ABSTRACT
Objectives
The present study aimed to analyze the association between the prescription of ivy leaf dry extract EA 575 (licensed under the trade name Prospan® in Germany) and the incidence of antibiotic use, incident bacterial complications, and days of sick leave in adult patients with cold diseases.
Methods
This retrospective cohort study was based on the IQVIA Disease Analyzer database and included adult patients from 1032 general practices in Germany with a documentation of common cold between 2017 and 2020 (index date) and prescription of either EA 575 or an antibiotic drug within 3 days after index date. 1:1 propensity score matching based on age, sex, index month, physician, health insurance status, and the Charlson Comorbidity Index was carried out. Univariable regression models were used to investigate the association between EA 575 prescription and defined outcomes.
Results
Data of 7034 patients treated with EA 575 and 7034 matched patients receiving an antibiotic were available. EA 575 prescription was associated with significantly lower odds of an antibiotic prescription in the time periods of 4–30 days (OR: 0.83; 95% CI: 0.72–0.96) and 31–365 days (OR: 0.44; 95% CI: 0.40–0.48) after the index date. EA 575 prescription was significantly associated with a lower rate of sick leave of more than 7 days (33.0% vs. 37.7%, OR: 0.81; 95% CI: 0.73–0.90) in patients with any sick leave, as well as with lower odds of a new cough diagnosis (OR: 0.91, 95% CI: 0.85–0.98) when compared to antibiotic prescription.
Conclusion
Our study provides further evidence that the use of phytopharmaceuticals, in particular ivy leaf dry extract EA 575, could contribute to a reduction in the number of inappropriate antibiotic prescriptions for respiratory infection with cough symptoms.
Introduction
The common cold is a benign, self-limiting syndrome that represents a group of diseases caused by members of several virus families. Symptoms generally relate to the infected mucosa of the upper respiratory tract, typically peak at 1–3 days after onset, and last for 7–10 days. They include a sore throat, rhinitis, rhinorrhea, cough, and malaise. The severity and type of symptoms vary among individuals and with different infective agents [Citation1].
Common colds and other upper respiratory tract infections are conditions with a high prevalence worldwide [Citation2]. In Germany, the 1-year average prevalence of common colds among children and adolescents amounts to approximately 89%. However, common colds occur less often in adults than in children [Citation2].
For common colds, patients typically seek medical counseling for reassurance and sick leave and are advised to use soothing measures [Citation3]. Diseases with the leading symptom cough are economically important, as respiratory diseases resulted in a total of 103.7 million sick leave days with estimated lost work time costs of 12.4 billion euros in Europe [Citation3].
Although both experts and nonprofessionals know that cold diseases are primarily caused by viruses and are mostly self-limiting, antibiotics are prescribed in too many cases [Citation4]. Most patients suffering from cold diseases do not benefit from antibiotic treatment, and many patients experience side effects such as diarrhea and rash. Furthermore, unnecessary antibiotic prescriptions contribute to increasing bacterial resistance to standard antibiotics [Citation5]. This issue is recognized as an ongoing problem for society and health economics that is also perceived at the policy level. The recently (2021) updated joint EU interagency report from EFSA, EMA, and ECDC on antibiotic consumption and antibiotic resistance concluded that further interventions to reduce and improve antimicrobial consumption would have a beneficial impact on the occurrence of antimicrobial resistance and underlines the need to promote prudent use of antimicrobial agents [Citation6].
The common cold is predominantly self-diagnosed and self-medicated with over-the-counter (OTC) medicines, including herbal products [Citation7]. In Europe, several herbs of different plant species have been used against the flu and common cold. The efficacy and safety of herbal medicines have been evaluated and confirmed in clinical trials [Citation8].
Ivy leaf (Hederae helicis folium, Hedera helix L.) dry extract EA 575 has been well established in treating different respiratory diseases [Citation9] and has also been recognized by the Germany Pulmonology Society (DGP) in its guidelines for cough treatment in adults [Citation10]. The results of a randomized, controlled clinical trial (RCT) demonstrated a significant superiority of EA 575 compared to placebo, as well as a rapid onset of action after 48 hours, in the treatment of acute cough [Citation11]. In 2019, Schaefer et al. published the results of another RCT corroborating their previous results and demonstrated the superiority of EA 575 given either two or three times per day over placebo during and at the end of treatment, as measured using bronchitis severity score [Citation12]. In a non-interventional study performed in Latin America in 9657 patients under normal clinical practice conditions and including a large population with a wide age range (0–98 years), EA 575 was found to be generally effective and safe in the treatment of patients with bronchitis [Citation13]. This study also found that concomitant antibiotic use, which was still widespread in Latin America at the time of this study, may increase the relative risk for the occurrence of adverse events, which would clearly support a preference for monotherapy for this indication.
