ABSTRACT
Patients with chronic kidney disease (CKD) and type 2 diabetes (T2D) are at high risk of CKD progression and cardiovascular (CV) disease. Prevalence of CKD in patients with T2D is currently around 40% and continues to grow. The increasing number of people with CKD and T2D will ultimately have a significant impact upon health resource use and costs of care for people with T2D. Management of CKD in patients with T2D aims to preserve kidney function to reduce the risk of end-stage kidney disease, CV events, and mortality. Evidence-based recommendations for the treatment of patients with CKD and T2D are provided by several international and national organizations and recommend several lifestyle and pharmacological approaches to help prevent or delay the progression of CKD in patients with T2D. Guidelines include regular screening of patients with T2D for CKD using spot urine albumin-to-creatinine ratio (UACR) and estimated glomerular filtration rate (at least annually and at least twice a year if UACR >300 mg/g). Additionally, assessment of vascular complications, together with interventions designed to improve glycemic control and lipid levels, maintain healthy body weight, and optimize blood pressure should be performed. Medications shown to slow progression of CKD include renin–angiotensin system inhibitors, sodium-glucose cotransporter-2 inhibitors, glucagon-like peptide 1 receptor agonists, and, more recently, selective, non-steroidal mineralocorticoid receptor antagonists. This review highlights the ongoing challenges facing primary care providers in the management of CKD in patients with T2D including the consideration of comorbidities, adoption of new treatment options, and implementation of individualized care. Achieving consensus for optimal treatment of this disease is critical in providing consistent and appropriate care for all patients. Strategies to improve outcomes should also include use of clear referral criteria, use of a multi-disciplinary approach, and patient education.
PLAIN LANGUAGE SUMMARY
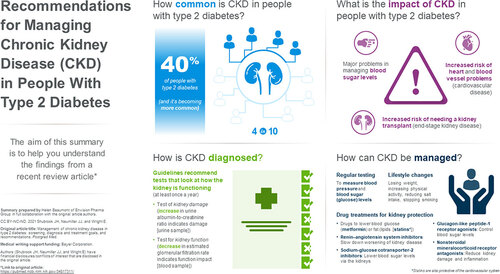
This review describes the diagnosis and management of chronic kidney disease in people who live with type 2 diabetes (T2D). About 40% of people with T2D experience long-term kidney problems. This is linked to a high risk of developing heart and blood vessel (cardiovascular) problems. Treatment guidelines recommend testing for kidney damage by calculating the urine albumin to creatinine ratio (increase indicates damage) and kidney function by measuring the estimated glomerular filtration rate (the kidneys’ filtration [waste removal] rate) from a blood sample. People should have these tests performed at least once a year. In people with T2D and chronic kidney disease, losing weight, increasing physical activity, and controlling blood sugar (glucose) levels and blood pressure can improve health. Clinicians may prescribe medicines that lower fat (lipid) levels, which can help reduce cardiovascular-related problems. Several medications may help slow progression (worsening) of chronic kidney disease and reduce cardiovascular risk. People with signs of abnormal kidney function or damage should take a medication called an angiotensin-converting enzyme inhibitor or an angiotensin II receptor blocker (both slow worsening of kidney disease). If these abnormalities persist despite people taking the maximum tolerated dose of either drug type, clinicians may consider adding a sodium-glucose cotransporter-2 inhibitor (to reduce glucose levels and help the kidneys) or the non-steroidal mineralocorticoid receptor antagonist, finerenone (reduces kidney damage and inflammation). Clinicians, nurses, pharmacists, kidney specialists and heart specialists should work together and discuss treatment options with each individual. See for an infographic version of this summary.
Introduction
Chronic kidney disease in patients with type 2 diabetes mellitus
Chronic kidney disease (CKD) in patients with type 2 diabetes mellitus (T2D) is associated with an increased risk of cardiovascular disease (CVD) and end-stage kidney disease (ESKD) [Citation1]. While CVD in T2D patients with CKD is generally due to the aggregation of numerous risk factors, a large body of evidence strongly suggests that CKD is an important and independent risk factor for adverse CV outcomes [Citation2]. CKD in patients with T2D also accounts for most patients with ESKD globally and is associated with high morbidity, mortality, and poor quality of life [Citation1]. CKD in patients with T2D is defined by elevated urine albumin excretion and/or a reduced glomerular filtration rate in the absence of other kidney pathologies and is a growing concern among clinicians involved in the management of T2D [Citation3,Citation4]. In recent years, there has been a marked increase in the population living with T2D and CKD, as well as the proportion of these individuals requiring kidney replacement therapy (KRT), inclusive of dialysis and/or kidney transplantation [Citation4,Citation5]. The prevalence of CKD in patients with T2D is currently around 40% and is predicted to increase to approximately 50% by the year 2025 [Citation4,Citation6,Citation7]. The increasing number of people with CKD and T2D requiring KRT will ultimately have a considerable impact upon health resource use and costs of care for people with T2D [Citation4].
Implications of CKD in patients with T2D
Glycemic management becomes increasingly complicated in patients with CKD and T2D. There is an increased risk of hypoglycemia in patients with CKD and T2D whose estimated glomerular filtration rate (eGFR) is <60 mL/min/1.73 m2, due in part to the decreased clearance of glucose-lowering medications and decreased gluconeogenesis by the kidney [Citation8]. Therefore for many drugs for diabetes, dose adjustments are required when used in patients with CKD and T2D [Citation8]. Moreover, the HbA1c test, which reflects average blood glucose levels over the previous 2 or 3 months, has limitations due to its imprecision in patients with CKD. As eGFR falls, red blood cell survival times become shorter, resulting in a reduction in measured HbA1c. In addition, treatment with erythrocyte-stimulating agents lower HbA1c further. For this reason, HbA1c results should be interpreted carefully in patients with CKD and T2D [Citation8].
The progressive nature of CKD in patients with T2D and its association with an increased risk of CV and adverse kidney outcomes leads to ever-worsening quality of life for these patients and mounting management costs. Health-related quality of life is significantly diminished with progression of CKD. Compared to people without diabetes those with T2D are more likely to experience poor physical and mental health outcomes [Citation9]. Timely intensified comprehensive diabetes care, that includes both therapeutic lifestyle and pharmacologic interventions has been shown to reduce the risk of poor kidney and CV outcomes. In the Steno-2 study, intensified multifactorial disease management increased median lifespan by 7.9 years and delayed CVD by a median of 8.1 years compared with a multifactorial intervention based upon standard of care at the time. A cost analysis of these treatment strategies over 21.2 years of follow-up revealed that there was no difference in total direct costs between these two interventions [Citation10].
Screening and diagnosis of CKD in patients with T2D
Urinary albumin:creatinine ratio and estimated glomerular filtration rate
In the absence of specific biomarkers and diagnostic tools, an increased urinary albumin: creatinine ratio (UACR; also known as microalbuminuria or urine microalbumin) i.e. UACR > 30 mg/g and declining eGFR are currently recognized as the most appropriate and accurate early indicators of kidney disease () [Citation11–13]. Studies have shown that combining eGFR and UACR levels is more accurate when predicting the risk of CKD progression and CVD or mortality in patients with T2D [Citation14]. These tests should be used to inform and guide clinical practice in line with the standards defined by guidelines and will enable more individualized and effective approaches to CKD management in patients with T2D.
