ABSTRACT
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death in the United States. Elevated low-density lipoprotein cholesterol (LDL-C) is a major causal risk factor for ASCVD. Current evidence overwhelmingly demonstrates that lowering LDL-C reduces the risk of secondary cardiovascular events in patients with previous myocardial infarction or stroke. There is no lower limit for LDL-C: large, randomized studies and meta-analyses have found continuous benefit and no safety concerns in patients achieving LDL-C levels <25 mg/dL. As ‘Time is plaque’ in patients with ASCVD, early, sustained reductions in LDL-C are critical to slow or halt disease progression. However, despite use of lipid-lowering medications, <30% of patients with ASCVD achieve guideline-recommended reductions in LDL-C, resulting in a substantial societal burden of preventable cardiovascular events and early mortality. LDL-C goals are not met due to several factors: lipid-lowering therapy is not initiated and intensified as directed by clinical guidelines (clinical inertia); most patients do not adhere to prescribed medications; and high-risk patients are frequently denied access to add-on therapies by their insurance providers. Promoting patient and clinician education, multidisciplinary collaboration, and other interventions may help to overcome these barriers. Ultimately, achieving population-level guideline-recommended reductions in LDL-C will require a collaborative effort from patients, clinicians, relevant professional societies, drug manufacturers, and payers.
1. Background
Cardiovascular (CV) disease (CVD) remains the leading cause of morbidity and mortality worldwide [Citation1,Citation2]. In the United States, 24 million adults are expected to have coronary heart disease by 2035 [Citation3], and elevated low-density lipoprotein (LDL) cholesterol (LDL-C) is a major contributor to this epidemic [Citation4].
Elevated LDL-C is a major causal risk factor for atherosclerotic cardiovascular disease (ASCVD) [Citation5]. LDL particles can move from plasma into the arterial subendothelial space, contributing to an inflammatory response and formation of atherosclerotic plaques [Citation6]. As atherosclerotic plaques become more inflamed and unstable, they can rupture, promote the formation of overlying thrombus and arterial obstruction, and induce acute ischemia [Citation7].
LDL-C elevation duration and magnitude determines risk for ASCVD. Total atherosclerotic plaque burden is proportional to an individual’s cumulative, lifetime exposure to LDL and other apo B-containing lipoproteins [Citation8]. Elevated LDL-C is also associated with earlier plaque formation, onset of ASCVD, and mortality [Citation4,Citation8,Citation9] ().
Figure 1. Effects of lifetime exposure to LDL-C on ASCVD risk. ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction. [Figure reproduced with permission from Ference BA et al. J Am Coll Cardiol 2018;72:2980–2995 [Citation8] Copyright © 2018, Elsevier].
![Figure 1. Effects of lifetime exposure to LDL-C on ASCVD risk. ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction. [Figure reproduced with permission from Ference BA et al. J Am Coll Cardiol 2018;72:2980–2995 [Citation8] Copyright © 2018, Elsevier].](/cms/asset/36e41088-fc0a-448d-96bb-d81fd5365a6b/ipgm_a_2117498_f0001_b.gif)
Lifestyle changes, including adopting a healthy diet and getting regular physical exercise, are important first steps toward reducing the risk of ASCVD at all ages. However, while these changes are effective, many patients will also require pharmacological treatment [Citation10]. Sustained reductions in LDL-C reduce the risk of CV events and ASCVD [Citation4,Citation11–17]. These results are achieved with statins, a mainstay of hyperlipidemia treatment, and other LDL-C-lowering agents, including ezetimibe and proprotein convertase subtilisin kexin 9 inhibitors (PCSK9i), confirming that reductions in CV events are attributable to reductions in LDL-C [Citation4,Citation18–22]. Larger reductions in LDL-C produce larger reductions in CV risk [Citation23]. For each 38.7 mg (1 millimole) per liter reduction in LDL-C with a statin, patients experience a 22% reduction in CV events over 5 years () [Citation18]. LDL-C is also strongly and linearly associated with the yearly event rate, which establishes LDL-C as the most readily modifiable risk factor for ASCVD [Citation4,Citation24,Citation25].