The majority of previously published studies with EA 575 have been clinical trials or non-interventional studies based on relatively small patient cohorts. Martin et al. have shown the effectiveness of different phytopharmaceuticals in the treatment of patients with acute infections of the lower and upper respiratory tract [Citation14]. However, they compared patients with phytopharmaceutical treatments to untreated patients without medical treatment, and antibiotic therapy was excluded [Citation14]. Yet, an in-depth analysis of health-care data for EA 575 treatment of adults and the relationship between disease progression and antibiotics has not yet been conducted.
The objective of the present study was to analyze the association between the prescription of EA 575 in Germany and the incidences of antibiotic use, incident bacterial complications, and days of sick leave in adult patients with acute respiratory infections.
Methods
Data source
This analysis was performed using the IQVIA Disease Analyzer (DA) database, which contains case-based information provided by office-based physicians (both GPs and specialists) in Germany. Information is available on patient demographics, drug prescriptions, concomitant medication, comorbid conditions, sick leave, and referrals to hospitals. Data analyses solely consider data from those sites that have continuously delivered data to the DA panel in the past. DA contains data from more than six million patients in the time period between 2016 and 2020. Nearly 3000 office-based physicians, representing approximately 3% of all German practices, provide information (DA status date: March 2021). Practices can be categorized into 10 classes according to the physician’s specialty (GPs and various specialists). The sample of practices included is representative of Germany with regard to geography, covering eight major German regions.
The database has been proven suitable for pharmaco-epidemiological and pharmaco-economic studies [Citation15,Citation16]. IQVIA ensures the accuracy, consistency, and completeness of the data. Evident data limitations are immediately communicated by IQVIA, and the analysis can thus be adapted accordingly.
Ethical statement
The database used includes only anonymized data in compliance with the regulations of the applicable data protection laws. German law allows the use of anonymous electronic medical records for research purposes under certain conditions. According to this legislation, it is not necessary to obtain informed consent from patients or approval from a medical ethics committee for this type of observational study that contains no directly identifiable data. Because patients were only queried as aggregates and no protected health information was available for queries, no Institutional Review Board approval was required for the use of this database or the completion of this study.
Study population
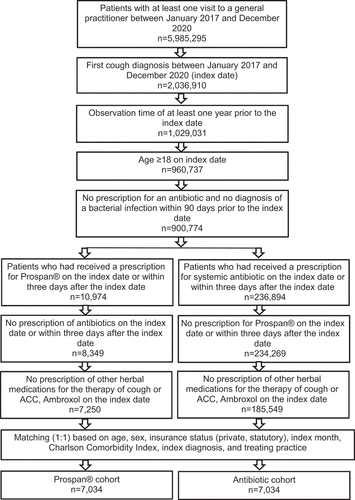
This study included adult patients (18 years or older) in general practices in Germany. To be eligible for the analysis, patients needed to meet the following inclusion criteria: (1) documentation of ICD-10 common cold disease codes such as viral infection of unspecified site (B34), acute nasopharyngitis [common cold] (J00), acute upper respiratory infections of multiple and unspecified sites (J06), acute bronchitis (J20), non-specified bronchitis (J40), or cough (R05) between 1 January 2017 and 31 December 2020. The first diagnosis documented in this time period is considered the index date; (2) prescription of EA 575 or of an antibiotic drug (ATC: J01) within 3 days after the day of the diagnosis (including the day of diagnosis); and (3) at least 12 months of observation prior to this diagnosis. Patients with an antibiotic prescription or a diagnosis of a bacterial infection within 90 days prior to the index date, as well as patients with prescription of other herbal medications for the therapy of cough, or n-acetylcysteine (NAC), or Ambroxol within 3 days after the day of the diagnosis, and patients who received both EA 575 and antibiotic within 3 days after the day of diagnosis were excluded from the analysis in order to prevent treatment interference from being included in our analyses.