Table 1. Categories used to describe albuminuria
It is critical that these screening tests are utilized in the early stages of diabetic kidney disease DKD as these patients are often asymptomatic [Citation15]. Timely screening allows for early identification of DKD before advanced disease and CV disease symptoms appear [Citation16]. It has been shown that up to half of patients with Stage 3 or 4 CKD are unaware of their kidney status [Citation17]. Interestingly, independent of each other and traditional risk factors, albuminuria, and eGFR have been shown to be significantly associated with increased CV risk () [Citation18,Citation19]. Protein-to-creatinine ratio (PCR) is still often used as an alternative to UACR. However, UACR is a more sensitive and specific marker of glomerular damage than PCR. Furthermore, the use of UACR in most predictive equations and in recent clinical trials of cardiorenal treatment make it the test of choice for measuring kidney damage and in assessing CV risk [Citation20].
Figure 1. Association of UACR and eGFR with cardiovascular mortality [Citation19]. Adjusted for each other (UACR or eGFR), age, gender, race, CVD history, systolic blood pressure, diabetes, smoking, and total cholesterol. Circles represent statistically significant, and triangles represent not significant.
![Figure 1. Association of UACR and eGFR with cardiovascular mortality [Citation19]. Adjusted for each other (UACR or eGFR), age, gender, race, CVD history, systolic blood pressure, diabetes, smoking, and total cholesterol. Circles represent statistically significant, and triangles represent not significant.](/cms/asset/624fcb0b-4048-4075-a6f4-effa4759f8e4/ipgm_a_2009726_f0001_c.jpg)
Guideline recommendations for the assessment of UACR and eGFR
Multiple guidelines recommend the assessment of kidney function in patients with diabetes using eGFR and kidney damage (albuminuria) using UACR [Citation12,Citation21,Citation22] (). eGFR is calculated using the CKD Epidemiology Collaboration (CKD-EPI) formula, which is recommended due to its superior performance in the eGFR range 60–90 mL/min/1.73 m2 [Citation23]. A recent Task Force of the National Kidney Foundation (NKF) and the American Society of Nephrology (ASN) has recommended a new race-free approach to diagnose kidney disease, via the adoption of the new EGFR 2021 CKD-EPI equation that estimates kidney function without a race variable [Citation24]. The Task Force also called for increased measurement of cystatin C, an alternative biomarker for kidney function, especially to confirm eGFR for clinical decision-making in adults who are at risk for or have CKD.
Figure 2. Guideline recommendations for the assessment of UACR and eGFR. a Calculated from serum creatinine (CKD-EPI); b With random spot urine sample; c Early morning urine sample is preferred.
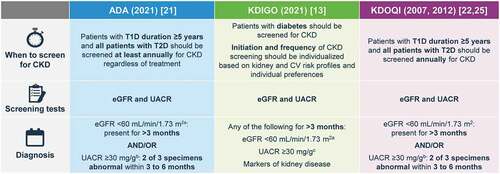
The recently updated American Diabetes Association (ADA) 2021 practice guidelines recommend that all T2D patients should have UACR and eGFR measured annually, at minimum, irrespective of treatment received. Furthermore, in those with diabetes and existing CKD, in whom risk of progression is higher, UACR and eGFR should be monitored more frequently to inform ongoing treatment decisions [Citation16,Citation21].
The US Kidney Disease Outcomes Quality Initiative (KDOQI) and Kidney Disease Improving Global Outcomes (KDIGO) groups also recommend UACR and eGFR assessments for patients with diabetes [Citation12,Citation13,Citation22]. Abnormalities in eGFR or UACR should be confirmed by repeat testing to make the diagnosis of DKD. Typically each of these tests will be repeated in 3 months to confirm the DKD abnormality [Citation25]. Similarly, two eGFR values <60 mL/min/1.73 m2 at least 3 months apart are required to confirm a diagnosis of CKD [Citation12]. KDIGO suggests the use of three categories to describe severity of albuminuria and that the terms micro- and macro-albuminuria should no longer be used. UACR values <30 mg/g are considered normal or mildly increased, with UACR values of 30 to 300 mg/g (corresponding to micro-albuminuria) referred to as ‘moderately increased,’ and UACR values of >300 mg/g (corresponding to macro-albuminuria) defined as ‘severely increased’ (). People with mild-to-moderate reductions in eGFR (eGFR 45–89 ml/min/1.73 m2) and/or moderately increased UACR should be tested/monitored at least once each year. People at greater risk of adverse outcomes, classified by a severe eGFR decrease (<30 mL/min/1.73 m2) and severely increased UACR (>300 mg/g) should be tested more frequently (2–4 times each year, depending on risk category) ().
Figure 3. KDIGO guide to frequency of monitoring by GFR and albuminuria category [Citation12]. Grid reflects the risk of progression by intensity of coloring (green: low risk [if no other markers of kidney disease, no CKD]; yellow: moderately increased risk; Orange: high risk; red/deep red, very high risk). The numbers in the boxes are a guide to the frequency of monitoring (number of times per year).
![Figure 3. KDIGO guide to frequency of monitoring by GFR and albuminuria category [Citation12]. Grid reflects the risk of progression by intensity of coloring (green: low risk [if no other markers of kidney disease, no CKD]; yellow: moderately increased risk; Orange: high risk; red/deep red, very high risk). The numbers in the boxes are a guide to the frequency of monitoring (number of times per year).](/cms/asset/b6b2932e-d30f-4359-bdf5-804dad0c4d30/ipgm_a_2009726_f0003_c.jpg)
While eGFR screening is widely utilized, rates of UACR testing in patients with T2D remain suboptimal. In one observational US study of patients with T2D it was reported that in the 15 months prior to study participation, more patients had received eGFR assessment than had UACR tested (85% vs 47%, respectively) [Citation26]. In another registry of patients with T2D at risk of CKD, this rate was even lower with only 8.7% having been tested for albuminuria at baseline [Citation27].
Both a drop in eGFR and increases in UACR are independent risk factors for adverse outcomes and higher rates of mortality. When abnormalities coexist in eGFR and UACR the resultant risk is multiplied [Citation19]. KDIGO guidelines for the assessment and management of CKD classify the stages of kidney disease and define the risk of CKD progression and CV events according to eGFR and UACR [Citation12,Citation13]. Depending on assigned GFR and albuminuria categories, the prognosis of CKD can be determined and graded by risk status ().
Management of CKD in patients with T2D
The major objectives in the management of CKD in patients with T2D are to preserve kidney function to reduce the risk of ESKD, CV events, and mortality. Treatment guidelines have been developed by several international and national organizations and recommend a wide range of lifestyle and pharmacological interventions to help prevent or inhibit progression of CKD in patients with T2D [Citation12,Citation13,Citation21,Citation22,Citation25]. Guidelines include regular evaluation of kidney and vascular complications, in addition to interventions that aim to improve glycemic control and lipid levels, maintain healthy body weight, and optimize blood pressure (BP). In the recently reported NID-2 study, multifactorial intensive treatment of main risk factors (including hypertension, hyperglycemia, and dyslipidemia) was significantly more effective than standard of care in reducing the risk of major adverse cardiovascular events in T2D patients with CKD [Citation28]. Clinical benefits were evident early during intervention and persisted long-term.
