has high internal consistency in measuring EDS in patients with OSA [Citation11,Citation40]. In general, studies have found the ESS to have good test‒retest reliability in controlled clinical trial settings or when administered in similar clinical settings, and less reliability when administered across different primary and secondary care settings (e.g. between a PCP and sleep specialist visit) [Citation11,Citation41-45]. Finally, the widespread use of the ESS has produced extensive evidence for normative scores in clinical and nonclinical samples, facilitating interpretation of results.
In addition to these strengths, there are several limitations. The main disadvantage of the ESS is its reliance on self-report, making it susceptible to patient’s self-awareness of their sleepiness. Indeed, ESS scores tend to be higher when scored by the patient’s bed partner than when scored by the patient, suggesting that patients may underestimate their sleepiness. Accuracy may be enhanced when the ESS is completed as a consensus between the patient and partner [Citation46]. Recent data have questioned the reliability of the ESS [Citation43,Citation44,Citation47,Citation48]. One study found variability in ESS scores even when the assessment was repeated on the same day, suggesting that situational sleepiness (i.e. time of test administration) may influence ESS scores [Citation48].
The Stanford Sleepiness Scale (SSS) [Citation49] and Karolinska Sleepiness Scale (KSS) [Citation50] are other self-report assessment tools that can be used to quantify EDS; however, both measure state EDS at a specific point in time [Citation49]. As such, these measures are more often used in research settings and are, for the most part, impractical for clinic settings [Citation49,Citation50].
2.4. Key points
The ESS is the best option available for clinicians to implement into day-to-day practice to assess EDS, although it is limited by a patient’s perception of their sleepiness and the potential for lower test‒retest reliability along a clinical pathway from primary care to specialist settings
Some of these limitations can be overcome if the ESS is completed by both the patient and patient’s partner [Citation46]
The ESS should be administered with the clinical interview to all patients treated for OSA
3. Differential diagnosis of residual EDS
If a patient who is treated for OSA has an ESS score >10, there are several potential explanations for EDS, including suboptimal OSA treatment (including inadequate adherence), lifestyle factors, comorbidities, and concomitant medications.
3.1. Suboptimal OSA therapy
The first step to making a differential diagnosis of residual EDS associated with OSA is to ensure that EDS is not due to inadequate adherence to primary OSA therapy or suboptimal OSA treatment (i.e. non-normalization of AHI and associated sleep fragmentation or ineffective device). The clinician should first assess whether the patient is adherent to his/her therapy. Although some patients who are adherent to CPAP continue to experience EDS, residual EDS has been shown to significantly improve with increased CPAP use [Citation14]. However, an estimated 30 to 40% of patients with OSA do not use CPAP as recommended (i.e. are nonadherent) [Citation51,Citation52]. To determine whether a patient is using their device as recommended, telemonitoring devices are available for those using CPAP that can provide patients and clinicians with objective data on daily usage, AHI, and leaks [Citation31]. The American Thoracic Society has described how to interpret CPAP tracking data [Citation53]. The patient can be advised to review these data (typically available on an app) as a tool to monitor daily use. When objective data are not available, the clinician can question the patient and bed partner on the frequency and duration of use (i.e. how many hours/night and nights/week the device is used), although self-report is less reliable. While insurance-mandated adherence is considered use ≥4 h/night on ≥70% of nights, patients should be educated that longer use per night is associated with better improvements in EDS in a dose-response manner [Citation14,Citation16,Citation17]. A variety of interventions are available to help improve adherence, such as troubleshooting technical issues (e.g. airway leaks, pressure adjustments, humidity settings), improving side effects (e.g. mask discomfort, dry mouth), patient engagement tools, telemedicine, and cognitive-behavior therapies (e.g. framing) [Citation54]. Detailed approaches to implementing these interventions have been published [Citation54,Citation55]; involvement of a psychologist, patient’s family/friends, or road traffic authorities (i.e. to suspend driving license) may be helpful to improve adherence. Some patients may not be sufficiently adherent to CPAP despite best efforts by both patient and clinician. In these cases, referral to a sleep specialist may be appropriate where second-line, alternative, and experimental therapies can be considered.
After adherence to therapy has been confirmed, the clinician should determine whether the patient is receiving optimal treatment for OSA. For patients using CPAP, this can be assessed via telemonitoring report of AHI. Determining the cause of an elevated AHI may require technical troubleshooting (e.g. airway leaks) and referral to a sleep specialist (e.g. in cases of complex sleep apnea/treatment-emergent central sleep apnea, which may occur in up to 20% of CPAP-treated patients [Citation56-58]). It is also important to determine whether the therapy being used has been adequately evaluated for efficacy. This may be particularly important for non-CPAP therapies, such as mandibular advancement devices or positional therapies which typically do not have telemonitoring capabilities. Adherence and effectiveness may also be improved by switching to a more appropriate ventilatory support [Citation59].