Figure 2. Effects of statin versus control therapy and more versus less statin on major vascular events. CABG, coronary artery bypass graft; CHD, coronary heart disease; CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; RR, rate ratio. [Figure reproduced with permission from Baigent C et al. Lancet. 2010;376(9753):1670–1681 [Citation18] Copyright © 2010, Elsevier].
![Figure 2. Effects of statin versus control therapy and more versus less statin on major vascular events. CABG, coronary artery bypass graft; CHD, coronary heart disease; CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; RR, rate ratio. [Figure reproduced with permission from Baigent C et al. Lancet. 2010;376(9753):1670–1681 [Citation18] Copyright © 2010, Elsevier].](/cms/asset/8698a3b9-ceb1-419c-b048-72dd76678170/ipgm_a_2117498_f0002_b.gif)
Atherosclerotic plaque progression can be arrested, and might regress, in patients treated with statins who achieve LDL-C < 70 mg/dL [Citation26–28]. Many patients benefit from reductions in LDL-C to as low as 10 mg/dL, with no increase in adverse events (AEs) [Citation29–32]. Importantly, there is no basis to the concern that too low of an LDL-C causes neurocognitive impairment; the brain does not rely on hepatically derived lipoproteins to produce cholesterol [Citation33], and aggressive reduction in LDL-C with combination statin and PCSK9i therapy does not affect brain function [Citation34]. Further, healthy populations have physiologically normal LDL-C (neonates: 30–70 mg/dL; hunter-gatherers: 50–75 mg/dL) [Citation35], and attainment of very low LDL-C is safe and effective in the elderly [Citation36,Citation37]. Overall, maximum, sustained reduction in LDL-C produces the greatest CV risk reduction for secondary prevention patients without increasing risk for AEs. There is therefore growing consensus in favor of maximum, sustained reduction in LDL-C ().
Table 1. Clinical consensus for maximum, sustained reductions in LDL-C.
LDL particles constitute a vascular toxin [Citation4], as they are the end product of lipoprotein metabolism, induce endothelial and macrophage dysfunction, amplify the inflammatory response, and constitute a direct reservoir of lipids used for plaque expansion [Citation41,Citation42]. Consequently, genetic polymorphisms that elevate LDL-C augment risk for ASCVD [Citation4,Citation43]. As LDL particles are not precursors to endogenous cholesterol synthesis [Citation44] and humans do not require a certain quantity of plasma LDL to drive intermediary metabolism [Citation43], reducing LDL-C is the optimal approach to reduce risk for ASCVD [Citation29].
1.1 Objective
There is a substantial unmet need worldwide to provide secondary prevention patients with the best possible risk reduction through maximum, sustained reductions in LDL-C. Patient outcomes can be improved through appropriate risk assessment and therapy selection based on patient characteristics and preferences. This review summarizes the current state of LDL-C management for secondary prevention in the United States, identifying barriers to optimal lipid management, and proposing practical solutions to continue improving clinical practice (). Due to its societal heterogeneity, the US patient population may to some extent be representative of a global patient population and some of the considerations in this review may extend beyond the United States.
Table 2. Barriers and strategies to achieving target, sustained LDL-C reductions.