Lifestyle interventions
Non-pharmacological interventions should play a crucial role in improving outcomes in patients with CKD and T2D and should include weight loss, increased physical activity, reduced intake of dietary sodium, and smoking cessation. Conducting a detailed dietary assessment with subsequent patient support and education may be a useful initial tool in slowing kidney function decline [Citation4].
In patients with mild-to-moderate kidney disease, as defined by UACR or eGFR, intentionally losing weight may reduce urinary albumin excretion, improve BP, and potentially benefit the kidneys. Increased physical activity and dietary modifications in obese patients who have eGFR ≥30 mL/min/1.73 m2 is recommended. However, it is important to consider the individual patient, as weight loss may not be appropriate for all patients with advanced CKDfor example, patients undergoing dialysis [Citation13].
The KDIGO and KDOQI guidelines recommend protein intake of 0.8 g per kg per day in patients with CKD and T2D and avoiding high-protein intake (>1.3 g/kg/day or ≥20% of total daily calories) [Citation12,Citation21,Citation22,Citation25]. Moreover, the ADA recommends ‘usual’ (not high; 16–18% of total calories) dietary protein intake [Citation21]. These guideline recommendations are based on several studies that on the whole show that low protein diets can slow the decline of GFR and progression to ESKD [Citation11]. However, more recently the kidney protective effects of low protein diets in patients with CKD and diabetes have been questioned, as ultra-low protein diets may increase the risk of mortality in these patients [Citation29]. Limitations on potassium consumption are less consistent. KDIGO guidelines state that measures to control high potassium levels should include moderation of potassium intake, with specific counseling advice [Citation13]. In contrast, KDOQI state that recommendations for potassium should be the same as for CKD with and without diabetes and that intake should be restricted to 2 to 4 g/day [Citation25].
Support and advice regarding smoking cessation and pharmacological interventions where appropriate may also be helpful [Citation4]. Overall, it is important for individuals to be actively involved in their own management and to be provided help in achieving mutually agreed upon treatment targets.
Glycemic control
Hyperglycemia in patients with CKD and T2D should be managed with a multifactorial approach. Lifestyle changes and metformin remain the recommended first-line therapy for patients with diabetes [Citation30]. However, regular reviews of glycemic control, including HbA1c, and prescribed glucose-lowering medications are also important. ADA guidelines recommend a target HbA1C level of <7% for many adults. A lower HbA1C target (e.g. <6% vs. 7% to 8%) has been associated with a reduction in CKD in patients with T2D but at the cost of potentially more hypoglycemic events, polypharmacy, and increased mortality [Citation21]. KDIGO guidelines recommend an individualized HbA1c target ranging from <6.5% to <8.0% in patients with diabetes and CKD not treated with dialysis [Citation13]. This represents a target range that can be individualized, i.e. depending on type of diabetes, risk of hypoglycemia, stage of CKD and whether the patient is on dialysis, while maintaining the balance between the long-term benefits of glycemic control and the short-term risks of hypoglycemia in each patient. If prevention of complications is a key goal, a lower HbA1c target (<6.5% or <7.0%) may be preferred. Alternatively, in patients with multiple comorbidities or in patients with an increased hypoglycemia burden, a higher target (<7.5% or <8.0%) may be selected [Citation13]. As discussed earlier, caution is warranted when interpreting HbA1c in patients with CKD and T2D as measurements may be unreliable in this population [Citation8]. Recent data have shown that continuous glucose monitoring (CGM) had similar correlations with HbA1c and glycated albumin in individuals with diabetes and CKD, and may offer the potential for more accurate monitoring and treatment adjustments to allow improved glycemic management when used to complement HbA1c measurement in these patients [Citation31,Citation32]. In particular, CGM may be an important tool for those with KRT, in whom measurement of HbA1c may not be as reliable; a limitation of HbA1c, especially in those having undergone KRT, is its inability to detect hypoglycemia, a significant risk for this population. Evidence suggests that tight glycemic control, avoiding hypoglycemia, allows not only a general reduction in CV risk among patients with T2D, but also a better prognosis during acute coronary events that are frequent in T2D subjects with CKD [Citation33,Citation34].
Hypertension
BP control is essential in preventing and/or slowing the progression of CKD in patients with T2D. A patient’s BP should be monitored at every visit. There is some variation in guideline recommendations for target BP in patients with diabetes and CKD. To reduce rates of microvascular disease, the ADA recommend that in patients with diabetes and hypertension at a lower risk for CV disease (10-year atherosclerotic CV disease [ASCVD] risk <15%) systolic BP should be maintained at <140 mmHg and diastolic BP should be maintained at <90 mmHg [Citation21]. For patients with diabetes and hypertension with higher CV risk (i.e. existing ASCVD or 10-year ASCVD risk ≥15%), lower BP targets (130/80 mm Hg) may be appropriate if they can be achieved without significant treatment burden or adverse effects [Citation21].
Lipid lowering
The onset of kidney disease in people with diabetes is associated with a significant increase in the risk of CV mortality, and as such, aggressive lipid-lowering therapy with statins is warranted in all patients [Citation21,Citation22]. There is ongoing debate as to whether lipid lowering therapy has any direct benefit in retarding progression of CKD in patients with T2D [Citation35], but in many ways this point is moot as all DKD patients should receive lipid-lowering therapy for CV risk reduction given their very high baseline risk [Citation36]. KDOQI guidelines recommend using lipid-lowering medicine, such as statins or statin/ezetimibe combination to reduce the risk of major atherosclerotic events in patient with diabetes and CKD, including patients who have received a kidney transplant [Citation22]. ADA guidelines also recommend statin treatment for patients with diabetes with or without atherosclerotic disease or in patients with risk factors for atherosclerotic disease [Citation21].
Kidney protection
Despite effective treatment of glucose and BP levels, in progression of CKD still occurs in many patients [Citation37]. Agents that may provide additive clinical benefits in slowing CKD progression and reducing the incidence of CVD are important in any effective treatment strategy.
Angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers
Agents that block the renin-angiotensin-aldosterone system (RAAS) pathway (angiotensin-converting enzyme inhibitor [ACEi] and angiotensin II receptor blocker [ARB] drugs) have demonstrated kidney protective effects in people with T2D. Inhibition of the RAAS is a cornerstone in the management of CKD in patients with T2D [Citation38]. In patients with T1DM, landmark studies have clearly demonstrated the beneficial effects of ACEi [Citation39]. Despite being standard of care for several decades, RAAS inhibitors are underutilized [Citation27]. In T2D, the strongest evidence for RAAS inhibition comes from studies of ARBs, in particular in the IDNT trial of irbesartan and in the RENAAL trial of losartan in patients with nephropathy [Citation40,Citation41].
