?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Introduction
This study aimed to quantify patients’ preferences for benefits and risks associated with treating degenerative mitral regurgitation (DMR) via open heart surgical repair versus a beating heart surgical approach.
Methods
A D-efficient main effects discrete choice experiment (DCE) survey with 10 choice tasks that involved trade-offs across six attributes varying between two and four levels each (procedure invasiveness, recovery intensity, risk of disabling stroke, risk of new onset atrial fibrillation, risk of symptom reappearance and risk of reintervention) was administered online to either clinically confirmed (n = 30) or self-reported DMR (n = 88) patients recruited from either cardiovascular clinics or online clinical patient databases. The error component logit (ECL) analysis combined both patient cohorts after performing a Swait-Louviere scale test. Patient trade-offs across attributes were estimated in relation to either an open-heart surgery (OHS) treatment profile or a beating heart approach.
Results
Patients demonstrated clear preferences across all attributes for the beating heart treatment. 76.0% (95% CI: 68.1,83.9) of patients would prefer a ‘beating heart’ intervention relative to the ‘open heart’ approach despite the higher likelihood of symptom recurrence and reintervention. In exchange for the combined net benefits associated with a ‘beating heart’ treatment, on average, participants were willing to accept a maximum acceptable risk (MAR) of 34.6 percentage points (95% CI: 23.8,45.4) for increased risk of symptom reappearance or 22.6 percentage points (95% CI: 14.7,30.4) increased risk of reintervention.
Conclusion
This study of US adults with DMR provides quantitative measures of risk tolerance for tradeoffs related to repair by a beating heart approach relative to conventional open-heart surgery (standard of care). These results may inform DMR treatment choices from regulatory agencies, payers, clinicians, and patients considering a beating heart repair or treatments with similar attributes as potential new alternatives to conventional surgery.
Introduction
Chronic mitral regurgitation (MR) occurs when the mitral leaflets, ventricle or atrium are impaired such that blood leaks back from the atrium to the ventricle. Degenerative (or primary) MR (or DMR) stems from disease of the mitral apparatus while functional (or secondary) MR (or FMR) results from a diseased ventricle or atrium[Citation1]. The potential consequences of MR can be severe and include pulmonary edema, hypotension, cardiogenic shock, myocardial damage, heart failure and death[Citation1]. Moderate to severe chronic MR affects more than 2 million American adults and is the most common valve disease observed in the United States[Citation2]. Despite its prevalence, MR can be asymptomatic and is frequently undertreated [Citation3,Citation4].
When repair (rather than replacement) is indicated for treatment of DMR, a frequently used approach is open-heart surgery involving a large incision, cardiopulmonary bypass, a lengthy procedure time, post-operative pain, an extended hospital stay, and a prolonged recouperation period prior to a return to normal activity[Citation5]. The HARPOON® beating heart mitral valve repair system (HARPOON® System; Edwards Lifesciences, Irvine, Ca, USA) was developed to enable surgeons to anchor expanded polytetrafluoroethylene (ePTFE) cords with a transventricular approach on prolapsed mitral valve leaflets on the beating heart under transesophageal echocardiographic image guidance. It currently represents the least invasive surgical approach to mitral valve (MV) repair indicated for DMR resulting from posterior leaflet prolapse, and is associated with potentially faster/easier recovery and fewer adverse events (e.g. stroke or new onset atrial fibrillation), but potentially higher rates of DMR recurrence and reintervention [Citation6,Citation7]. Therefore, it is important to understand patients’ perspectives on the tradeoff between a beating heart surgical repair option that may not permanently repair their DMR versus an option with greater certainty of more permanent repair requiring an open-heart procedure.
The application and science related to patient preferences in medical decision-making and clinical trial design has grown recently, in part as a function of increased interest from the US Food and Drug Administration (FDA), patient advocacy organizations and research institutes [Citation8–10]. Assessments are being conducted across the medical product life cycle from ‘bench to bedside’ with applicability from discovery, pre-clinical studies, design of clinical trials, product approval/marketing authorization to post-marketing studies [Citation9]. Depending on the application, a wide range of preference elicitation methods and model types (especially statistical models which incorporate choice preferences) can conform to the objective of particular studies [Citation11,Citation12]. With respect to understanding patient risk/benefit valuation, the discrete choice experiment (DCE) is a well-accepted, frequently-adopted technique used across a variety of disciplines such as economics, sociology and political science [Citation13–15]. Patient risk/benefit preferences based on DCEs have previously been incorporated to inform potential patient-relevant clinical trial endpoints – one of the motivations for conducting this study [Citation16,Citation17]. As a result, after consultation with and input from FDA, the study sponsors concluded that the DCE method would be most appropriate for this purpose.