Study outcomes
During this study, the following outcomes were investigated..
(1) Proportion of patients with new antibiotic prescriptions in the time periods of 4–30 days and 31–365 days following the index date, respectively.
(2) Proportion of patients with documentation of bacterial infections in the time period 4–30 days following the index date. The bacterial infection diagnoses included pneumococcal pharyngitis/staphylococcal pharyngitis (ICD-10: J02.0), streptococcal tonsillitis (ICD-10: J03.0), acute tonsillitis due to other specified organisms (ICD-10: J03.8), pneumonia due to Streptococcus pneumoniae (ICD-10: J13), pneumonia due to Haemophilus influenzae (ICD-10: J14), bacterial pneumonia not elsewhere classified (ICD-10: J15), acute streptococcal bronchitis (ICD-10: J20.2), streptococcus and staphylococcus as the cause of diseases classified elsewhere (ICD-10: B95), and other specified bacterial agents as the cause of diseases classified elsewhere (ICD-10: B96).
(3) Duration of sick leave in the time period 1–30 days following the index date. The mean duration of sick leave in the time period 1–30 days after the index date and the proportions of patients with a sick leave duration of at least 3 days and at least 7 days were calculated. The logistic regression model is used to test whether EA 575 prescription was negatively associated with a defined sick leave duration. These analyses were performed for all patients aged 18–65 and separately for patients with at least 1 day of sick leave.
(4) Proportion of patients with repeated virus infections in the time period 8–365 days following the index date (we assume that the common cold usually lasts no longer than 7 days).
Statistical Methods
To control for confounding factors, 1:1 matching was carried out based on a propensity score that was constructed as the conditional probability of being prescribed EA 575 as a function of age, sex, index month, physician, health insurance status, and Charlson Comorbidity Index (CCI) (logistic regression). The CCI is a method for categorizing comorbidities of patients based on the International Classification of Diseases (ICD) diagnosis codes found in administrative data, such as hospital abstracts. The higher the score, the more likely the predicted outcome will result in mortality or higher resource use [Citation17].
Propensity score matching was performed by choosing a patient treated with EA 575 with a propensity score minimally different from the propensity score of a patient treated with an AB. Descriptive statistics were given, and group differences (EA 575 vs. AB) were assessed using the Wilcoxon test or the McNemar’s test after propensity score matching.
We adopted univariable logistic regression models to investigate the association between EA 575 prescription and new antibiotic prescription, new bacterial diagnoses, as well as sick leave duration of at least 3 and 7 days. Time until the new cough diagnosis was shown using Kaplan–Meier curves. As the proportional hazard assumption in the analysis of new viral infections was not fully met, we further used univariate logistic regression models to estimate the association between EA 575 prescription and new viral infection in the time period 8–365 days after the index date. To compare the sick leave duration in days, univariate linear regression was used. All outcomes were analyzed stratified by patient age (18–30, 31–40, 41–50, 51–60, 61–70, and >70 years) and sex. For illustrative purposes, p-values <0.05 were considered statistically significant without adjustment for multiplicity.
Results
Baseline characteristics
Prior to the matching, 7250 EA 575 patients and 185,549 antibiotic patients in the database met the selection criteria above. After the matching, 7034 EA 575 patients and 7034 matched antibiotic patients from 1032 general practices were available for analysis (). shows the baseline characteristics of the study patients. Due to the matching process, there were no significant differences between patients receiving EA 575 or antibiotics in terms of age (51 years), sex (54.6% female), or CCI (mean value: 1.4). Acute bronchitis was diagnosed in 18.7%, unspecified bronchitis in 12%, and acute upper respiratory infections of multiple and unspecified sites in 41.5% of patients in both cohorts ().
Table 1. Basic characteristics of study patients
Proportion of patients with new antibiotic prescription
At least one new (further) antibiotic prescription was issued within 4–30 days after the index date for 5.1% of patients with EA 575 prescription and 6.1% of patients with antibiotic prescription. The results of the multivariate logistic regression model are displayed in . EA 575 prescription was associated with significantly lower odds of a new antibiotic prescription in the time period 4–30 days after the index date (OR: 0.83; 95% CI: 0.72–0.96). While this negative association was observed in all the investigated subgroups, it was significant only in men and patients aged >60 ().