Based on KDIGO guidelines, people with T2D and CKD should be on the maximally tolerated dose of an ACEi or ARB. ACEis and ARBs should not be used in combination due to an association with increased risk of adverse events [Citation42]. If a person does not tolerate an ACEi, or ARB, it is recommended that treatment with the other agent be attempted. The KDOQI guidelines recommend starting ACEi/ARBs at a low dose and titrating to higher doses in patients with an eGFR >45 mL/min/1.73 m2. It is important to monitor a patient’s, eGFR and serum potassium levels within the first few weeks of starting an ACEi/ARB [Citation43].
RAAS drugs reduce the relative risk of kidney events (based on a composite endpoint of doubling of serum creatinine, ESKD, or death) by around 20% in the RENAAL and IDNT studies [Citation40,Citation41]. Moreover, in the RENAAL study, losartan reduced the incidence of doubling of serum creatinine by 25% (p = 0.006) and the risk of ESKD by 28% (p = 0.002) but had no effect on the rate of death [Citation41]. Despite this, a residual risk remains for people with T2D [Citation4].
Aldosterone appears to play a role in the initiation and progression of CKD independent of arterial blood pressure and plasma angiotensin II level. Evidence suggests that continuous ACEi therapy does not necessarily result in sustained reduction of plasma aldosterone levels, which may remain high or even increase during long-term use (aldosterone escape) [Citation44]. Aldosterone escape has been reported in up to 40% of patients with T2D with early nephropathy despite the use of ACEis, and has been associated with elevation in urinary albumin excretion or an enhanced decline in GFR in diabetic kidney disease (DKD) [Citation45].
Sodium-glucose cotransporter-2 inhibitors
The sodium-glucose cotransporter-2 (SGLT-2) typically accounts for around 90% of filtered glucose reabsorption in the proximal tubule, and its inhibitors are used in T2D to treat hyperglycemia by reducing renal glucose reabsorption and increasing urinary glucose excretion [Citation46]. SGLT-2 inhibitors (SGLT-2is) have demonstrated beneficial effects on kidney and CV outcomes. Evidence from large-scale randomized trials support the use of SGLT2is to reduce the overall rate of kidney function decline and adverse kidney events among those with T2D [Citation47–51]. ADA (2021) and KDIGO (2020) guidelines each recommend the use of SGLT2i treatments for people with T2D and eGFR ≥30 mL/min/1.73 m2 where the approved indication allows [Citation13,Citation21]. Recommendations are based on subgroup analyses from SGLT2i CV outcome trials as well as more recent data from the CREDENCE trial (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation), the first study to show efficacy of an SGLT2i agent on a background of maximally tolerated ACEi/ARB doses. CREDENCE reported a relative risk reduction of 34% with canagliflozin versus placebo in the kidney-specific composite endpoint of ESKD, doubling of serum creatinine or death from renal or CV causes in patients with T2D and CKD [Citation48]. Consistent results were also reported in the DAPA-CKD study, which demonstrated the benefit of dapaglifozin versus placebo for improving kidney and CV outcomes in patients with diabetes and extended the findings to those without diabetes [Citation52].
It should be noted that the kidney protective effects of SGLT2i are largely independent of their glycemic effects. They may therefore be used at an eGFR level below which their glycemic effects are apparent, for kidney protection. SGLT2 inhibition results in glycosuria and a reduction in fasting and postprandial plasma glucose levels, but does not stimulate insulin secretion and therefore does not increase the risk of hypoglycemia [Citation53]. Putative non-glycemic mechanisms for kidney protection with SGLT2is include prevention of hemodynamic changes and readjustment of BP control, weight loss, prevention of oxidative damage via amelioration of free radical generation, inhibition of inflammatory responses and fibrotic processes, and reductions in plasma uric acid levels, RAAS activation, and natriuretic peptides [Citation54].
Patients initiating SGLT2i should be educated on potential adverse effects, including polyuria and genital infections, and euglycemic diabetic ketoacidosis (DKA) () [Citation55]. Clinicians should be aware that DKA associated with SGLT2i may present with symptoms of abdominal pain, nausea, vomiting, fatigue, or metabolic acidosis and normal or only modestly elevated blood glucose levels. These medications can have a ‘glucoretic’ effect and therefore attention to volume status is recommended. This could include monitoring blood pressure and volume status. If a person becomes hypotensive, reduction or cessation of diuretic therapy, if in use, may be necessary [Citation55]. While SGLT2is are unlikely to lead to hypoglycemia themselves, when they are added to insulin and sulfonylureas these medications may need to be reduced or stopped if hypoglycemia occurs [Citation55].
Table 2. Summary of risk mitigation strategies for SGLT2is, GLP-1 RAs, and MRAs in DKD
Glucagon-like peptide-1 receptor agonists
Glucagon-like peptide 1 (GLP-1) receptor agonists improve glucose metabolism by increasing glucose-dependent insulin secretion and suppressing the release of glucagon [Citation56]. A series of clinical trials have demonstrated beneficial effects of GLP-1RAs on secondary kidney outcomes in patients with T2D [Citation57–59]. For example, results of the AWARD-7 showed that treatment with the GLP-1 RA dulaglutide was associated with slowed eGFR decline and reduced UACR compared with insulin glargine in patients with T2D and moderate-to-severe CKD [Citation58]. While AWARD-7 indicated a potential slowing of eGFR decline with GLP-1 RA use, the preponderance of evidence to date indicates that the kidney protective effects seen in large GLP-1 RA outcome trials are mainly driven by reductions in albuminuria. These data are mostly originated from secondary outcomes of CV endpoint trials of relatively short duration. These studies have generally reported small differences in glycemic control between intervention and control groups, and it is therefore not obvious whether effects are due to differences in glycemia or whether other mechanisms of action are more important. The phase III FLOW trial (NCT03819153) is investigating the effect of the GLP-1RA, semaglutide, versus placebo on the progression of kidney impairment in patients with T2D and CKD. Primary kidney outcome data are pending.
Patients should be made aware that GLP-1RAs can cause gastrointestinal adverse events, including vomiting, diarrhea, and predominantly nausea, which generally improve over time () [Citation60]. Although GLP-1RA do not cause hypoglycemia when given alone, there is risk of hypoglycemia when a GLP-1RA is added to background insulin or insulin secretagogues [Citation60].
The ADA recommends that, irrespective of baseline A1C or individualized A1C goal, a patient with heart failure or albuminuric CKD should be treated with an SGLT2i with evidence of reducing heart failure and/or CKD progression provided the eGFR is adequate for the use of an SGLT-2i. It should be noted that the lower eGFR threshold for glycemic effects is different from the eGFR threshold for kidney effects. Therefore, while the glucose-lowering effects of SGLT2is are dampened in patients with T2D with eGFR <45 mL/min/1.73 m2, kidney and cardiovascular benefits are observed down to eGFR levels of 30 mL/min/1.73 m2 and below with no significant change in glucose [Citation21]. While SGLT2is are not currently recommended to be initiated if eGFR is 30 mL/min/1.73 m2, KDIGO guidelines state that once an SGLT2i has been started, it is reasonable to continue treatment even if the eGFR falls below 30 mL/min/1.73 m2, unless it is not tolerated or until progression to KRT [Citation13]. If an SGLT-2i is not tolerated or if the eGFR is less than can be used with an SGLT-2i, then a GLP-1 RA should be used [Citation21]. KDIGO guidelines recommend an SGLT2i for treating individuals with T2D, CKD, and an eGFR ≥30 mL/min/ 1.73 m2, and if metformin and SGLT2is are not effective in meeting personal glycemic targets or are unusable due to a contraindication or tolerability issue, a long-acting GP-1RA is recommended [Citation13].