The objective of this study was to identify patients’ preferences for different treatment options to address DMR. We implement a discrete choice experiment (DCE) on a subset of DMR patients (including medically verified or self-reported DMR based on the patients’ understanding of their diagnosis) to determine how they trade off costs and benefits of key attributes associated with treatment of DMR. Specifically, we focus on those attributes associated with standard open-heart procedures and the beating heart option. The DCE approach yields quantitative estimates (regression coefficients) reflective of the utility associated with different treatment attributes. This allows for a measure of the ‘relative attribute importance’ (RAI) between an open-heart procedure and a beating heart procedure. The study also estimates the benefit/risk tolerance thresholds or the ‘maximum acceptable risk’ (MAR) of lower efficacy or durability in exchange for fewer complications and avoidance of an open-heart procedure. Finally, the study establishes a predicted probability that DMR patients will opt for a beating heart treatment versus open heart surgery.
Methods
Given the importance of survey development and participant selection in DCEs, this cross-sectional survey study consisted of three stages: 1) survey design, 2) patient recruitment and data collection, 3) analytic modeling and data analysis. Details for each of the stages are described below.
Stage I: Survey Design
Attributes that characterize MR treatments were initially based on a targeted review of clinical trials, FDA documents related to MR repair procedures, input from patients and expert opinion eliciting key features that differentiate open-heart and beating heart DMR repair. These attribute descriptions were reviewed with patients (n = 19) and cardiothoracic surgeons (n = 5) to assess relevance, accuracy and clarity.
A draft survey was developed and pre-tested by DMR patients (n = 10) and adjusted to reflect their input (Appendix ). Based on this analysis, the final DCE survey included six attributes with two to four levels each: procedure invasiveness, procedure recovery, risk that DMR symptoms reappear, risk of needing a reintervention, risk of disabling stroke and risk of new onset atrial fibrillation (, Full Descriptions Appendix ). The research was approved by the Western Institutional Review Board (IRB) as it was determined the study was exempt from human subject review.
Table 1. Final DCE Attributes and Levels
A D-efficient main effects experimental design with 10 choice tasks determined how the attribute levels were varied across treatment profiles. Participants also completed a dominance task and a holdout taskFootnote1 [Citation18,Citation19]. The survey also included three evaluation questions to assess participants’ ease with understanding, answering, and truth of answering[Citation20]. Further details on DCE development are provided in the supplement (Section 2: DCE Development Description and Appendix ).
Treatment Profiles of Interest
In order to estimate preferences corresponding to a choice between a beating heart procedure and the comparator of open-heart surgical repair, treatment profiles with specific attributes and levels were defined to approximate the beating heart procedure (the beating heart procedure-like profile) and an open-heart surgery procedure (the open-heart surgery-like profile) ().
Table 2. Treatment Profiles
Stage II: Participant Recruitment and Data Collection
After Western Institutional Review Board (IRB) determined that the study was exempt from human subject review, two cohorts of participants were recruited in the US. The first cohort included patients who had a physician-confirmed clinical diagnosis of severe DMR and were recruited via cardiovascular clinics. The second cohort of patients had self-reported moderate to severe DMR diagnosis and were recruited via e-mail invitation across multiple patient databases or social media platforms with help from patient advocacy organizations (See Supplement section 3: ‘Recruitment Details and Participant Screeners’ for additional information). Inclusion and exclusion criteria are summarized in Appendix . In the case of the clinically diagnosed cohort, the cause for MR had to be degenerative/primary valve disease and in both cohorts non-severe DMR diagnosis and those with MR due to functional/secondary valve disease were excluded. Several symptom-based screening questions, designed by expert cardiovascular surgeons, were asked to help increase confidence in the likelihood that clinically un-confirmed (self-reported) patients experienced severe DMR (treated or untreated).
Remuneration for participation was $25 and patients were free to withdraw at any time. In addition to the DCE section, the online survey (a cross-sectional representation of patients’ status and preferences) included sections on A) disease history (clinical characteristics and physical functioning) B) treatment history for MR (e.g. prior mitral procedure) and C) sociodemographic characteristics (age, gender, working status, etc.). Surveys were fielded between October 2019 and January 2020. Informed consent documents were imbedded in the survey link for participants to print and sign electronically.
Demographic and clinical history data were summarized using descriptive statistics, presenting the mean, standard deviation (SD), median, interquartile range (IQR), minimum (min) and maximum (max) for continuous variables, and frequencies and percentages for categorical variables (Appendix ). The diagnosis cohorts were compared using appropriate statistical tests, i.e. t-test (or Mann-Whitney-Wilcoxon test if warranted e.g. due to extreme influential outliers) for continuous variables and Chi-squared test (or Fisher’s exact test, if one or more cells had expected counts less than 5) for categorical variables. Given the separate cohorts, the two-step Swait-Louviere scale test procedure assessed whether the diagnosis cohorts could be pooled for DCE analysisFootnote2[Citation21].
Stage III: Analytic Modeling and Data Analysis
The DCE analyses rely on a random utility model (Eq. 1) in which the utility (U) a participant obtains from choosing a treatment (i) depends on the utility the participant obtains from the characteristics/attributes of this treatment (X) and a random error (ε). In our study the treatment vector is comprised of procedure invasiveness, procedure recovery, risk that symptoms reappear, risk of reintervention, risk of disabling stroke, and risk of new onset atrial fibrillation (X).