3.2. Lifestyle factors
After ensuring the underlying airway obstruction is being optimally treated, the clinician should evaluate whether lifestyle factors may be contributing to EDS. The most common cause of EDS in the general population is insufficient sleep, with more than one-third of American adults sleeping less than is recommended to prevent cognitive, health, and safety-related impairments [Citation60,Citation61]. Indeed, patients with OSA who do not get enough sleep have a 4.6-fold increased risk of having residual EDS () [Citation4,Citation5,Citation18]. Clinicians should advise patients to:
Get ≥7 h of sleep each night [Citation61]
Maintain a consistent sleep/wake schedule (i.e. go to bed/wake up at the same time every day, including weekends)
Create a comfortable sleep environment (e.g. dark, quiet bedroom with a cooler temperature; electronics, including cell phones, turned off/silenced)
Practice good sleep hygiene (e.g. avoid electronics, arousing/stressful activities [e.g. social media, e-mail, finances, action movie], alcohol, caffeine, nicotine, meals, and exercise prior to bed) Notably, there are inter-individual differences in sleep need. Recommending a sleep extension trial, even in patients who get ≥7 hours of sleep, may help eliminate insufficient sleep as the cause [Citation62]. Sleep specialists and psychologists can help manage poor sleep habits. The clinician should also evaluate a patient’s diet (e.g. caffeine consumption) and exercise habits. Self-reported sleepiness has been shown to worsen after both high-carbohydrate and high-fat meals [Citation63,Citation64]. It has been proposed that a high-fat, high-calorie diet could contribute to regularly occurring EDS [Citation64]. Moreover, several studies have demonstrated an association between obesity and EDS and the ability of exercise to significantly reduce EDS [Citation64,Citation65].
3.3. Comorbidities/concomitant medications
Beyond lifestyle factors, EDS can have many other etiologies, such as medications, substance abuse, psychiatric disorders, medical disorders, or other sleep disorders () [Citation66]. For instance, EDS is a common side effect of prescription (e.g. sedating antidepressants, opioids) and nonprescription medications (e.g. first-generation antihistamines) [Citation67]. Depression is another common cause of EDS. In addition to EDS, OSA and depression can have many overlapping symptoms, such as poor concentration, irritability, psychomotor impairments, and weight gain [Citation68].
Table 3. Differential diagnosis of EDS
EDS is also associated with other sleep disorders that can co-occur with OSA. Insomnia is commonly comorbid with OSA, occurring in 39–55% of patients [Citation69,Citation70]. Narcolepsy, which can occur with (type 1) or without (type 2) cataplexy, idiopathic hypersomnia, and Kleine-Levin syndrome are central disorders of hypersomnolence where the primary complaint is EDS. The presence of cataplexy can help distinguish narcolepsy type 1 from other hypersomnolence disorders, whereas idiopathic hypersomnia and Kleine-Levin syndrome can be identified by consistent or episodes of long sleep times, respectively. Circadian rhythm sleep-wake disorders are characterized by misalignment between one’s biological clock and desired/required sleep-wake schedule (e.g. shift work, jet lag); wakefulness during the biological nighttime can lead to EDS. Finally, restless legs syndrome and periodic limb movement disorder, characterized by an urge to move the legs during rest/inactivity and frequent limb movements during sleep, respectively, can cause sleep disturbance-induced EDS [Citation62].
As these etiologies require different treatment approaches, it is critical for the clinician to determine the cause of EDS to ensure each patient receives the most appropriate and comprehensive care. In most cases, a differential diagnosis can be made based on a clinical consultation; however, further testing with questionnaires, laboratory tests, or objective assessments may be needed in other cases. If another sleep disorder is suspected, the clinician should refer the patient to a sleep specialist, who can determine what additional testing is needed. Involvement of a psychologist may be valuable in other cases, particularly if depression or insomnia are suspected.
3.4. Objective assessments for EDS
Several objective assessments are available to better characterize EDS (). Unfortunately, most are time consuming, expensive, and complex to administer. For these reasons, it is not feasible to use these assessments to assess EDS in routine practice; however, they may be helpful when attempting to make a differential diagnosis [Citation71,Citation72], justify use of pharmacotherapy, or evaluate response to treatment. These assessments are typically conducted and interpreted by sleep specialists in a sleep center and, therefore, are only briefly described below.
The Multiple Sleep Latency Test (MSLT) and Maintenance of Wakefulness Test (MWT) are the most commonly used objective assessments for characterizing an individual’s ability to fall asleep in a sleep-inducing environment or stay awake while seated in a dark, quiet environment, respectively (). For both assessments, the outcome of interest is mean sleep onset latency (the average amount of time it takes the patient to fall asleep) as determined by electroencephalography. The MSLT is often used to rule out other sleep disorders as the etiology for EDS, such as narcolepsy. The American Academy of Sleep Medicine (AASM) advises that the MWT should not be used for diagnostic purposes [Citation71]. Instead, it may be useful to evaluate the response to treatment or assess whether an individual’s ability to remain awake poses a public or personal safety risk, such as those employed in public transportation [Citation72].
Although less standardized, there are tests that use methods other than EEG to objectively quantify EDS. The Oxford Sleep Resistance (OSLER) test [Citation73] and Psychomotor Vigilance Task (PVT) [Citation74] use simple behavioral-based methods to measure sleep latency and sustained vigilance, respectively [Citation73,Citation74]. In addition, home-based wearables, such as wrist-worn activity monitors, can provide insight into sleep and wakefulness patterns in the home environment and may serve as a useful tool when attempting to rule out insufficient sleep as a cause for EDS, although there is uncertainty about their ability to accurately estimate sleep [Citation75].