2. Nonattainment of target LDL-C reduction: the scale of the problem
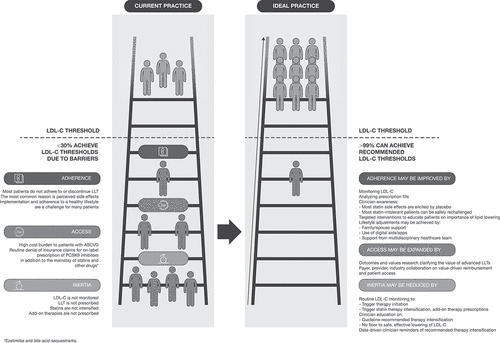
Most secondary prevention patients do not achieve guideline-recommended LDL-C [Citation45–52] and a recent retrospective cohort study showed that approximately 50% of patients with established ASCVD are not on any statin [Citation53]. Recent studies have found that 72 to 84% of adults with ASCVD have LDL-C ≥ 70 mg/dL [Citation45,Citation51]. However, in patients treated on lipid-lowering therapy (LLT), LDL-C < 70 mg/dL was achieved by only 21% of patients with baseline LDL-C ≥ 100 mg/dL and 34% of patients with baseline LDL-C 70–99 mg/dL [Citation52]. Achievement of LDL-C < 70 mg/dL was greatest in patients who were White or managed by a cardiologist (both: 30%) [Citation52]. Real-world studies have also found that <30% actually achieve that target [Citation45–47,Citation51]. There is therefore a substantial opportunity to help patients achieve guideline-recommended LDL-C through use of LLT.
Patients may not achieve target LDL-C due to lack of prescription for LLT. Data suggest that only 64% of patients with ASCVD take a statin [Citation51], and 53% of secondary prevention patients have no history of LLT [Citation45]. A report from the Patient and Provider Assessment of Lipid Management (PALM) registry found higher rates of statin use, with only around 18% of secondary prevention patients without diabetes not using a statin [Citation54]. However, 37% of patients in this study had no cholesterol monitoring in the previous 2 years, providing a possible reason for why some patients were not prescribed LLT [Citation54].
Most secondary prevention patients are not prescribed optimal doses of LLT. In real-world and registry analyses, only 35 to 42% of eligible patients were prescribed a high-intensity statin or statin type consistent with guideline recommendations [Citation55,Citation56]. Further, among patients with ASCVD and LDL-C ≥ 100 mg/dL, only 22% had LLT intensified at 2 years of follow-up; 6.4% had their statin intensified, 6.8% had ezetimibe added, and 6.3% had a PCSK9i added [Citation52].
Even if a patient is prescribed an appropriate dose of LLT, they may not take the medication as prescribed. For example, one study found that 57% of patients were not adherent to LLT within 6 months of experiencing a myocardial infarction (MI) [Citation57]. Another study showed that <25% of patients who started on statins were still taking them after 5 years [Citation58]. Low adherence to LLT is associated with an increased risk of morbidity and mortality [Citation57].
LLT is essential to achieving maximum, sustained reductions in LDL-C and reducing the risk of CV events in secondary prevention patients. Clinical inertia, medication adherence, and access to therapy represent critical barriers to achieving maximum, sustained reductions in LDL-C [Citation59–61]. Implementing strategies to overcome these barriers is essential in reversing the increasing trajectory of mortality due to ASCVD ().
3. Barriers and strategies to achieving sustained target LDL-C reductions ()
3.1 Barrier #1: clinical inertia
Effective treatment of hyperlipidemia requires clinicians to recognize and understand guideline-recommended targets for LDL-C. After LLT is initiated, LDL-C levels should be checked routinely and LLT intensified until LDL-C falls below guideline-recommended targets (i.e. 70 mg/dL) [Citation10,Citation62,Citation63].
Clinicians may not appropriately initiate or intensify treatment due to a variety of factors, including clinical inertia and time constraints in clinical encounters. Clinical inertia occurs when a clinician is aware that their patient’s LDL-C is elevated but does not initiate or intensify treatment [Citation59]. The time involved in counseling, administration, and evaluation of drug-drug interactions is a barrier to medication initiation or dose optimization, and the more complicated the patient visit, the less likely it is that a therapy will be initiated or adjusted [Citation64].
Clinicians may overestimate their adherence to guidelines or their ability to correctly identify patients who require treatment initiation or intensification [Citation59]. Clinicians may also avoid intensifying therapy because of perceptions that LDL-C is improving with current therapy, patient desire to reduce LDL-C with lifestyle changes versus LLT, or patient perception that intensifying therapy will result in increased AEs [Citation59]. Concerns regarding treatment versus risk become pronounced for elderly patients with comorbidities. A recent study found that less than half of adults aged >75 years were prescribed even moderate-intensity statins [Citation65]. Frequent guideline changes and inconsistencies between different lipid guidelines may further hinder LLT changes in routine clinical practice [Citation66]. This pattern is especially true with cerebrovascular or peripheral artery diseases [Citation67], and it impacts clinicians who manage health maintenance and/or other patient concerns during limited clinical encounters.