Mineralocorticoid receptor antagonists
Increasing evidence has demonstrated that pathophysiological overactivation of the mineralocorticoid receptor (MR) results in inflammation and fibrosis and is a major driver of progression of CKD and associated morbidity [Citation61]. Blockade of the MR has therefore been investigated as a novel treatment approach to slow progression of CKD [Citation61]. Combination of the steroidal MR antagonists (MRAs) spironolactone or eplerenone with an ACEi/ARB have been shown to provide reductions in BP and in albuminuria in patients with diabetes and kidney disease. However, this combination is associated with an increased incidence of hyperkalemia and acute kidney injury, resulting in high rates of treatment discontinuation, and, for spironolactone, antiandrogenic adverse effects such as gynecomastia [Citation62,Citation63].
A selective non-steroidal MRA has also been shown to reduce albuminuria in clinical trials in patients with CKD in T2D [Citation61]. Finerenone is a novel, nonsteroidal, selective MRA with a high affinity for the MR and a unique binding mode shown to reduce inflammation and fibrosis in animal models [Citation63–65], and reduce albuminuria in short-term trials involving patients with CKD and T2D while being associated with lower rates of hyperkalemia reported with other MRAs [Citation61,Citation66]. In the ARTS study, treatment with finerenone resulted in a significantly smaller increase in serum potassium than spironolactone but was at least as effective in reducing albuminuria in patients with stable chronic heart failure and mild or moderate CKD [Citation67]. In addition, the incidence of adverse events related to worsening of renal function was lower with finerenone than with spironolactone. Finerenone has a shorter half-life (2–3 hours in patients with kidney failure) than spironolactone or eplerenone and has no active metabolites [Citation68]. In contrast, spironolactone is a prodrug with biologically active metabolites with long half-lives that can accumulate over time. Eplerenone has a half-life of 4–6 hours [Citation68].
The combined used of SGLT2is and finerenone is supported by the distinct but complementary mechanisms of action of these agents [Citation69]. More trials should be conducted to further assess the effects of these agents in combination for the treatment of DKD [Citation69].
In the Phase 3 FIDELIO-DKD study, finerenone significantly reduced risk of the composite kidney endpoint of time to kidney failure, a sustained decrease in eGFR of 40% or more from baseline, or renal death in patients with CKD and T2D [Citation70]. The effects of finerenone on the primary endpoint were generally consistent among prespecified subgroups, regardless of CKD severity, baseline BP control, or baseline blood glucose control. Finerenone also significantly reduced the risk of the composite CV endpoint of time to CV death, non-fatal myocardial infarction or hospitalization for heart failure, compared with placebo, and this effect was seen as early as 1 month into the trial and persisted throughout the study. More recently, results from the FIGARO-DKD study demonstrate that the addition of finerenone to standard medical therapy significantly reduced the risk of the composite of death from CV causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure compared with placebo in patients with CKD and T2D [Citation71]; the difference was predominantly due to the lower rate of heart failure hospitalizations with finerenone.
In both FIDELIO and FIGARO, finerenone treatment was associated with a higher mean serum potassium level than placebo; a maximal difference was observed at month 4 in FIDELIO and month 1 in FIGARO and the difference remained largely stable thereafter. Permanent discontinuation of finerenone was low in both studies (FIDELIO, 2.3%; FIGARO, 1.2%).
A second nonsteroidal MRA, esaxerenone (currently only available in Japan for the treatment of hypertension), also reduces albuminuria in patients with CKD and T2D. However, trials of esaxerenone report higher rates of hyperkalemia than those examining finerenone, and the effects of esaxerenone on mortality and ESKD are unknown [Citation72].
Optimal treatment recommendations
In the management of patients with T2D, it is essential to screen eGFR and UACR at least annually per ADA and KDIGO guidelines () [Citation13,Citation21]. If eGFR and/or UACR is abnormal, recheck within 3 months to confirm a diagnosis of DKD and to address albuminuria; at this stage, nephrology referral can be considered based on patient-specific characteristics and laboratory findings. For people with normal to mildly increased albuminuria (UACR <30 mg/g and eGFR >60 mL/min/1.73 m2), no immediate treatment to reduce CKD progression is required, but such individuals should be rescreened within a year, and guideline-directed therapy should be optimized to prevent micro and macrovascular complications, including glucose, blood pressure, and cholesterol management in addition to smoking cessation when applicable. People with moderately increased albuminuria (UACR 30–300 mg/g) and severely increased albuminuria (UACR >300 mg/g) should be started on an ACEi or an ARB and titrated as tolerated to the recommended renal dose.
Figure 4. Optimal treatment recommendations for CKD associated with T2D. a Referring clinicians may wish to discuss with their nephrology service depending on local arrangements regarding monitoring or referring. KDIGO guidelines provide direction on when to consider referral to a nephrologist based on eGFR and UACR levels (); b For people with normal to mildly increased albuminuria (UACR <30 mg/g and eGFR >60 mL/min/1.73 m2), no immediate treatment to reduce CKD progression is required, but such individuals should be rescreened within a year, and guideline-directed therapy should be optimized to prevent micro and macrovascular complications. Refer to ADA guidelines to improve glycemic control and lipid levels, maintain healthy body weight, and optimize blood pressure; c ACEi or ARB therapy should be maintained even if eGFR is reduced.
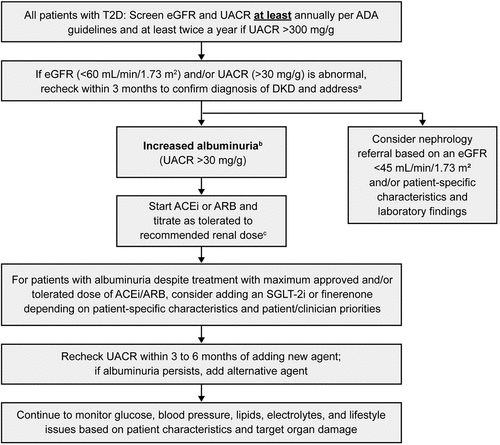
For people whose albuminuria persists despite treatment with maximum approved and/or tolerated dose of ACEi or ARB, the addition of an SGLT-2 inhibitor or finerenone should be considered, with agent selection guided by patient-specific characteristics and patient/clinician priorities. UACR should be rechecked within 3 to 6 months of starting either agent; and if baseline UACR >300 mg/g and albuminuria persists then addition of the alternative agent (either SGLT-2 inhibitor or finerenone depending on which agent the patient is already receiving, and provided there is no contraindication to therapy) should be considered. Individuals should be continued to be monitored for glucose, blood pressure, lipids, electrolytes, and lifestyle issues based on patient characteristics and target organ damage.