Subjective symptoms and self-report measures of EDS often do not correlate with objective measures. In patients with OSA, complaints of sleepiness, tiredness, lack of energy, and fatigue have been found to have no association with MSLT mean sleep latency [Citation34]. In addition, the ESS and MWT are only moderately associated, whereas the ESS and MSLT are weakly associated [Citation11]. There are several possible explanations for these low agreements. As previously discussed, patients may simply be unaware of the severity of their sleepiness, especially if their baseline reference for feeling ‘normal’ has gradually shifted. On the other hand, patients may be reluctant to report negative feelings associated with disease, for fear of work- or driving-related implications. Indeed, research has shown that close relatives often describe the patient’s sleepiness as more severe than the patient reports [Citation76]. Another reason may be that these assessments measure different dimensions of EDS. For example, it has been postulated that the PVT and ESS may predict impaired performance, whereas the MSLT may predict physiologic sleep propensity [Citation77].
3.5. Key points
If a patient has EDS, the clinician should first determine if the patient is adherent to primary OSA therapy via self-report or telemonitoring data and evaluate effectiveness of device
Referral to a sleep specialist may be needed to determine cause of elevated AHI in adherent patients, confirm effectiveness of current treatment in adherent patients, or explore alternative treatments in nonadherent patients
Next, the clinician should evaluate lifestyle factors and other conditions that could be causing EDS
Referral to a sleep specialist may be needed for cases with multiple potential causes
Objective assessments can be useful when attempting to make a differential diagnosis, justify pharmacotherapy, or document response to treatment
If the clinician suspects their use is necessary, the patient should be referred to a sleep specialist
4. Pharmacotherapy for residual EDS
After a diagnosis of residual EDS and altered quality of life associated with OSA has been confirmed and other contributing conditions have been ruled out, pharmacotherapy with a wake-promoting agent or stimulant can be considered. The decision to prescribe a trial of pharmacotherapy should typically be made by a sleep specialist and can be based upon EDS severity, as characterized by the ESS or objective assessments. In general, ESS scores >10 indicate that the patient’s EDS is burdensome and interfering with daily life; however, this threshold is not absolute [Citation38,Citation39]. Clinical judgment should be made on an individual basis, especially for patients whose occupations may pose a personal and/or public safety risk (e.g. public transportation, healthcare, childcare). If pharmacologic treatment is determined to be appropriate, it is important to note that pharmacotherapy for EDS should not replace primary treatment of the underlying airway obstruction. Clinicians should monitor patients’ CPAP use after initiating pharmacotherapy to confirm adherence. Although pharmacotherapy for residual EDS may be prescribed more often in a specialist setting than in primary care, it is useful for clinicians to be familiar with the available options. Key information for the treatment options discussed below is summarized in [Citation78-86].
Table 4. Pharmacotherapy options for EDS in OSA
4.1. Modafinil and armodafinil
Modafinil (Provigil®; Teva Pharmaceuticals, North Wales, PA) and armodafinil (Nuvigil®; Teva Pharmaceuticals) are approved in the United States, but not in Europe, to improve wakefulness in adults with EDS associated with narcolepsy, OSA, or shift work disorder [Citation78,Citation79]. Both agents have demonstrated efficacy in reducing EDS and improving wakefulness in patients with OSA in short-term randomized controlled trials (RCTs) [Citation87-90] and long-term open-label extension trials [Citation91,Citation92], improving ESS scores by ~2 points and MWT sleep latency by ~3 minutes compared with placebo [Citation91-94]. Modafinil had previously been approved for OSA in the European Union; however, in 2011, the European Medicines Agency (EMA) withdrew this indication from the marketing authorization due to a poor benefit/risk profile. Specifically, the EMA concluded that the risk of serious cardiovascular disorders, neuropsychiatric disorders, and skin and hypersensitivity disorders outweighed any potential benefit for EDS in patients with OSA [Citation78,Citation79,Citation95].
The most common side effect experienced with both agents is headache, although armodafinil tends to be associated with a lower rate of headache than modafinil [Citation96]. Other common side effects include nausea, dizziness, insomnia, upper respiratory tract infection, and anxiety [Citation96]. Serious skin rashes, such as Stevens-Johnson syndrome and toxic epidermal necrolysis, and hypersensitivity, including angioedema and anaphylactoid reaction, have been reported [Citation78,Citation79]. The EMA withdrew approval due to potential adverse cardiovascular effects and the FDA issued a warning for use in patients with known cardiovascular disease [Citation78,Citation79,Citation95]. A recent meta-analysis concluded that modafinil and armodafinil may slightly increase blood pressure [Citation93]. Clinicians should monitor patients’ blood pressure prior to initiating and routinely during treatment. Modafinil and armodafinil can reduce the effectiveness of birth control and have been associated with fetal congenital malformations, including congenital cardiac anomalies [Citation97]; if prescribed to premenopausal women, alternative methods of contraception are recommended and these risks should be discussed.
4.2. Solriamfetol
Solriamfetol (Sunosi™; Jazz Pharmaceuticals, Inc, Palo Alto, CA) is a dopamine/norepinephrine reuptake inhibitor approved in the United States and European Union to improve wakefulness in adults with EDS associated with narcolepsy or OSA [Citation80,Citation98]. Solriamfetol has demonstrated efficacy in reducing EDS and improving wakefulness for up to 9 h in patients with OSA in short-term RCTs [Citation99,Citation100] and a long-term open-label extension trial [Citation101], improving ESS scores by ~2 to 5 points and MWT sleep latency by ~5 to 13 min compared with placebo [Citation80,Citation101].