The preference estimate, , represents utility the participant gets from the treatment attributes. The error term, εi, is assumed to follow an independently and identically distributed type 1 extreme-value distribution. Given this assumption (logit model), the probability of choosing alternative i relative to j total alternatives is:
Our primary specification for analysis was an error component logit model (ECL) in which only the error component varies randomly. Specifically, the error component was defined as an alternative-specific intercept (constant), i.e. a dummy variable for one of the two treatment options, with a random normal distribution around a fixed mean of 0 (therefore producing an estimate only for the SD of this error component, not the mean).Footnote3 Only responses to the 10 experimental design choice tasks (so not to the dominanAppendixce or holdout task) were included in the analytic model.
Linearity for Numerical Attributes
Based on visual assessment, it was determined that a linear assumption was reasonable for preferences derived for risk that symptoms reappear and risk of reintervention; therefore, these risk attributes were treated as linear continuous variables in the final analytic models. Procedure invasiveness, recovery, risk of disabling stroke and risk of new onset atrial fibrillation were specified as categorical variables.
Relative Attribute Importance
Relative attribute importance (RAI) percentages were used to rank attributes. The RAI and ranking data reflect how patients prioritize the attributes. Attributes with a higher ranking and a greater relative importance are more important in the decision-making process, on average.
The (average) relative importance of each attribute RAI was calculated with respect to a choice/comparison between the ‘beating heart’ base case treatment profile and the ‘open-heart surgery-like’ profile by determining the absolute (positive) value difference between the ECL model coefficients corresponding to the ‘beating heart’ and ‘open-heart surgery-like’ profile levels for each attribute. For attributes that were coded as continuous, RAI was calculated by taking the absolute value of the coefficient for a 1-unit change in the attribute and multiplying it by the unit change between the ‘beating heart’ and ‘open-heart surgery-like’ profile levels for that attribute. For example, for risk of symptom recurrence which was defined as 10% for ‘beating heart’ and 1% for ‘open-heart surgery-like’ profiles (a 9-percentage point difference), RAI was calculated by multiplying the coefficient of the continuously specified symptom recurrence attribute by 9. A 95% confidence interval for each RAI percentage was estimated via the delta method.
Tradeoffs: Marginal Rate of Substitution and Maximum Acceptable Risk
Marginal rate of substitution (MRS) refers to the rates at which participants would be willing to trade between different attributes, i.e. how much change in one attribute is required for the preference to be equivalent to preference for a 1-unit (or particular amount of) change in the other attribute. This allows all treatments/attributes to be assessed on the same scale. The MRS is calculated using the following equation:
where Y and X are attributes, and d represents the change in attribute Y with respect to X. MRS estimates were calculated using each of the continuous attributes (risk of symptom recurrence and risk of reintervention) as the denominator, with 95% confidence intervals estimated using the delta method.
Specifically, analyses were performed to estimate (separately) the maximum increased risk of symptom recurrence and reintervention (each) that participants would be willing to accept, on average, in exchange for each of the hypothesized/estimated benefits associated with valve repair by the ‘beating heart’ alternative relative to open-heart surgery. The MRS for an increased risk (in exchange for a given amount of benefit) is also referred to as maximum acceptable risk (MAR).
For each MAR attribute (risk that symptoms reappear and risk of reintervention), the individual MAR/MRS estimates for trade-offs between that MAR attribute and each of the other 5 attributes were then summed to calculate estimates of the ‘combined MAR.’ The combined MAR represents the total increased risk (of symptom recurrence or reintervention) participants would be willing to accept in exchange for a ‘beating heart’ treatment relative to ‘open-heart surgery-like’ treatment.
Choice Probabilities
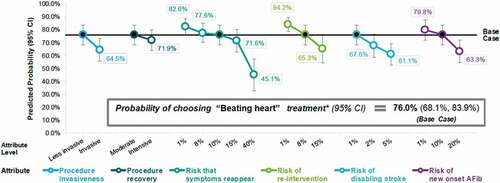
Based on the preference estimates of the ECL choice model, the predicted probability that participants would choose the ‘beating heart’ base case treatment profile over the ‘open-heart surgery-like’ treatment profile (as presented in ) was calculated using Equation 2, with 95% CIs estimated using the delta method.
Sensitivity analysis calculations were then performed to estimate the predicted probability that participants would choose a ‘beating heart’ treatment profile differing from the base case by one attribute level at a time (versus ‘open-heart surgery-like’ treatment). These sensitivity analysis calculations allow an assessment of how predicted choice probability for the ‘beating heart’ profile (relative to the ‘open-heart surgery-like’ profile) would change if one of the risks associated with the ‘beating heart’ alternative was underestimated (or overestimated) in the base case profile.
Results
Participant Characteristics
145 subjects completed the survey (n = 34 Clinically Confirmed MR, n = 111 Self-reported MR), but 27 patients were dropped after excluding those who either A) failed the dominance test, B) ‘straight-lined’ their responses or C) completed the survey in less than 7 minutes. The final analytic sample (n = 118) included 30 clinically confirmed MR patients and 88 self-reported MR patients (Appendix ), but a sensitivity analysis was conducted on the full sample.