Table 2. Association between EA 575 prescription and probability of antibiotic prescription in the period 4–30 days and 31–365 after the index date [EA 575 versus antibiotic]
Within 31–365 days after the index date, at least one new antibiotic prescription was issued for 15.0% of patients with EA 575 prescription and 28.8% of patients with antibiotic prescription. The results of the multivariate logistic regression model are displayed in . EA 575 prescription was associated with significantly lower odds of an antibiotic prescription in the time period 31–365 days after the index date (OR: 0.44; 95% CI: 0.40–0.48). These negative associations were observed in all the investigated subgroups (p < 0.001) ().
Table 3. Association between EA 575 prescription and sick leave duration in days [EA 575 versus antibiotic]
Proportion of patients with documentation of bacterial infections
Diagnoses of bacterial infections were very rare in both cohorts. Any bacterial infection was documented in only five patients treated with EA 575 (=0.07%) and 11 patients treated with antibiotics (=0.16%) within 4–30 days after the index date. Although the incidence of bacterial infection was twice as high in the antibiotic cohort, the absolute numbers were too small to observe statistical significance, with an OR of 0.45 (95% CI: 0.16–1.31). In subgroup analyses, the numbers were small and confidence intervals were very broad, without significant effects.
Duration of sick leave
In this study, 5329 patients treated with EA 575 and 5325 patients treated with antibiotics were 18–65 years old and could be included for sick leave analyses. Of them, 64.0% of patients treated with EA 575 and 50.5% of patients treated with antibiotics took at least 1 day of sick leave. When including those who did not take sick leave into the computation using a sick leave duration of 0 days, averages for EA 575 were 5.0 (SD: 5.7) and 4.1 (SD: 6.4) days, respectively. The difference was −0.9 days in favor of antibiotics (p < 0.001) ().
In the logistic regression model, EA was significantly associated with higher odds of sick leave of at least 7 days (48.8% vs. 39.5%, OR: 1.46; 95% CI: 1.35–1.58). There was also a mild association for >7 days (21.1% vs. 19.0%, OR: 1.14; 95% CI: 1.04–1.25). When only participants with at least 1 day of sick leave were analyzed (EA 575: n = 3410; antibiotics: n = 2687), patients treated with EA 575 were on sick leave for an average of 7.8 (SD: 6.9) days compared to 8.1 (SD: 6.9) days in the antibiotic cohort. The difference was −0.3 days in favor of EA 575 (p = 0.086). The sick leave duration difference was higher in women (−0.5 days, p = 0.061) than in men (−0.1 day, p = 0.569). The highest difference was observed in people aged >60 (−1.4 days, p = 0.064) ().
In the logistic regression model on this patient subset, EA 575 prescription was significantly associated with lower odds of sick leave of at least 7 days (33.0% vs. 37.7%, OR: 0.81; 95% CI: 0.73–0.90). For >3 days, the association was weaker and not significant (76.3% vs. 78.3%; OR: 0.89; 95% CI: 0.79–1.01; p = 0.065).
New virus infections (cough diagnoses)
Of the study patients, 29.2% treated with EA 575 and 31.1% treated with antibiotics received at least one new cough diagnosis within 8–365 days after the index date (). EA 575 prescription was significantly associated with lower odds of a new cough diagnosis (OR: 0.91, 95% CI: 0.85–0.98). The association was strongest and significant in women (OR: 0.85, 95% CI: 0.77–0.93), as well as in persons >60 years (OR: 0.85, 95% CI: 0.74–0.97). In men and persons aged ≤40 and 41–60, no significant associations were observed ().
Figure 2. Cumulative incidence of new cough diagnoses stratified by index therapy (EA 575 versus antibiotic).