Referral criteria
The core diabetes team should ideally consist of at least a primary care provider (PCP), a pharmacist, a patient educator, and a nutrition expert. However, given the complexity of the disease and the risk of poor outcomes, nephrology specialists can be of particular assistance when treating patients at different stages of CKD.
Early referral to a nephrologist can help determine the etiology of CKD, recommend specific disease-related therapy, suggest treatments to slow progression of disease in patients non-responsive to conventional therapies, recognize and treat for disease-related complications, and plan for KRT. In fact, appropriate referral to a nephrologist for specialist assessment is specifically recommended in guidelines as it has been shown to improve quality of care, delay dialysis, and decrease costs [Citation12,Citation21,Citation73,Citation74]. Co-management of patients with T2D and CKD by their PCP and a nephrologist have also been associated with significantly increased rates of eGFR testing and prescription of an ACEi/ARB [Citation75]. Research shows that late referral to nephrology is associated with significantly higher rates of mortality within the first 90 days of dialysis [Citation73].
Referral to nephrology is based on KDIGO guidelines and is generally indicated when the eGFR is <30 mL/min/1.73 m2 or when there is a rapid decline of eGFR, severely increased UACR (>300 mg/g) or other ‘alert’ signs such as the presence of urinary red blood cell casts () [Citation12]. Despite this, the complex nature of CKD in patients with diabetes means that referral guidelines cannot account for all clinical scenarios, and so individualization and common sense need also to be applied.
Figure 5. KDIGO referral decision making by eGFR and albuminuria [Citation12]. *Referring clinicians may wish to discuss with their nephrology service depending on local arrangements regarding monitoring or referring.
![Figure 5. KDIGO referral decision making by eGFR and albuminuria [Citation12]. *Referring clinicians may wish to discuss with their nephrology service depending on local arrangements regarding monitoring or referring.](/cms/asset/1560a61f-a3c3-40a1-97de-0cd4fd0ddb6b/ipgm_a_2009726_f0005_c.jpg)
Criteria for referral to nephrology need to be easy to follow to facilitate the clinician’s decision-making task () [Citation12]. It has been suggested that tools such as smartphone apps could complement the referral criteria and reduce the complexity for more accurate recommendations, making assessment more straightforward for non-nephrologist clinicians, and minimizing delayed referrals to nephrology [Citation76]. Such criteria can assist clinicians in determining when a preemptive referral is required to prevent advanced CKD stages and ESKD in their patients. Moreover, because patients with CKD can be at high risk for adverse CV outcomes, referral to cardiology should also be considered.
Table 3. Current KDIGO criteria of nephrology referral in CKD [Citation12]
Multi-disciplinary approach
It is important to recognize that patients with T2D and CKD are at high risk for other comorbidities, complications and excessive healthcare costs [Citation1,Citation77]. Prevention of development and progression of CKD is paramount as DKD can have a significant impact on a person’s quality of life [Citation78]. In view of the predicted global increase in the prevalence of CKD in patients with T2D [Citation78], healthcare systems are faced with major financial consequences that call for novel management strategies for slowing the progression of disease, enhancing self-care activities, and improving patients’ health-related outcomes.
As the treatment landscape has evolved, so have the requirements to manage this complex multisystem disease by multiple healthcare providers in both primary care and specialist settings. Systematic reviews of the literature and meta-analyses in other chronic diseases such as heart failure and diabetes, in general, support the effectiveness of multidisciplinary management [Citation79–81]. It is important that clinicians working across primary care, diabetes services, and kidney and cardiovascular services act as a team to ensure that the diagnosis and ongoing management of CKD in patients with T2D are prioritized and that colleagues are appropriately supported to implement this work. Many have advocated for comprehensive multidisciplinary care that would optimize the care of patients with CKD and T2D via structured care plans developed in collaboration by more than one healthcare discipline, including primary care physicians, nephrologists, cardiologists, nurses, pharmacists, dietitians, or health educators [Citation82,Citation83]. Such a multidisciplinary disease management strategy can contribute to preserving kidney function and delaying disease progression [Citation82]. The NKF recently held a scientific workshop to discuss optimizing use of evidence-based therapies to improve outcomes in patients in with T2D and CKD. Participants of the workshop concluded that dedicated effort is needed to provide information and around use of existing and emerging therapies for CKD in T2D, and that clinical pharmacists as members of a multidisciplinary care team are well placed to help close critical gaps in CKD care by ensuring adequate patient follow-up and continuity of care [Citation83]. Physicians and HCPs involved in the care of patients with CKD and T2D face several challenges in the future including the management of other comorbidities, the implementation of multi-treatment options, and individualized care. This evolving treatment landscape means that the place of multidisciplinary teams will continue to be pivotal to the success of any future management approaches.
Patient education
Patient involvement is critical to effective management of CKD in patients with T2D and minimizing complications and should not be overlooked. It is imperative that people with diabetes receive education to raise awareness of their condition and the importance of disease management, with the aim in helping them understand that the course of CKD can be changed with early intervention so that it becomes an enduring but non-lethal condition that most people die with and not directly from.
A significant proportion of diabetes management, such as lifestyle modifications and treatment adherence, is the responsibility of the patient, and physicians have limited control over how patients manage their condition outside of visits. Patients with CKD may be responsible for taking complex medication regimens, monitoring blood glucose and BP, and adhering to altered lifestyle regimes, such as undertaking physical activity and dietary requirements [Citation84]. For this reason, it is important that physicians consider the numerous variables that are outside their influence but impact disease management and educate patients accordingly to empower them to take an active role in their care.
Education programs appear to have beneficial effects on improving patients’ knowledge of diabetes and some self-management behavioral changes for patients with diabetes on dialysis or with microalbuminuria. For example, in contrast to those lacking knowledge, patients aware of BP goals have demonstrated improved BP [Citation84]. Practical tools and materials are available from local and national organizations to help support these initiatives.
The KDIGO guidelines recommend that a structured self-management educational program be implemented in the care of people with diabetes and CKD, particularly when implemented as part of multidisciplinary integrated care. Specifically, the guidelines note that special education and counseling should be undertaken for nutrition, weight reduction, foot care, and stress management. The key objectives of an effective self-management education program are to improve disease-related knowledge and skills, self-management and self-motivation, emotional and mental well-being, treatment satisfaction and quality of life, and vascular risk factors. Additional aims are to encourage the adoption and maintenance of a healthy lifestyle, to increase engagement with mediation, glucose monitoring, and complication screening programs, and to reduce the risk of complications [Citation13].
Research from focus groups has shown that patients considered the complexity of diabetes care was too great for a single provider and were more open to a multidisciplinary approach [Citation85]. When asked, patients identified key barriers in the current care model as the lack of physician time to attend to complex management, and the lack of co-ordination among many healthcare professionals. Other studies have also reported that patients with diabetes can be overwhelmed by referral to many health professionals with a perception of fragmentation of care, particularly when those providers were not co-located [Citation86].
To improve management strategies for CKD in patients with T2D, it is essential to consider the patient perspective, and ensure that implementation of a care plan incorporates lessons learned from patients. Active patient engagement that emphasizes the importance of early treatment of CKD rather than at the latter stages of disease and KRT and involves shared decision making are critical for improved outcomes.