Among the 118 participants in the final analytic sample, across both cohorts, the mean age was 64 (with a range of 22 to 92 years), with nearly equal proportions of men and women and the large majority (94%) identifying as white (Appendix ). The clinically confirmed and self-reported diagnosis cohorts had similar (i.e. not statistically significantly different) distributions in terms of gender, ethnicity, race, employment status, marital status, parental status and terminal illness.
The overall sample was diagnosed an average of 15.9 (median 9.5) years ago. Comorbidities and complications most frequently reported by participants were atrial fibrillation (51%), diabetes (18%) and coronary artery disease (15%). Almost one third (30%) of the sample reported having no comorbidities (Appendix ).
Two thirds (69%) of the sample reported having received a previous mitral repair or replacement procedure, requiring a mean of 8.3 (median 6) nights in the hospital, while 48% were currently taking medications for their DMR symptoms (Appendix ).
DCE Evaluation
Overall, 92% of participants strongly agreed or agreed that the choice tasks were easy to understand, 77% strongly agreed or agreed that they were easy to answer, 97% strongly agreed or agreed they responded according to their preferences, and 89% strongly agreed or agreed that the questions were relevant to them. Participant’s evaluations of the DCE choice tasks were similar between the clinically confirmed and self-reported diagnosis cohorts. (Appendix )
Scale Test
The Swait-Louviere test indicated that it was appropriate to pool the two diagnosis cohorts as we could not reject the hypothesis that the underlying preferences in the two cohorts were the same. (Appendix ).
DCE Main Model Results
presents the preference estimates (with 95% confidence intervals) from the error component logit model. Preference estimates for all attribute levels were statistically significant and in the expected directions. Less invasive procedures, moderate recovery, lower risks of disabling stroke and new onset atrial fibrillation were more preferred (as indicated by positive estimates), while higher risks of symptom recurrence and reintervention were less preferred (as indicated by negative estimates). Compared with the highest levels of the categorical risk attributes (5% risk of stroke and 20% risk of atrial fibrillation), participants preferred the lowest risk levels (1% for both attributes) more than the middle risk levels (2% risk of stroke and 10% risk of atrial fibrillation) as indicated by larger positive estimates; however, the preferences for reductions between these adjacent risk levels were not statistically significantly different from each other as indicated by overlapping confidence intervals. Results for alternative model specifications using multinomial logit and/or categorical variable definitions are reported in (Appendix ).
Table 3. Error component logit model results (main effects, n = 118)
Relative Attribute Importance
The mean relative importance (RAI) percentage weight and ranking of each attribute, as calculated for the comparison of a ‘beating heart’ treatment with ‘open-heart surgery-like’ treatment based on the ECL model estimates, is presented in . On average, for treatment options with attribute levels differing by values representing a ‘beating heart’ profile versus an ‘open-heart surgery-like’ profile, ‘risk of disabling stroke’ was the most important attribute, followed sequentially by ‘risk of new onset atrial fibrillation,’ ‘procedure invasiveness,’ ‘risk of reintervention,’ ‘risk that symptoms reappear’ and finally ‘procedure recovery.’
Table 4. Relative attribute importance (RAI) for differences in attribute levels based on ‘beating heart’ versus ‘open-heart surgery-like’ treatment profiles (n = 118)
Overlap between many of the RAI confidence intervals indicated only a few statistically significant differences. On average, ‘procedure recovery’ (7.7% [95% CI: 2.0%, 12.2%]) was significantly less important than all other attributes except ‘risk that symptoms reappear.’ ‘Risk of disabling stroke’ (23.3% [95% CI: 16.6%, 30.1%]) was also significantly more important than ‘risk that symptoms reappear’ (13.5% [95% CI: 11.2%, 15.8%]). The successive magnitudes of RAI weights were similar and confidence intervals overlapped among attributes within all other groupings/pairs.
Tradeoffs: Marginal Rate of Substitution and Maximum Acceptable Risk
Results of marginal rates of substitution (MRS)/maximum acceptable risk (MAR) calculations, in terms of trade-offs between the ‘beating heart’ and ‘open-heart surgery-like’ treatment profiles, are presented in . MARs of symptoms reappearing were larger than MARs of reintervention. For example, in exchange for a less invasive (versus invasive) procedure (with all other attributes equal between treatments), on average, participants were willing to accept a maximum of 12.4 percentage points increased risk of symptoms reappearance or 7.5 percentage points increased risk of reintervention. The lower MARs for reintervention indicated its potentially greater relative importance compared with the reappearance of symptoms, although the differences were not statistically significant.
Table 5. Maximum (or minimum) acceptable risk (MAR) thresholds for A) symptoms reappearing and B) reintervention
According to the combined MAR estimates, in exchange for a ‘beating heart’ treatment relative to ‘open heart surgery-like’ treatment, on average, participants were willing to accept a maximum of 34.6 percentage points (95% CI: 23.8, 45.4) increased risk that symptoms reappear or 22.6 percentage points (95% CI: 14.7, 30.4) increased risk of reintervention (beyond the corresponding risk associated with an ‘open-heart surgery-like’ treatment). Point estimates and confidence intervals for these combined MAR estimates excluded the estimates for actual increased risks (9 percentage point increased risk that symptoms reappear and 7 percentage point increased risk of reintervention) associated with the beating heart alternative to open-heart surgery to avoid double-counting the risk level.