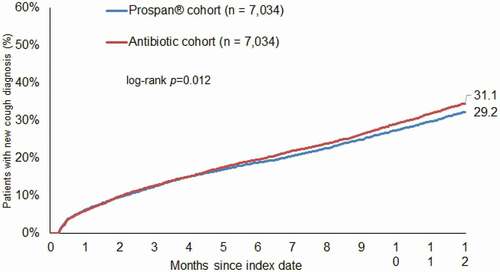
Table 4. Association between EA 575 prescription for new virus infections (cough diagnoses) in the time period 8–365 days after the index date [EA 575 versus antibiotic]
Discussion
The results of this retrospective study, based on a large sample of over 14,000 patients in Germany, show that prescriptions of EA 575 were significantly associated with reduced (re-)prescription of antibiotics in the further course of the disease, as well as with a decreased incidence of new cough diagnoses in the next 12 months after the therapy. Moreover, we found no consistent evidence that antibiotic prescriptions were associated with fewer days of sick leave when compared to EA 575. Interestingly, associations were mostly present in both women and men and in different age groups although not always significant due to available patient counts.
As in the present study, patients treated with EA 575 were propensity-score matched with patients receiving antibiotics in order to exclude confounding factors, the association between the prescription of EA 575 and lower antibiotic use and lower frequency of new cough diagnoses cannot be explained by sex, insurance status, cough diagnosis, or secondary diagnoses of these patients.
It may still be possible that patients who received antibiotics had more severe symptoms and that physicians decided to prescribe antibiotics to avoid the risk of complications. The general expectation would be that a curative drug like AB would lead to fewer additional AB prescriptions during the same disease episode. However, the data reported here show that in these cases the initial prescription was not as effective as expected, and this could be another indicator for the well-known fact that antibiotics are mainly useless in cases of acute respiratory infections.
The study published by Kern and Kostev clearly reported that the ‘habitual’ preference of some physicians for antibiotics had a stronger effect on the decision to prescribe antibiotics than all investigated, patient-related variables like age, diagnoses, or symptoms [Citation18]. In the study of Zweigner et al., a small number of physicians were responsible for a disproportionally large portion of the antibiotic prescriptions underlining the difference in physician preference. Moreover, the fact that the cohort of adult patients analyzed in this study visited a physician rather than only buying an OTC drug in a pharmacy may indicate that their symptoms were more severe [Citation19]. This is particularly important in the case of EA 575, which is marketed as an OTC drug and is not generally reimbursed by health insurances for adults even when prescribed. Sales data show that the vast majority of sales of EA 575 formulations occur without a prescription. Therefore, it could be assumed that the patients in this study, who received EA 575 after visiting a physician, were among the more severely ill, whereas patients with milder manifestations likely obtain EA 575 without a prescription and can thus not be studied using physicians’ medical records.
Similar to previous studies [Citation14,Citation20], this investigation showed that the documentation of bacterial infection diagnoses is rare and diagnoses of viral and undefined infections are very frequent. Since the decision on therapy has to be made quickly, and the lab results are usually available only several days after a patient visit, laboratory tests for distinguishing between infections with viral and bacterial origin are rarely performed in clinical practice.
Among the 900,774 adult patients in the database with a cough diagnosis during the study period, an observation time of at least 1 year, and no bacterial infection or antibiotic use during the period 90 days prior to the index date, approximately one-quarter received antibiotics on the day of diagnosis or within 3 days thereafter (). As cough is primarily caused by viral infections [Citation4], antibiotics are prescribed in too many patients, which can contribute to increasing antibiotic resistance [Citation5]. Moreover, antibiotic administration can weaken immune memory, with the consequence that patients become increasingly susceptible to new viral infections [Citation21]. In our study, patients who received antibiotics had a higher incidence of new infections in the time between 8 and 365 days after the index date than patients treated with ivy leaf dry extract EA 575. Whether the patients treated with antibiotics had a weaker immune system could not be tested in this study and thus cannot be ruled out. However, it is also possible that doctors were more inclined to prescribe an antibiotic to a patient they knew to be more susceptible to infections. Both hypotheses would have to be tested in other suitable data sets or in interventional trials.
In the present study, patients treated with EA 575 had a slightly shorter average duration of sick leave and a slightly lower risk of at least a 7-day-long sick leave compared to patients treated with AB when only patients with any days of sick leave were analyzed. However, when including patients without sick leave (sick leave duration of 0 days), average sick leave duration was somewhat shorter for patients taking AB, and patients taking EA 575 had a slightly higher risk of at least a 7-day-long sick leave.