Conclusions
The increasing number of people living with T2D and CKD will have a substantial impact on health resource use and costs of care in the future. Although our understanding of this disease continues to improve and new treatments to prevent progression of CKD in patients with T2D are emerging, physicians still face several challenges. These include, but are not limited to, the management of comorbidities, implementation of new treatment options, and adoption of individualized care. Reaching a consensus for the optimal treatment of this disease is vital in providing consistent and appropriate care for all patients. Practical approaches that prevent or delay the progression of this serious and costly condition are urgently required. Strategies to improve care should also include use of clear screening and detection criteria, management and treatment approaches, referral criteria, use of a multi-disciplinary approach, and patient education.
Transparency statements
Author contributions
All authors participated in the selection and interpretation of the articles and in the drafting, critical revision, and approval of the final version of the manuscript.
Disclosure of financial/other conflicts of interest
Bayer Corporation was involved in the development of the concept for this manuscript but had no role in the selection or interpretation of articles to be included or preparation of the manuscript.
Jay H Shubrook has served on an advisory board for Astra Zeneca, Bayer, and Novo Nordisk. Joshua J. Neumiller serves on the Speaker’s Bureau for Dexcom and as an Advisory Board member for Sanofi. Eugene E. Wright has served on Advisory Boards and Speakers Bureaus for About Diabetes Care, Bayer, Boehringer Ingelheim, Eli Lilly and Sanofi.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Medical writing assistance was provided by John Bilbruck PhD, of Envision Pharma Group, and was funded by Bayer. Envision Pharma Group’s services complied with international guidelines for Good Publication Practice (GPP3).
Additional information
Funding
References
- Rangaswami J , Bhalla V , de Boer IH , et al. Cardiorenal protection with the newer antidiabetic agents in patients with diabetes and chronic kidney disease: a scientific statement from the american heart association. Circulation. 2020;142(17):e265–e86.
- Go AS. Cardiovascular disease consequences of CKD. Semin Nephrol. 2016;36(4):293–304.
- Gheith O , Farouk N , Nampoory N , et al. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2016;5(1):49–56.
- Wheeler DC , James J , Patel D , et al. SGLT2 inhibitors: slowing of chronic kidney disease progression in type 2 diabetes. Diabetes Ther. 2020;11(12):2757–2774.
- Assogba FG , Couchoud C , Hannedouche T , et al. Trends in the epidemiology and care of diabetes mellitus-related end-stage renal disease in France, 2007-2011. Diabetologia. 2014;57(4):718–728.
- Wu B , Bell K , Stanford A , et al. Understanding CKD among patients with T2DM: prevalence, temporal trends, and treatment patterns-NHANES 2007-2012. BMJ Open Diabetes Res Care. 2016;4(1):e000154.
- Kainz A , Hronsky M , Stel VS , et al. Prediction of prevalence of chronic kidney disease in diabetic patients in countries of the European Union up to 2025. Nephrol Dial Transplant. 2015;30(Suppl 4):iv113–8.
- Neumiller JJ , Hirsch IB . Management of hyperglycemia in diabetic kidney disease. Diabetes Spectr. 2015;28(3):214–219.
- Aggarwal HK , Jain D , Pawar S , et al. Health-related quality of life in different stages of chronic kidney disease. QJM. 2016;109(11):711–716.
- Gaede J , Oellgaard J , Ibsen R , et al. A cost analysis of intensified vs conventional multifactorial therapy in individuals with type 2 diabetes: a post hoc analysis of the Steno-2 study. Diabetologia. 2019;62(1):147–155.
- Tuttle KR , Bakris GL , Bilous RW , et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37(10):2864–2883.
- Kidney Disease Improving Global Outcomes . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–163.
- Kidney Disease Improving Global Outcomes . KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int Suppl. 2020;98(45):1–120.
- Fung CS , Wan EY , Chan AK , et al. Association of estimated glomerular filtration rate and urine albumin-to-creatinine ratio with incidence of cardiovascular diseases and mortality in Chinese patients with type 2 diabetes mellitus - a population-based retrospective cohort study. BMC Nephrol. 2017;18(1):47.
- Levey AS , Becker C , Inker LA . Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313(8):837–846.
- Christofides EA , Desai N . Optimal early diagnosis and monitoring of diabetic kidney disease in type 2 diabetes mellitus: addressing the barriers to albuminuria testing. J Prim Care Community Health. 2021;12:21501327211003683.
- Chu CD , McCulloch CE , Banerjee T , et al. CKD awareness among us adults by future risk of kidney failure. Am J Kidney Dis. 2020;76(2):174–183.
- Stehouwer CD , Smulders YM . Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol. 2006;17(8):2106–2111.
- Chronic Kidney Disease Prognosis C , Matsushita K , van der Velde M , Astor BC , et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081.
- Lamprea-Montealegre JA , Shlipak MG , Estrella MM . Chronic kidney disease detection, staging and treatment in cardiovascular disease prevention. Heart. 2021;107(16):1282–1288.
- American Diabetes Association . Standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S1–S232.
- National Kidney Foundation . KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850–886.
- Levey AS , Stevens LA , Schmid CH , et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612.
- Delgado C , Baweja M , Crews D , et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol. 2021;32:1305–1317.
- National Kidney Foundation . KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49:S1–S180.
- Szczech LA , Stewart RC , Su HL , et al. Primary care detection of chronic kidney disease in adults with type-2 diabetes: the ADD-CKD Study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease). PLoS One. 2014;9(11):e110535.
- Tuttle KR , Alicic RZ , Duru OK , et al. Clinical characteristics of and risk factors for chronic kidney disease among adults and children: an analysis of the CURE-CKD registry. JAMA Network Open. 2019;2(12):e1918169.
- Sasso FC , Pafundi PC , Simeon V , et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: a randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc Diabetol. 2021;20(1):145.
- Yamada S . First, do no harm: critical appraisal of protein restriction for diabetic kidney disease. Diabetology. 2021;2:51–64.
- Marin-Penalver JJ , Martin-Timon I , Sevillano-Collantes C , et al. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. 2016;7(17):354–395.
- Galindo RJ , Beck RW , Scioscia MF , et al. Glycemic monitoring and management in advanced chronic kidney disease. Endocr Rev. 2020;41(5):756–774.
- Wang F , Wang D , Lyu XL , et al. Continuous glucose monitoring in diabetes patients with chronic kidney disease on dialysis: a meta-analysis. Minerva Endocrinol. 2020. DOI:10.23736/S0391-1977.20.03284-8
- Marfella R , Sasso FC , Cacciapuoti F , et al. Tight glycemic control may increase regenerative potential of myocardium during acute infarction. J Clin Endocrinol Metab. 2012;97(3):933–942.
- Sasso FC , Rinaldi L , Lascar N , et al. Role of tight glycemic control during acute coronary syndrome on CV outcome in type 2 diabetes. J Diabetes Res. 2018;2018:3106056.
- Esmeijer K , Dekkers OM , de Fijter JW , et al. Effect of different types of statins on kidney function decline and proteinuria: a network meta-analysis. Sci Rep. 2019;9(1):16632.