Choice Probabilities
Choice probabilities for the ‘beating heart’ base case treatment profile and sensitivity analysis calculations are presented in . The predicted probability that participants would choose the ‘beating heart’ base case treatment profile over the ‘open-heart surgery like’ treatment profile was 76.0% (95% CI: 68.1%, 83.9%). The predicted choice probability (and 95% CI) for ‘beating heart’ treatment remained above 50% for every one-attribute level change from the base case profile except when risk that symptoms reappear was increased to 40%.
Subgroup Analyses
While the study was not appropriately powered to conduct comprehensive subgroup analyses, exploratory analyses based on gender, age (under 65, 65 and over), working status, prior treatment and physical functioning were examined. Preferences were similar regardless of gender and employment/working status, but some preferences differed across age, previous treatment status and physical functioning level. Details are available in Section 4 (‘Subgroup Analyses’) in the Supplement.
Discussion
This study provides new quantitative information on patients’ benefit-risk preferences for treatment of DMR by surgical MV repair. Of the 6 attributes considered, patients weigh the risk of disabling stroke as most important and procedure as recovery least important, but most attributes tested contributed fairly equally in participants’ decisions. The model predicted that 76% of participants would choose a ‘beating heart’ (versus ‘open-heart surgery-like’) treatment profile despite potential risks. The results of predicted choice probability sensitivity analyses suggest that even if one of the risks associated with the ‘beating heart’ alternative was underestimated in the base case profile, most patients would still prefer the ‘beating heart’ alternative despite the higher risk level (at least up to 15% for risk that symptoms reappear) if the other attributes were defined correctly.
Limitations
Several important limitations should be considered in the interpretation of results from this study.
In addition to a relatively small sample size, both the clinically confirmed and self-reported diagnosis cohorts were limited in their population representativeness. The self-reported diagnosis cohort had additional limitations in terms of ability to confirm certain eligibility criteria and clinical information. However, the self-diagnosis cohort were selected on the basis of carefully crafted screening questions designed with MR expert clinician input (as seen in the ‘Appendix: Patient Survey’ section, key questions included MR diagnosis, MR severity, type of MR, procedural history, NYHA classification and type of mitral prolapse). This helped provide significant assurance that respondents met the study inclusion criteria. Neither cohort was selected with a probability-based or random approach to ensure representativeness of all patients with MR; both cohorts were subject to biases including self-selection and nonresponse.
The self-reported diagnosis cohort was a different sample than the clinically confirmed diagnosis cohort. Without physician confirmation it was not feasible to guarantee the same eligibility criteria were met including MR: severity, meeting clinical guidelines for surgery, and having primary/degenerative versus secondary/functional MR type. Encouragingly, results of the Swait-Louviere scale test showed that it was appropriate to pool data from the two cohorts.
Furthermore, the calculations based on the ‘beating heart’ versus ‘open-heart surgery-like’ treatment profiles are only applicable to the specific differences in attribute levels as estimated for these profiles. If the performances of (difference in risks between) actual beating heart procedures and open-heart surgery are different than the assumptions in our survey, and if those new values were applied in calculating RAI, MAR, and predicted choice probability estimates, the results of such calculations would change. Going forward, efforts to elicit responses from larger, more representative samples with clinically confirmed DMR will improve understanding of patient preferences.
Policy Implications
These results may help inform MR treatment decision-making by regulatory agencies, payers, clinicians, and patients considering a beating heart approach as a potential new alternative to conventional open-heart surgical repair. From a patient perspective, these findings identify and quantify the complex trade-offs involving multiple treatment modalities for MR. The results may also improve the ‘sorting’ of patients into different treatment options based on their ex-ante preferences for key outcomes.
Increasingly, healthcare decision-making bodies are accounting for patient preferences in the process of evaluating clinical trial outcomes. With respect to HARPOON®, this DCE study, in combination with additional analyses, increased the acceptable level of reoperation associated with ‘beating heart’ technologies. Naturally, these types of tradeoffs may extend to numerous other medical treatments. As such, further research using patient preference elicitation methods can impact whether, and under what circumstances, novel medical technologies enter markets. While adoption of new innovations may impact costs associated with patient management (e.g. potential for reinterventions), resources are more likely to be directed in ways in which the end-user arbitors of value, patients and health systems that represent them, prefer. Patient preference studies may also help refine clinical trial administration and recruitment to concentrate on those patients with the greatest perceived preference for particular technologies. Lastly, as payors aim to provide high value care to patients, patient preference research can uncover value and help influence coverage and price determination for technologies after market entry of new (or existing) products.
Conclusion
Patients with chronic MR are willing to accept higher rates of reintervention or symptom recurrence in return for the benefits associated with less invasive surgical treatment procedures. Physicians and stakeholders involved in either the treatment or development of new technologies for MR should account for patient preference accordingly. Future research initiatives can expand on our understanding of patient preferences for novel treatments or different patient types.