The most common side effects with solriamfetol treatment include headache, nausea, decreased appetite, insomnia, and anxiety [Citation80]. In addition, solriamfetol has been associated with small increases in heart rate and blood pressure in patients with OSA, although increases were greatest for the 300 mg/day dose, which is not FDA- or EMA-approved [Citation99]. Patients’ hypertension should be controlled prior to initiating solriamfetol, and heart rate and blood pressure should be monitored periodically during treatment. The FDA advises avoiding use in patients with unstable cardiovascular disease, serious heart arrhythmias, or other serious heart problems. Solriamfetol is contraindicated with monoamine oxidase inhibitors [Citation80].
4.3. Pitolisant (off-label)
Pitolisant (Wakix®; Harmony Biosciences, LLC, Plymouth Meeting, PA) is a histamine-3 receptor antagonist approved in the United States to improve wakefulness and cataplexy in adults with narcolepsy and in the European Union for the treatment of narcolepsy with or without cataplexy in adults [Citation81,Citation102]. Recent phase 3 RCTs suggest pitolisant may reduce EDS in patients with OSA [Citation86,Citation103], but it is not currently approved for use in OSA [Citation81,Citation102].
4.4. Stimulants (off-label)
Stimulants, such as methylphenidate (Ritalin®; Novartis Pharmaceuticals Corporation, East Hanover, NJ) and amphetamines (Adderall® [Teva Select Brands, Horsham, PA] or Dexedrine® [Teva Pharmaceuticals]), have also been used off-label to treat EDS associated with OSA. Importantly, no RCTs or published data exist to support their use in this population. Their extensive side-effect profiles and potential for abuse/addiction limit their use.
4.5. Follow-up
After initiating a trial of pharmacotherapy, the sleep specialist or PCP should follow-up with the patient to evaluate efficacy, side effects, and adherence to primary OSA therapy. To determine efficacy, the clinician can re-administer the ESS and evaluate improvement in quality of life and productivity. Determining whether a patient has responded to treatment based on ESS scores can be considered based on the absolute score or the degree of change. In the first case, ESS scores may have decreased within the normal range (≤10); however, improvement this significant may not be observable until after many weeks of stable treatment [Citation86,Citation88,Citation104]. In the second case, the clinician can assess the percent reduction in ESS scores relative to baseline, using a reduction in ESS score ≥25% from baseline as a threshold for a clinically meaningful response to treatment [Citation105,Citation106]. Alternatively, some data suggest that a 2- to 3-point reduction in ESS score, corresponding to an effect size of 0.5, represents the minimal clinically important difference [Citation86,Citation107].
In addition to evaluating efficacy, the clinician should monitor for adverse events, particularly cardiovascular side effects (e.g. hypertension). Heart rate and blood pressure should be monitored prior to initiating and periodically throughout treatment with any wake-promoting agent or stimulant. With modafinil and armodafinil, some side effects, such as rashes, may occur weeks after initiation. With solriamfetol, some common early-onset side effects (those that occur during the first week of treatment) subside within ~10 days of treatment (e.g. headache and nausea), although others can persist for >2 months (e.g. decreased appetite) [Citation108].
4.6. Key points
Choosing a pharmacologic agent should be individualized to the patient, taking into account the evidence available regarding an agent’s efficacy and safety profile, cost, and patient characteristics
Regardless of which agent is chosen, the clinician, with support from a sleep specialist, should assess a patient’s response to treatment during follow-up visits
Efficacy with the ESS
Tolerability with adverse events
Blood pressure (every 6 months) and heart rate (a 12-lead ECG annually)
Adherence to primary OSA therapy
Whether to treat patients who struggle with adherence to primary OSA therapy with a pharmacologic agent warrants discussion. Treatment of EDS with a wake-promoting agent or stimulant is not a replacement for treatment of the underlying airway obstruction. For every patient, best efforts should be made to optimize adherence to CPAP or other airway therapies. Even in patients who are adherent, pharmacologic treatment has the potential to reduce motivation to use CPAP. Modafinil and armodafinil have been associated with a trend toward reduction in CPAP use, although both short- and long-term data suggest that adherence remains stable during treatment with solriamfetol [Citation96,Citation99,Citation109]. It is unknown whether pitolisant impacts primary OSA therapy adherence [Citation103]. Sometimes, despite best efforts by clinician and patient, patients remain nonadherent to primary OSA therapy. In these cases, the clinician should use his/her best clinical judgment and, in this specific situation, it is recommended to seek guidance from a sleep specialist. In some cases, it may not be feasible to optimally treat contributing causes, and it may be appropriate to temporarily prescribe a pharmacologic agent while working to improve adherence.
5. When to refer to a sleep specialist
In cases when the clinician may be unable to evaluate or manage a patient’s EDS, referral to a sleep specialist for further evaluation and/or testing may be necessary (see ). This could be when a patient is not sufficiently adherent to CPAP, despite best efforts by both clinician and patient, and the clinician suspects other therapies should be considered. Another appropriate case for referral would be if a clinician needs help determining the cause of an elevated AHI or wants to confirm that OSA is being optimally treated before prescribing wake-promoting pharmacotherapy. Finally, referral may be necessary when a clinician is uncertain about the cause of EDS (e.g. in cases that may have multiple etiologies) or suspects the cause is related to another sleep disorder. In these cases, additional testing (e.g. MSLT, MWT, overnight polysomnography) in a sleep clinic would likely be warranted.