Both results are biased by the fact that the proportion of patients with any sick leave was substantially larger in the EA 575 cohort (64%) when compared to the AB cohort (50.5%). As sick leave was prescribed during the visit when treatment was initiated, it cannot have been related to treatment efficacy but likely reflects the pretreatment status of the patient at the index visit. Sick leave itself may be a sign of disease severity. In patients without a sick leave prescription, there may either not have been a medical indication for this measure, or they may not have required a sick certificate because they were not employed (e.g. students, self-employed, homemakers, and early pensioners). Assuming the latter reason to be randomly distributed between the EA 575 and AB cohorts, the sick leave rate differences between the two could likely reflect a larger proportion of more severely affected patients in the EA 575 group (even though this could not be tested on the basis of the available data). This is consistent with the observation that, unlike AB use, the majority of EA 575 sales for adults are a result of self-medication.
With regard to sick leave duration, the results of this study are not conclusive as they are likely confounded with the proportions of sick leave prescriptions and, in turn, with severity of illness for which no other data were available for analysis. However, there was also no evidence that AB prescriptions may have led to shorter sick leave when compared to EA 575.
The main strength of this study is the large sample size as no clinical trials to date have included such samples. The second strength is the use of longitudinal data, including both diagnoses and prescriptions, as well as sick leave information. The third strength is the comparison of EA 575 with AB therapy in a matched-pair design, as the application of this method in a treatment comparison is novel. Moreover, in most studies on ivy leaf extract, the control group was given a placebo [Citation22].
However, retrospective primary care database analyses are generally limited by the availability, validity, and completeness of data. Since our study was based on standardized, anonymized information obtained from routine medical records, analyses are necessarily limited to information that can be derived from such records, whereas other potentially interested aspects cannot be analyzed if not documented routinely and systematically: first, assessments are based on ICD codes entered by general practitioners, and some diagnoses may be underreported. Furthermore, some diagnostic codes do not allow for a univocal separation of viral and bacterial infections (for example, acute bronchitis). Second, no diagnosis severity and no information on the recovery date or possible side effects is available, especially in acute diseases (in a naturalistic setting, patients usually do not return to their physician after having recovered and report adverse effects only when in need of a medical intervention). The association between disease severity and AB prescription cannot be ruled out. Third, to buy herbal medicines, which are usually OTC drugs, patients mostly do not need a prescription from physicians. The database does not include data on the use of herbal medicines that patients buy without prescriptions; however, as already mentioned, patients consulting a physician for the treatment of an acute respiratory disorder may on average feel more severely ill than those who exercise self-medication. Fourth, data on socioeconomic status and lifestyle-related risk factors (smoking, alcohol, and physical activity) are not systematically available and can therefore not be analyzed as potential covariates. Fifth, patients can only be observed in a single practice; when they receive a diagnosis or prescription from another physician, such prescriptions are not documented.
Conclusion
Our study provides further evidence that the use of phytopharmaceuticals, specifically ivy leaf dry extract EA 575, could contribute to a reduction in the number of inappropriate antibiotic prescriptions for respiratory infection with cough symptoms. Nevertheless, this article does not claim to replace an individual clinical assessment and is not intended as such. It is very important to identify the cases in which antibiotic prescription could be life-saving. In other cases, the prescription of such evidence-based herbal medicines as EA 575 remains a valuable treatment option.
Disclosure of financial/other conflicts of interest
GS and AV declare that they have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. FL and CS are employees of
Engelhard Arzneimittel GmbH & Co. KG. KK is an employee of IQVIA. The authors have no other relevant conflicts of interest to disclose. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014 Feb 18;186(3):190–199.
- Eckel N, Sarganas G, Wolf IK, et al. Pharmacoepidemiology of common colds and upper respiratory tract infections in children and adolescents in Germany. BMC Pharmacol Toxicol. 2014 Aug 9;15(1):44.
- German College of General Practitioners and Family Physicians, DEGAM-Leitlinie Nr. 11: Akuter und chronischer Husten. DEGAM 2021.
- Altiner A, Bell J, Duerden M, et al. More action, less resistance: report of the 2014 Summit of the Global Respiratory Infection Partnership. Int J Pharm Pract. 2015;23(5):370–377.
- Köchling A, Löffler C, Reinsch S, et al. Reduction of antibiotic prescriptions for acute respiratory tract infections in primary care: a systematic review. Implement Sci. 2018 Mar 20; 13(1):47.