Author Contributions
Transparency Statements
Declaration of Funding
Financial support for study provided by Edwards Lifesciences, The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethics Statement and Consent
All research reported in this paper has been conducted in an ethical and responsible manner, and fully complies with all relevant codes of experimentation and legislation. All patient level data that informed the analysis are completely deidentified. Our study follows the principles of the Declaration of Helsinki.
Acknowledgements
Edwards Lifesciences provided financial support for this research. No other assistance in the preparation of this article isdeclared.
Disclosure Statement
Dr. Keuffel and Dr. Rizzo each serve as consultants to Edwards Lifesciences. Dr. Janssen was an employee of ICON PLC, a consultancy contracted with Edwards Lifesciences at the time of the analysis. Mr. Liden and Ms. Hanna are both employees of Edwards Lifesciences. The authors have no other conflicts of interest to declare. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Notes
1. A dominance task asks the participant to choose between a dominant option (across all attributes) and an inferior option. This task helps ensures that the participant is engaged and not choosing randomly or without comprehension of the choices. The holdout task has the participants directly choose between the options which most closely resemble the ‘beating heart-like’ option and the ‘open heart surgery-like’ option.
2. In the Swait-Louviere scale test the first step tests whether it is appropriate to pool the data from two population samples (based on a lack of difference in their underlying preferences). Separate logit models for each cohort were compared to a pooled model allowing the scale factor for each cohort to vary (known as a scale-adjusted or heteroscedastic model) using a likelihood ratio test. If the null hypothesis of this test (equality of preferences between the two samples) is rejected, the two samples have different preferences and should not be pooled. If equality cannot be rejected, then the second step can be performed which tests whether there are differences in the scale of preferences between the two samples (that the pooled model should thus adjust for). This test compares two pooled models, the scale-adjusted/heteroscedastic model and a model with equal scale factors. If the null hypotheses of this test (equality of scale parameters) are rejected, then the scale-adjusted/heteroscedastic pooled model should be used. If equality of scale parameters cannot be rejected, then the pooled model with equal scale factors is appropriate.
3. Given four numeric attributes could either be analyzed as continuous or discrete measures, coefficient estimates from an ECL model with all attributes coded categorically were assessed for linearity in relationships between preferences and risk levels. If coefficients for any of the categorical risk attributes appeared approximately linearly distributed (e.g. for risk of reintervention with 1% risk as the reference level, if the coefficient for 15% risk [a 14-unit risk increase] is two times greater than the coefficient for 8% risk [a 7-unit risk increase]), the model was re-specified with those attributes coded as linear continuous variables instead of categorical variables.
References
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2021;77(4):e25–e197.
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011.
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American heart association. Circulation. 2010;121(7):e46–e215.
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28(11):1358–1365.
- De Oliveira JF, Antunes MJ. Mitral valve repair: better than replacement. Heart. 2006;92(2):275–281.
- Gammie JS, Bartus K, Gackowski A, et al. Beating-heart mitral valve repair using a novel ePTFE cordal implantation device: a prospective trial. J Am Coll Cardiol. 2018;71(1):25–36.
- Gammie JS, Wilson P, Bartus K, et al. Transapical beating-heart mitral valve repair with an expanded polytetrafluoroethylene cordal implantation device: initial clinical experience. Circulation. 2016;134(3):189–197. Circulation.
- Hunter NL, O’Callaghan KM, Califf RM. Engaging patients across the spectrum of medical product development: view from the US food and drug administration. Jama. 2015;314(23):2499–2500.
- Van Overbeeke E, Janssens R, Whichello C, et al. Design, conduct, and use of patient preference studies in the medical product life cycle: a multi-method study. Front Pharmacol. 2019;10:1395.
- Oehrlein EM, Harris J, Balch A, et al. Improving access and quality of health care in the United States: shared goals among patient advocates. Patient-Patient-Centered Outcomes Res. 2021;14(5):687–690.
- Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19(4):300–315.
- Soekhai V, Whichello C, Levitan B, et al. Methods for exploring and eliciting patient preferences in the medical product lifecycle: a literature review. Drug Discov Today. 2019;24(7):1324–1331.
- Ryan M, Gerard K, Amaya-Amaya M. Using discrete choice experiments to value health and health care. Vol. 11. Dordrecht, The Netherlands: Springer Science & Business Media; 2007.
- Johnson FR, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16(1):3–13.
- Soekhai V, de Bekker-grob EW, Ellis AR, et al. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–226.
- Fairchild AO, Katz EG, Reed SD, et al. Patient preferences for ketamine-based antidepressant treatments in treatment-resistant depression: results from a clinical trial and panel. Neurol Psychiatry Brain Res. 2020;37:67–78.
- Hollin IL, Peay H, Fischer R, et al. Engaging patients and caregivers in prioritizing symptoms impacting quality of life for duchenne and becker muscular dystrophy. Qual Life Res. 2018;27(9):2261–2273.