6. Conclusions
EDS negatively impacts patients’ ability to function in daily life and is a risk factor for patient and public health. As such, it is important for any physician to be able to recognize, evaluate, and manage EDS in patients with OSA. Due to the high prevalence, it falls to the clinician to assess patients treated for OSA for residual EDS with the ESS. If a patient is suspected of having EDS, the clinician should first confirm the underlying airway obstruction is being optimally treated. If the patient continues to experience EDS despite adherence to OSA therapies, the patient should be reviewed in the clinic and, when appropriate, with questionnaires, physical examination, laboratory tests, or objective assessments to rule out other potential causes of EDS. After a differential diagnosis of residual EDS associated with OSA has been confirmed, pharmacologic treatment using a wake-promoting agent may be considered. Consistent use of this proposed assessment and management approach can help clinicians identify patients with EDS, improve diagnostic accuracy, enhance management strategies in the primary care setting, and make optimal use of secondary and tertiary resources in specialist centers.
Transparency
Declaration of funding
This review was supported by Jazz Pharmaceuticals. Jazz Pharmaceuticals has worldwide development, manufacturing, and commercialization rights to solriamfetol, excluding certain jurisdictions in Asia. SK Biopharmaceuticals, the discoverer of the compound (also known as SKL-N05), maintains rights in 12 Asian markets, including Korea, China, and Japan.
Declaration of financial/other relationships
R Rosenberg has received consultancy fees from Eisai; honoraria from Merck; research funding from Jazz Pharmaceuticals, Merck, Actelion, Eisai, and Philips Respironics; and has served on the speakers’ bureau for Merck and as an advisory board member for Jazz Pharmaceuticals. PK Schweitzer has received consultancy fees from Jazz Pharmaceuticals, Her institution has received research funding from Jazz Pharmaceuticals, Apnimed, Balance Therapeutics, Avadel-Flamel, Harmony Biosciences, Inspire Medical Systems, and Suven Life Sciences. J Steier has received consultancy fees from Jazz Pharmaceuticals, Sanofi, travel support from Fisher Paykel, GSK, and research funding from ResMed, British Lung Foundation, Actelion, Phillips Respironics. J Steier’s contributions were partially supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London, UK. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. J-L Pepin has received lecture fees or conference traveling grants from Resmed, Perimetre, Philips, Fisher and Paykel, AstraZeneca, Jazz Pharmaceuticals, Agiradom, and Teva, and has received unrestricted research funding from ResMed, Philips, GlaxoSmithKline, Bioprojet, Foundation de la Recherche Medicale (Foundation for Medical Research), Direction de la Recherche Clinique du CHU de Grenoble (Research Branch Clinic CHU de Grenoble), and fond de dotation ‘Agir pour les Maladies Chroniques’ (endowment fund ‘Acting for Chronic Diseases’). Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Biographical notes
Russell P. Rosenberg, PhD, is currently the Chief Science Officer and CEO of NeuroTrials Research, Inc. and the Director of the Atlanta School of Sleep Medicine and Technology in Atlanta, GA. Paula K. Schweitzer, PhD, is director of research at St. Luke’s Sleep Medicine and Research Center in Chesterfield, MO. Professor Joerg Steier works as Consultant Respiratory Physician at the Lane Fox Unit, a tertiary service for weaning and noninvasive ventilation, and the British Sleep Society (BSS) accredited Sleep Disorders Centre at King’s Health Partners; he is an executive committee member and honorary secretary of the BSS, and a task force member of the European Respiratory Society. Jean-Louis Pepin, MD, PhD, is currently a Professor of Clinical Physiology at University of Grenoble-Alpes (UGA) (exceptional class), a member of the national council of universities (CNU, 44-02: Clinical Physiology), Vice-Dean of the Grenoble School of Medicine in charge of research, and Director of the HP2 Laboratory (Inserm U1300; Hypoxia Pathophysiology).
Acknowledgments
Under the direction of the authors, Hannah K. Ritchie, PhD, Jeannette Fee, and Christopher Jaworski of Peloton Advantage, LLC, an OPEN Health company, provided medical writing and editorial support for this review article, which was funded by Jazz Pharmaceuticals.
References
- Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698.
- American Academy of Sleep Medicine. Obstructive sleep apnea, adult. International Classification of Sleep Disorders. 3rd ed. ed. Darien, IL: American Academy of Sleep Medicine; 2014. p. 53–62.
- Bailly S, Destors M, Grillet Y, et al. Obstructive sleep apnea: a cluster analysis at time of diagnosis. PLoS One. 2016;11(6):e0157318.
- Koutsourelakis I, Perraki E, Economou NT, et al. Predictors of residual sleepiness in adequately treated obstructive sleep apnoea patients. Eur Respir J. 2009;34(3):687–693. .
- Kapur VK, Baldwin CM, Resnick HE, et al. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep. 2005;28(4):472–477.
- Smith S, Rossdale J, Serry Y, et al. Multiple dimensions of excessive daytime sleepiness. J Thorac Dis. 2018;10(Suppl 1):S170–s176.
- Keenan BT, Kim J, Singh B, et al. Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: a cluster analysis. Sleep. 2018;41(3):3.
- Lal C, Weaver TE, Bae CJ, et al. Excessive daytime sleepiness in obstructive sleep apnea: mechanisms and clinical management. Ann Am Thorac Soc. 2021;18(5):757–768.