- European Centre for Disease Prevention and Control (ECDC), European Food Safety Authority (EFSA) and European Medicines Agency (EMA). Third joint inter-agency report on integrated analysis of consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals in the EU/EEA, JIACRA III. 2016–2018. Stockholm, Parma, Amsterdam: ECDC, EFSA, EMA; 2021. https://doi.org/10.2900/056892
- Raal A, Volmer D, Sõukand R, Hratkevitš S, and Kalle R. Complementary Treatment of the Common Cold and Flu with Medicinal Plants – Results from Two Samples of Pharmacy Customers in Estonia. PLoS One. 2013; 8(3): e58642.
- Mousa HA-L. Prevention and treatment of influenza, influenza-like illness, and common cold by herbal, complementary, and natural therapies. J Evid Based Complementary Altern Med. 2017;22(1):166–174.
- Lang C, Röttger-Lüer P, Staiger C. A valuable option for the treatment of respiratory diseases: review on the clinical evidence of the ivy leaves dry extract EA 575®. Planta Med. 2015 Aug;81(12/13):968–974.
- Kardos P, Dinh QT, Fuchs KH, Gillissen A, Klimek L, Koehler M, Sitter H, and Worth H. Guidelines of the German Respiratory Society for Diagnosis and Treatment of Adults Suffering from Acute, Subacute and Chronic Cough. Pneumologie. 2019 Mar;73(3):143–180
- Schaefer A, Kehr MS, Giannetti BM, et al. A randomized, controlled, double-blind, multi-center trial to evaluate the efficacy and safety of a liquid containing ivy leaves dry extract (EA 575®) vs. placebo in the treatment of adults with acute cough. Pharmazie. 2016 Sep 1;71(9):504–509.
- Schaefer A, Ludwig F, Giannetti BM, et al. Efficacy of two dosing schemes of a liquid containing EA 575 versus placebo in the treatment of acute bronchitis in adults. ERJ Open Res. 2019 Dec 8;5(4):00019–2019.
- Fazio S, Pouso J, Dolinsky D, Fernandez A, Hernandez M, Clavier G, and Hecker M. Tolerance, safety and efficacy of Hedera helix extract in inflammatory bronchial diseases under clinical practice conditions: a prospective, open, multicentre postmarketing study in 9657 patients. Phytomedicine 2009 Jan;16(1):17–24
- Martin D, Konrad M, Adarkwah CC, et al. Reduced antibiotic use after initial treatment of acute respiratory infections with phytopharmaceuticals – a retrospective cohort study. Postgrad Med. 2020 Jun;132(5):412–418.
- Becher H, Kostev K, Schröder-Bernhardi D. Validity and representativeness of the ”Disease Analyzer” patient database for use in pharmacoepidemiological and pharmacoeconomic studies. Int J Clin Pharmacol Ther. 2009 Oct;47(10):617–626.
- Rathmann W, Bongaerts B, Carius H-J, et al. Basic characteristics and representativeness of the German disease analyzer database. Int J Clin Pharmacol Ther. 2018 Oct;56(10):459–466.
- Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, and Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. American Journal of Epidemiology 2011;173(6):676–682.
- Kern WV, and Kostev K. Prevalence of and Factors Associated with Antibiotic Prescriptions in Patients with Acute Lower and Upper Respiratory Tract Infections-A Case-Control Study. Antibiotics (Basel). 2021 Apr 16;10(4):455
- Zweigner J, Meyer E, Gastmeier P, et al. Rate of antibiotic prescriptions in German outpatient care – are the guidelines followed or are they still exceeded? GMS Hyg Infect Control. 2018;13:Doc04. Published 2018 Mar 13.
- Höller M, Steindl H, Abramov-Sommariva D, Wagenlehner F, Naber KG, and Kostev K. Treatment of Urinary Tract Infections with Canephron® in Germany: A Retrospective Database Analysis. Antibiotics (Basel). 2021 Jun 8;10(6):685
- Benoun JM, Labuda JC, and McSorley SJ. Collateral Damage: Detrimental Effect of Antibiotics on the Development of Protective Immune Memory. mBio. 2016;7(6):e01520–16
- Sierocinski E, Holzinger F, Chenot JF. Ivy leaf (Hedera helix) for acute upper respiratory tract infections: an updated systematic review. Eur J Clin Pharmacol. 2021;77(8):1113–1122.