- Janssen EM, Marshall DA, Hauber AB, et al. Improving the quality of discrete-choice experiments in health: how can we assess validity and reliability? Expert Rev Pharmacoecon Outcomes Res. 2017;17(6):531–542.
- Tervonen T, Schmidt-Ott T, Marsh K, et al. Assessing rationality in discrete choice experiments in health: an investigation into the use of dominance tests. Value Health. 2018;21(10):1192–1197.
- Bridges JF, Tsai J-H, Janssen E, et al. How do members of the duchenne and becker muscular dystrophy community perceive a discrete-choice experiment incorporating uncertain treatment benefit? an application of research as an event. Patient-Patient-Centered Outcomes Res. 2019;12(2):247–257.
- Swait J, Louviere J. The role of the scale parameter in the estimation and comparison of multinomial logit models. J Marketing Res. 1993;30(3):305–314.
Section 1: Appendix Tables
Table A1. Initial (version 1) DCE attributes and levels
Table A2. Full description of final attributes and levels
Table A3. Final DCE design
Table A4. Dominance test task
Table A5. Holdout task for comparison of choice probabilities
Table A6. Inclusion and exclusion criteria
Table A7. Participant sociodemographic characteristics (n = 118)
Table A8. Participant clinical characteristics (n = 118)
Table A9. Participant mitral regurgitation treatment history characteristics (n = 118)
Table A10. DCE data quality metrics for the full sample including all complete survey responses (n = 145)
Table A11. Participant evaluation of DCE (n = 118)
Table A12. Swait-Louviere scale test procedure results
Table A13. Conditional/multinomial logit model results (main effects, n = 118)
Table A14. Error component logit model results (main effects, n = 118) – categorical specification
Table A15. Conditional/multinomial logit model results (main effects, n = 118) – categorical specification
Section 2: DCE Development Description
We used a blocked D-efficient main-effects experimental design (Ngene Software, ChoiceMetrics)–a common approach–in order to efficiently identify each attribute level’s effect on treatment choice [Citation13,Citation14]. The design yielded 3 blocks with 8 paired comparisons along with a ‘dominance’ task and a ‘holdout’ task. As such, each DCE participant (in pretest and final surveys) would respond to 10 choice tasks (Appendix ). The dominance test presented a clearly preferable/superior (dominant) profile relative to an inferior profile (Appendix ). Participants who chose the clearly less preferable (dominated) treatment profile in this scenario were considered to be giving illogical responses. The other additional choice task was a ‘holdout’ task that presented a scenario comparing a treatment profile that approximates a minimally invasive procedure similar to the HARPOON® procedure and a treatment profile that approximates an open-heart surgery procedure (Appendix ). The dominance task and the holdout task were not used in main statistical evaluation of attributes, although we did compare the preferences from our model-derived probability estimates with the ‘holdout’ task results.
Section 3: Recruitment Details and Participant Screeners
For the self-reported diagnosis cohort participants were recruited from A) Edwards Lifesciences internal database of patients with aortic valve disease, B) Members of two patient advocacy organizations (Mended Hearts and Heart Valve Choice), C) a clinical research network (PMG Research), D) an online patient panel (Global Perspectives” and E) Internal Databases of market research firms (Schlesinger Group, Adler Weiner Research and Shugoll Research). The clinically confirmed diagnosis patients were referred from ten cardiovascular clinics.
E-Mail invitations including the online link to access the survey were sent to potential self-reported DMR patients. Through the survey link, patients were required to complete an online screener to confirm study eligibility.
Physician Screener
Mitral Valve Regurgitation Patient Survey
ELIGIBILITY SCREENER
Clinician Instructions
Please first review the eligibility screening questions on this form and answer those which do not require the patient’s input (‘pre-screen’ questions). If none of the pre-screen responses indicate ineligibility, please use the included script to introduce the survey and ask the remaining questions to determine the patient’s eligibility.
If the patient appears eligible and interested, then please complete the questions on the left side of the postcard as they relate to your patient’s condition. Tear off the right side of the postcard to retain for your records and give the left side to your patient.
Pre-Screen Questions – Inclusion Criteria
Please confirm that your patient meets all of the following criteria (i.e. all questions are answered ‘Yes’). If the answer is ‘No’ to any of the following questions, then please do not distribute a study postcard to the patient:
Is the patient 19 years old or older?
Yes
No [If No, not eligible, end screening]
Does the patient have a clinically confirmed diagnosis of current or previously surgically treated severe mitral valve regurgitation due to primary/degenerative mitral valve disease?
Yes
No [If No, not eligible, end screening]
Is the patient’s mitral valve regurgitation severe (or was previously, if treated surgically), meaning that the patient meets (or met) the clinical guidelines for surgical intervention? (The patient does not need to have been treated surgically yet.)
Yes
No [If No, not eligible, end screening]
The following criteria are allowed but not required. The patient may participate if they have been:
Treated surgically with mitral valve repair or replacement for severe MR due to primary/degenerative mitral valve disease
Treated for aortic stenosis surgically or with transcatheter devices
Pre-Screen Questions – Exclusion Criteria
Please confirm that your patient does not meet any of the following criteria (i.e. all questions are answered ‘No’). If the answer is ‘Yes’ to any of the following questions, then please do not distribute a study postcard to the patient:
Does the patient have a diagnosis of secondary/functional mitral valve regurgitation?