- Macey PM, Woo MA, Kumar R, et al. Relationship between obstructive sleep apnea severity and sleep, depression and anxiety symptoms in newly-diagnosed patients. PLoS One. 2010;5(4):e10211.
- Gabryelska A, Białasiewicz P. Association between excessive daytime sleepiness, REM phenotype and severity of obstructive sleep apnea. Sci Rep. 2020;10(1):34.
- Kendzerska TB, Smith PM, Brignardello-Petersen R, et al. Evaluation of the measurement properties of the Epworth Sleepiness Scale: a systematic review. Sleep Med Rev. 2014;18(4):321–331.
- Patil SP, Ayappa IA, Caples SM, et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2019;15(2):335–343.
- Epstein LJ, Kristo D, Strollo PJ Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276.
- Gasa M, Tamisier R, Launois SH, et al. Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J Sleep Res. 2013;22(4):389–397.
- Pepin JL, Viot-Blanc V, Escourrou P, et al. Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study. Eur Respir J. 2009;33(5):1062–1067.
- Antic NA, Catcheside P, Buchan C, Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34(1):111–119.
- Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719.
- Budhiraja R, Kushida CA, Nichols DA, et al. Predictors of sleepiness in obstructive sleep apnoea at baseline and after 6 months of continuous positive airway pressure therapy. Eur Respir J. 2017;50(5):5.
- Stepnowsky C, Sarmiento KF, Bujanover S, et al. Comorbidities, health-related quality of life, and work productivity among people with obstructive sleep apnea with excessive sleepiness: findings from the 2016 US National Health and Wellness Survey. J Clin Sleep Med. 2019;15(2):235–243.
- Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. 2012;141(6):1601–1610.
- Werli KS, Otuyama LJ, Bertolucci PH, et al. Neurocognitive function in patients with residual excessive sleepiness from obstructive sleep apnea: a prospective, controlled study. Sleep Med. 2016;26:6–11.
- Tramonti F, Maestri M, Carnicelli L, et al. Relationship quality of persons with obstructive sleep apnoea syndrome. Psychol Health Med. 2017;22(8):896–901.
- Mulgrew AT, Ryan CF, Fleetham JA, et al. The impact of obstructive sleep apnea and daytime sleepiness on work limitation. Sleep Med. 2007;9(1):42–53.
- Tregear S, Reston J, Schoelles K, et al. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5(6):573–581.
- Garbarino S, Guglielmi O, Sanna A, et al. Risk of occupational accidents in workers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2016;39(6):1211–1218.
- Mazza S, Pépin JL, Naëgelé B, et al. Driving ability in sleep apnoea patients before and after CPAP treatment: evaluation on a road safety platform. Eur Respir J. 2006;28(5):1020–1028.
- Philip P, Bailly S, Benmerad M, et al. Self-reported sleepiness and not the apnoea hypopnoea index is the best predictor of sleepiness-related accidents in obstructive sleep apnoea. Sci Rep. 2020;10(1):16267.
- Gottlieb DJ, Ellenbogen JM, Bianchi MT, et al. Sleep deficiency and motor vehicle crash risk in the general population: a prospective cohort study. BMC Med. 2018;16(1):44. .
- Jennum P, Castro JPC, Mettam S, et al. Socioeconomic and humanistic burden of illness of excessive daytime sleepiness severity associated with obstructive sleep apnoea in the European Union 5. Sleep Med. 2021; 84:46-55.
- Won CH, Bogan RK, Doghramji K, et al. Assessing communication between physicians and patients with excessive daytime sleepiness associated with treated obstructive sleep apnea: insights from an ethnographic study of in-office visits [abstract]. Am J Respir Crit Care Med. 2019;199:A1390.
- Pépin JL, Tamisier R, Hwang D, et al. Does remote monitoring change OSA management and CPAP adherence? Respirology. 2017;22(8):1508–1517.
- He K, Kapur VK. Sleep-disordered breathing and excessive daytime sleepiness. Sleep Med Clin. 2017;12(3):369–382.
- Javaheri S, Javaheri S. Update on persistent excessive daytime sleepiness in OSA [Review]. Chest. 2020;158(2):776–786.
- Chervin RD. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest. 2000;118(2):372–379.
- Vernet C, Redolfi S, Attali V, et al. Residual sleepiness in obstructive sleep apnoea: phenotype and related symptoms. Eur Respir J. 2011;38(1):98–105.
- Arand D, Bonnet M, Hurwitz T, et al. The clinical use of the MSLT and MWT. Sleep. 2005;28(1):123–144.
- Thomann J, Baumann CR, Landolt HP, et al. Psychomotor vigilance task demonstrates impaired vigilance in disorders with excessive daytime sleepiness. J Clin Sleep Med. 2014;10(9):1019–1024.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545.
- Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep. 1997;20(10):844–849.
- Lapin BR, Bena JF, Walia HK, et al. The Epworth Sleepiness Scale: validation of One-Dimensional Factor Structure in a Large Clinical Sample. J Clin Sleep Med. 2018;14(8):1293–1301.
- Rosenberg R, Babson K, Menno D, et al. Epworth Sleepiness Scale Test-Retest Reliability Analysis In Solriamfetol Studies [abstract]. Sleep. 2020;43(Suppl 1):A285–A286.
- Taylor E, Zeng I, O’Dochartaigh C. The reliability of the Epworth Sleepiness Score in a sleep clinic population. J Sleep Res. 2019;28(2):e12687.