- Visseren FLJ , Mach F , Smulders YM , et al. ESC guidelines on cardiovascular disease prevention in clinical practice. Eur J Prev Cardiol. 2021. DOI:10.1093/eurjpc/zwab154.
- Hahr AJ , Molitch ME . Management of diabetes mellitus in patients with chronic kidney disease. Clin Diabetes Endocrinol. 2015;1:2.
- Lozano-Maneiro L , Puente-Garcia A . Renin-angiotensin-aldosterone system blockade in diabetic nephropathy. Present evidences. J Clin Med. 2015;4(11):1908–1937.
- Lewis EJ , Hunsicker LG , Bain RP , et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The collaborative study group. N Engl J Med. 1993;329(20):1456–1462.
- Lewis EJ , Hunsicker LG , Clarke WR , et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860.
- Brenner BM , Cooper ME , de Zeeuw D , et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869.
- Kidney Disease Improving Global Outcomes . KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2021;99(35):1–92.
- Vassalotti JA , Centor R , Turner BJ , et al. Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. 2016;129(2):153–62e7.
- Schjoedt KJ , Andersen S , Rossing P , et al. Aldosterone escape during blockade of the renin-angiotensin-aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia. 2004;47(11):1936–1939.
- Sato A , Hayashi K , Naruse M , et al. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2003;41(1):64–68.
- Bays H . Sodium glucose co-transporter type 2 (SGLT2) inhibitors: targeting the kidney to improve glycemic control in diabetes mellitus. Diabetes Ther. 2013;4(2):195–220.
- Neal B , Perkovic V , Mahaffey KW , et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657.
- Perkovic V , Jardine MJ , Neal B , et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306.
- Wiviott SD , Raz I , Bonaca MP , et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357.
- Zinman B , Wanner C , Lachin JM , et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128.
- Heerspink HJL , Karasik A , Thuresson M , et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8(1):27–35.
- Heerspink HJL , Stefansson BV , Correa-Rotter R , et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446.
- Fioretto P , Zambon A , Rossato M , et al. SGLT2 inhibitors and the diabetic kidney. Diabetes Care. 2016;39(Suppl 2):S165–71.
- Yaribeygi H , Simental-Mendía LE , Banach M . The major molecular mechanisms mediating the renoprotective effects of SGLT2 inhibitors: an update. Biomed Pharmacother. 2019;120:109526.
- Zoungas S , de Boer IH . SGLT2 Inhibitors in Diabetic Kidney Disease. Clin J Am Soc Nephrol. 2021;16(4):631–633.
- Tran KL , Park YI , Pandya S , et al. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. Am Health Drug Benefits. 2017;10(4):178–188.
- Mann JFE , Orsted DD , Brown-Frandsen K , et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–848.
- Tuttle KR , Lakshmanan MC , Rayner B , et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–617.
- Gerstein HC , Colhoun HM , Dagenais GR , et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131–138.
- Michos ED , Tuttle KRGLP-1 . Receptor agonists in diabetic kidney disease. Clin J Am Soc Nephrol. 2021;16:1578–1580.
- Georgianos PIA R . Mineralocorticoid receptor antagonism in chronic kidney disease. Kidney International Reports 2021; In press.
- Currie G , Taylor AH , Fujita T , et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol. 2016;17(1):127.
- Haynes BA , Mookadam F , Currie G . Male gynecomastia. Mayo Clin Proc. 2009;84(8):672.
- Kolkhof P , Delbeck M , Kretschmer A , et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69–78.
- Barrera-Chimal J , Estrela GR , Lechner SM , et al. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int. 2018;93(6):1344–1355.
- Bakris GL , Agarwal R , Chan JC , et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884–894.
- Pitt B , Kober L , Ponikowski P , et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34(31):2453–2463.
- Agarwal R , Kolkhof P , Bakris G , et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42(2):152–161.
- Sridhar VS , Liu H , Cherney DZI . Finerenone-A new frontier in renin-angiotensin-aldosterone system inhibition in diabetic kidney disease. Am J Kidney Dis. 2021;78(2):309–311.
- Bakris GL , Agarwal R , Anker SD , et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–2229.
- Pitt B , Filippatos G , Agarwal R , et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021. DOI:10.1056/NEJMoa2110956.
- Ito S , Shikata K , Nangaku M , et al. Efficacy and safety of esaxerenone (CS-3150) for the Treatment of Type 2 Diabetes with microalbuminuria: a randomized, double-blind, placebo-controlled, Phase II trial. Clin J Am Soc Nephrol. 2019;14(8):1161–1172.
- Smart NA , Dieberg G , Ladhani M , et al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev. 2014;6:CD007333. DOI:10.1002/14651858.CD007333.pub2
- Jan MY , Wish JB . Balancing nephrology referrals with nephrologist capacity to decrease emergency dialysis starts. Kidney Int Rep. 2021;6(1):7–10.
- Samal L , Wright A , Waikar SS , et al. Nephrology co-management versus primary care solo management for early chronic kidney disease: a retrospective cross-sectional analysis. BMC Nephrol. 2015;16:162.
- Oliva-Damaso N , Oliva-Damaso E , Rodriguez-Perez JC , et al. Improved nephrology referral of chronic kidney disease patients: potential role of smartphone apps. Clin Kidney J. 2019;12(6):767–770.
- Kovesdy CP , Isaman D , Petruski-Ivleva N , et al. Chronic kidney disease progression among patients with type 2 diabetes identified in US administrative claims: a population cohort study. Clin Kidney J. 2021;14(6):1657–1664.
- Alicic RZ , Rooney MT , Tuttle KR . Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045.
- Musuuza J , Sutherland BL , Kurter S , et al. A systematic review of multidisciplinary teams to reduce major amputations for patients with diabetic foot ulcers. J Vasc Surg. 2020;71(4):1433–46e3.
- Bongaerts BW , Mussig K , Wens J , et al. Effectiveness of chronic care models for the management of type 2 diabetes mellitus in Europe: a systematic review and meta-analysis. BMJ Open. 2017;7(3):e013076.
- Holland R , Battersby J , Harvey I , et al. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005;91(7):899–906.
- Jain K , Mottl AK . Comprehensive care for people with diabetic kidney disease. Diabetes Spectr. 2015;28(3):187–192.
- Neumiller JJ , Shubrook JH , Manley T , et al. Optimizing use of SGLT2 inhibitors and other evidence-based therapies to improve outcomes in patients with type 2 diabetes and chronic kidney disease: an opportunity for pharmacists. Am J Health Syst Pharm. 2021. DOI:10.1093/ajhp/zxab271
- Narva AS , Norton JM , Boulware LE . Educating patients about CKD: the path to self-management and patient-centered care. Clin J Am Soc Nephrol. 2016;11(4):694–703.
- Berkowitz SA , Eisenstat SA , Barnard LS , et al. Multidisciplinary coordinated care for type 2 diabetes: a qualitative analysis of patient perspectives. Primary Care Diabetes. 2018;12(3):218–223.
- Maneze D , Dennis S , Chen HY , et al. Multidisciplinary care: experience of patients with complex needs. Aust J Prim Health. 2014;20(1):20–26.