Yes [If Yes, not eligible, end screening]
No
Does the patient have a cognitive or visual impairment that would interfere with their ability to participate in a 30 minute online survey?
Yes [If Yes, not eligible, end screening]
No
Does the patient have acute psychopathology that would interfere with their ability to participate?
Yes [If Yes, not eligible, end screening]
No
If the patient is eligible for the survey based on your answers up to this point, please follow the script on the next page.
Clinician Script
You have been invited to participate in a research survey about people with severe mitral valve regurgitation. A research company is conducting this research survey on behalf of Edwards Lifesciences, a medical device company. This survey is being done to understand your experience living with severe mitral valve regurgitation and your preferences for treatment of your severe mitral valve regurgitation.
The survey is being conducted online and will take approximately 30 minutes to complete. If you are eligible and decide to participate you will receive a $25 gift card after completing the survey.
I have already confirmed that your condition meets certain clinical criteria, and will ask you a few other questions to determine if you are eligible to participate in this research.
[Ask patient directly] Are you willing to participate?
Yes
No [If No, not eligible, end screening]
[Ask patient directly] Are you able to speak, read, and understand English?
Yes
No [If No, not eligible, end screening]
[Ask patient directly] Do you have access to a web interface on a computer or tablet for the purpose of completing the online survey? (Please note that the survey will not display properly on a phone.)
Yes
No [If No, not eligible, end screening]
[If ineligible]
I am sorry, but you do not match the criteria for participation in this survey. Thank you very much for your interest and for your time.
[If eligible]
[If the patient appears eligible and interested, then please complete the questions on the left side of the postcard as they relate to your patient’s condition. Tear off the right side of the postcard to retain for your records and give the left side to your patient.]
Thank you for answering my questions. You are eligible. Here is a postcard with the survey information and instructions for you to participate. Please keep track of this postcard until you have completed the survey. It contains the survey website link, a unique PIN code and some other information you’ll be asked to enter into the online form.
Patient Screener
Mitral Valve Regurgitation Patient Survey
Welcome!
Please make sure you are visiting this link from a computer or tablet. The survey requires a larger screen and will not display properly on a smart phone.
Thank you for your interest in participating in this survey. First, we need to ask you a few questions in order to determine if you are eligible to participate.
[Require responses to all screening questions]
What is your age?[Numeric field with range from 0 to 100] Years[If number entered is <19, not eligible – show message below]
Have you been diagnosed with mitral valve regurgitation?
- Not sure [If selected, not eligible – show message below](3) What is the severity of your mitral valve regurgitation, currently?
Mild
Moderate
Moderately severe
Severe
Not sure
Have you ever received surgery or a procedure to repair or replace your mitral valve?
Yes
No [If selected AND response to question S3 above = ‘Mild’, not eligible – show message below]
Not sure
What type is your mitral valve regurgitation?
Primary/degenerative
Secondary/functional [If selected, not eligible – show message below]
Not sure
What is your NYHA classification?
Class I
Class II
Class III
Class IV
Not sure
What is your type of mitral prolapse?
P2
Bi-leaflet
Anterior
Not sure
[If any responses above indicate the respondent is ineligible]
Unfortunately you do not match the criteria for participation in this survey. Thank you for your interest and for your time.
[End survey]
[If respondent is eligible, continue to next page]
Based upon the answers you provided, you are eligible to participate. A research company is conducting this survey on behalf of Edwards Lifesciences, a medical device company. We are trying to understand your experience living with mitral valve regurgitation. We also want to know about your preferences for treatment of your mitral valve regurgitation.
Participation is voluntary, and you are free to choose whether you would like to continue.
Findings from this survey will influence work to improve the treatment of people with mitral valve regurgitation.
Section 4: Subgroup Analyses Summary
Compared with younger patients, patients 65 and older assign more importance to a less invasive (versus invasive) procedure and more strongly prefer to avoid an increased risk that symptoms reappear. The results also suggest that, compared with previously treated patients, untreated patients may have a stronger preference for lower risk of disabling stroke and accept a higher maximum increased risk that symptoms reappear in exchange for decreased risk of stroke and the overall ‘beating heart’ (versus ‘open-heart surgery-like’) procedure profile.
The results further suggest that compared with more physically limited patients, patients with few/no physical limitations may have a stronger desire to avoid an increased risk that symptoms reappear and require a greater reduction in risk of reintervention in exchange for a higher probability that symptoms reappear. This effect may be driven partially by previous treatment experience such that patients whose functioning improved since treatment are more afraid of worsening again after finding relief or that they may be more opposed to considering a procedure less effective than the one they received. It may also be explained by patients with fewer limitations valuing remaining symptom-free because they want to continue functioning at the same level (whereas patients with more limitations may have adapted to their worse functional status). Further details and regression results are available from the authors.