- Campbell AJ, Neill AM, Scott DAR. Clinical reproducibility of the Epworth Sleepiness Scale for patients with suspected sleep apnea. J Clin Sleep Med. 2018;14(5):791–795.
- Nguyen ATD, Baltzan MA, Small D, Nguyen AT, Baltzan MA, Small D, et al. Clinical reproducibility of the Epworth Sleepiness Scale. J Clin Sleep Med. 2006;2(2):170–174.
- Ayeni A, Beghal GS, Pengo MF, et al. Self-reported sleepiness in the context of fitness-to-drive. Sleep Breath. 2019;23(4):1227–1232.
- Bonzelaar LB, Salapatas AM, Yang J, et al. Validity of the epworth sleepiness scale as a screening tool for obstructive sleep apnea. Laryngoscope. 2017;127(2):525–531.
- Hunasikatti M. Low repeatability of the Epworth Sleepiness Scale and the need to redefine the minimal clinically important difference. J Clin Sleep Med. 2020;16(10):1827.
- Grewe FA, Roeder M, Bradicich M, et al. Low repeatability of Epworth Sleepiness Scale after short intervals in a sleep clinic population. J Clin Sleep Med. 2020;16(5):757–764.
- Hoddes E, Zarcone V, Smythe H, et al. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10(4):431–436.
- Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52(1–2):29–37.
- Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45(1):43.
- Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: implications for future interventions. Indian J Med Res. 2010;131:245–258.
- Schwab RJ, Badr SM, Epstein LJ, et al. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med. 2013;188(5):613–620.
- Pengo MF, Czaban M, Berry MP, et al. The effect of positive and negative message framing on short term continuous positive airway pressure compliance in patients with obstructive sleep apnea. J Thorac Dis. 2018;10(Suppl 1):S160–S169.
- Weaver TE. Novel Aspects of CPAP Treatment and Interventions to Improve CPAP Adherence. J Clin Med. 2019;8(12):12.
- Neu D, Balkissou AD, Mairesse O, et al. Complex sleep apnea at auto-titrating CPAP initiation: prevalence, significance and predictive factors. Clin Respir J. 2017;11(2):200–209.
- Nigam G, Riaz M, Chang ET, et al. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86–91.
- Liu D, Armitstead J, Benjafield A, et al. Trajectories of Emergent Central Sleep Apnea During CPAP Therapy. Chest. 2017;152(4):751–760.
- Pépin JL, Woehrle H, Liu D, et al. Adherence to Positive Airway Therapy After Switching From CPAP to ASV: a Big Data Analysis. J Clin Sleep Med. 2018;14(1):57–63.
- Sleep and sleep disorders data and statistics: centers for Disease Control and Prevention; 2017 [2020 Oct 16]. Available from: https://www.cdc.gov/sleep/data_statistics.html.
- Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):843–844.
- Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394.
- Lowden A, Holmbäck U, Akerstedt T, et al. Performance and sleepiness during a 24 h wake in constant conditions are affected by diet. Biol Psychol. 2004;65(3):251–263.
- Panossian LA, Veasey SC. Daytime sleepiness in obesity: mechanisms beyond obstructive sleep apnea--a review. Sleep. 2012;35(5):605–615.
- Lins-Filho OL, Pedrosa RP, Gomes JML, et al. Effect of exercise training on subjective parameters in patients with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med. 2020;69:1–7.
- Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391–396.
- Pagel JF. Drug-Induced Hypersomnolence. Sleep Med Clin. 2017;12(3):383–393.
- Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17–25.
- Luyster FS, Buysse DJ, Strollo PJ Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2010;6(2):196–204.
- Ong JC, Crawford MR, Wallace DM. Sleep apnea and insomnia: emerging evidence for effective clinical management. Chest. 2021;159(5):2020–2028.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
- Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the Multiple Sleep Latency Test and the Maintenance of Wakefulness Test. Sleep. 2005;28(1):113–121.
- Bennett LS, Stradling JR, Davies RJ. A behavioural test to assess daytime sleepiness in obstructive sleep apnoea. J Sleep Res. 1997;6(2):142–145.
- Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;17(6):652–655.
- Goldstein C. Current GC. Future Roles of Consumer Sleep Technologies in Sleep Medicine. Sleep Med Clin. 2020;15(3):391–408.
- Li Y, Zhang J, Lei F, et al. Self-evaluated and close relative-evaluated Epworth Sleepiness Scale vs multiple sleep latency test in patients with obstructive sleep apnea. J Clin Sleep Med. 2014;10(2):171–176.
- Li Y, Vgontzas A, Kritikou I, et al. Psychomotor Vigilance Test and Its Association With Daytime Sleepiness and Inflammation in Sleep Apnea: clinical Implications. J Clin Sleep Med. 2017;13(9):1049–1056.
- Provigil [package insert]. North Wales, PA: Teva Pharmaceuticals; 2018 November. 2018.
- Nuvigil [package insert]. North Wales, PA: Teva Pharmaceuticals; 2018 November. 2018.
- Sunosi™ (solriamfetol) tablets Prescribing Information. June 2019. Palo Alto, CA: Jazz Pharmaceuticals, Inc; 2019.
- Wakix [package insert]. Plymouth Meeting, PA: Harmony Biosciences; 2020 October. 2020.
- Ritalin [package insert]. 2019 November. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2019.
- Concerta [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2017 January. 2017.
- Adderall [package insert]. Horsham, PA: Teva Pharmaceuticals; 2017 January. 2017.
- Dextroamphetamine sulfate [package insert]. North Wales, PA: Teva Pharmaceuticals; 2016 September. 2016.
- Dauvilliers Y, Verbraecken J, Partinen M, et al. Pitolisant for daytime sleepiness in obstructive sleep apnea patients refusing CPAP: a randomized trial. Am J Respir Crit Care Med. 2020;201(9):1135–1145.
- Pack AI, Black JE, Schwartz JR, et al. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164(9):1675–1681.
- Black JE, Hirshkowitz M. Modafinil for treatment of residual excessive sleepiness in nasal continuous positive airway pressure-treated obstructive sleep apnea/hypopnea syndrome. Sleep. 2005;28(4):464–471.
- Hirshkowitz M, Black JE, Wesnes K, et al. Adjunct armodafinil improves wakefulness and memory in obstructive sleep apnea/hypopnea syndrome. Respir Med. 2007;101(3):616–627.
- Roth T, White D, Schmidt-Nowara W, et al. Effects of armodafinil in the treatment of residual excessive sleepiness associated with obstructive sleep apnea/hypopnea syndrome: a 12-week, multicenter, double-blind, randomized, placebo-controlled study in nCPAP-adherent adults. Clin Ther. 2006;28(5):689–706.
- Hirshkowitz M, Black J. Effect of adjunctive modafinil on wakefulness and quality of life in patients with excessive sleepiness-associated obstructive sleep apnoea/hypopnoea syndrome: a 12-month, open-label extension study. CNS Drugs. 2007;21(5):407–416.
- Black JE, Hull SG, Tiller J, et al. The long-term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open-label extension study. J Clin Sleep Med. 2010;6(5):458–466.
- Chapman JL, Vakulin A, Hedner J, et al. Modafinil/armodafinil in obstructive sleep apnoea: a systematic review and meta-analysis. Eur Respir J. 2016;47(5):1420–1428.
- Chapman JL, Serinel Y, Marshall NS, et al. Residual Daytime Sleepiness in Obstructive Sleep Apnea After Continuous Positive Airway Pressure Optimization: causes and Management. Sleep Med Clin. 2016;11(3):353–363.
- European Medicines Agency. Modafinil - Article 31 referral - Annex I, II, III, IV London. United Kingdom: European Medicines Agency; 2011 2019 Jan 24. Available from https://www.ema.europa.eu/documents/referral/modafinil-article-31-referral-annex-i-ii-iii-iv_en.pdf.
- Sukhal S, Khalid M, Tulaimat A. Effect of wakefulness-promoting agents on sleepiness in patients with sleep apnea treated with CPAP: a meta-analysis. J Clin Sleep Med. 2015;11(10):1179–1186.
- Health Canada. ALERTEC (modafinil) and the risk of congenital anomalies: health Canada; 2019 [2020 Jan 8]. Available from: https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2019/70201a-eng.php.
- Sunosi™ (solriamfetol) tablets Summary of Product Characteristics. January 2020. Dublin, Ireland: Jazz Pharmaceuticals Ireland Ltd; 2020.
- Schweitzer PK, Rosenberg R, Zammit GK, et al. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): a randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421–1431.
- Strollo PJ Jr., Hedner J, Collop N, et al. Solriamfetol for the treatment of excessive sleepiness in OSA: a placebo-controlled randomized withdrawal study. Chest. 2019;155(2):364–374.
- Malhotra A, Shapiro C, Pepin JL, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020;43(2):zsz220.
- Wakix (pitolisant) Summary of Product Characteristics. January 2019. Paris, France: Bioprojet Pharm; 2019.
- Pépin JL, Georgiev O, Tiholov R, et al. Pitolisant for residual excessive daytime sleepiness in OSA patients adhering to CPAP: a randomized trial. Chest. 2021;159(4):1598–1609.
- Rosenberg R, Baladi M, Menno D, et al. Clinically relevant effects of solriamfetol on excessive daytime sleepiness: a post-hoc analysis of the magnitude of change in clinical trials in adults with narcolepsy or obstructive sleep apnoea [abstract]. Sleep Med. 2019;64(11):S325.
- Scrima L, Emsellem HA, Becker PM, et al. Identifying clinically important difference on the Epworth Sleepiness Scale: results from a narcolepsy clinical trial of JZP-110. Sleep Med. 2017;38:108–112.
- Lammers GJ, Bogan R, Schweitzer PK, et al. Thresholds for clinically meaningful changes on the Epworth Sleepiness Scale and Maintenance of Wakefulness Test sleep latency [abstract]. Sleep Med. 2019;64(suppl 1):S210.
- Patel S, Kon SSC, Nolan CM, et al. The Epworth Sleepiness Scale: minimum clinically important difference in obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197(7):961–963.
- Rosenberg R, Schweitzer PK, Malhotra A, et al. Incidence and duration of common adverse events in a solriamfetol (JZP-110) phase 3 study for treatment of excessive daytime sleepiness in obstructive sleep apnea [abstract 0569]. Sleep. 2019;42(suppl 1):A226–A227.
- Schweitzer PK, Strohl KP, Mayer G, et al. Effects of solriamfetol in a long-term trial of participants with obstructive sleep apnea who are adherent or nonadherent to airway therapy. J Clin Sleep Med. 2021;17(4):659–668.