Clinical inertia is a pervasive problem in ASCVD. A recent study found that, among secondary prevention patients without target LDL-C or use of PCSK9i, only 44% were prescribed a high-intensity statin, and only 9% were prescribed ezetimibe, despite guidelines recommending use of both therapies [Citation10,Citation62,Citation68]. In addition, real-world data have shown that 48% of patients are not treated according to guidelines for statin therapy, further confirming that not using recommended high-intensity therapy in patients with ASCVD is a widespread problem [Citation69]. Importantly, recent results from the PALM registry found that 59% of patients not on a statin had never been offered one, confirming that clinical inertia, rather than therapy discontinuation or patient resistance to therapy intensification, underlies under-treatment in a substantial percentage of cases [Citation60].
LLT is also not intensified when indicated. A retrospective study of patients with coronary heart disease (or a risk equivalent) found that only 25.3% of managed care organizations up-titrated statin therapy and only 4.5% added ezetimibe [Citation70]. In the same dataset, 24% of patients with elevated LDL-C who were prescribed a high-intensity statin and concomitant ezetimibe achieved LDL-C < 70 mg/dL, while only 3.6% of patients prescribed statin monotherapy without up-titration did so, demonstrating that more appropriate intensification of LLT would have improved LDL-C threshold attainment and outcomes [Citation70]. Importantly, while guidelines recommend regular lipid monitoring to identify patients who are not meeting recommended lipid thresholds and need therapy intensification, data from the PALM registry indicate that one-third of secondary prevention patients or patients at high risk of ASCVD are being treated without provider knowledge of LDL-C levels in the previous 2 years [Citation54]. This evidence indicates that providers are failing to intensify LLT, and in a substantial portion of cases, this occurs because LDL-C is not being routinely monitored.
3.2 Solutions to clinical inertia
Multiple interventions have been proposed to overcome clinical inertia. Emphasizing routine monitoring of LDL-C in secondary prevention practices may be a critical first step to prompting appropriate therapy intensification when guideline-directed LDL-C levels have not been met using statin monotherapy. In addition, several intervention studies have attempted to combat clinical inertia by implementing clinical decision support systems, which issue electronic reminders to clinicians when they do not initiate or intensify LLT in eligible patients [Citation71–73]. However, in studies to date, such systems did not significantly improve statin prescribing [Citation71,Citation72]. Other strategies for combating inertia could include more frequent LDL-C monitoring, and polypill approaches to reduce the burden on clinicians of managing multiple comorbidities. Use of a polypill combining LLT and antihypertensive medication (with or without aspirin) has been shown to improve medication adherence and produce significantly greater reductions in LDL-C versus usual care [Citation74,Citation75].
Overcoming inertia will require a substantial investment from clinicians and health systems in order to improve the management of patients with ASCVD. It can be difficult to educate patients on the benefits of proactively managing lipids, especially when there is an urgent need to manage comorbidities. However, using treatment algorithms to support decision-making around therapy intensification could enable up to 99% of patients to meet guideline-recommended LDL-C. Continued development of strategies to combat clinical inertia and clinician support of available support systems are essential to achieve maximum, sustained LDL-C reductions in patients with ASCVD.
3.3 Barrier #2: Adherence to LLT
Lifestyle management is a mainstay intervention to decrease blood lipid concentrations and the risk of ASCVD. A diet rich in fruits, vegetables, and healthy protein sources and low in red meat and sugar is recommended, along with smoking and alcohol cessation and an appropriate calorie intake to avoid weight gain or promote weight loss in obese patients. Implementation of and adherence to a healthy lifestyle along with adjunct pharmacological treatment with LLT are the recommended strategies for the secondary prevention of ASCVD [Citation76].
Nonadherence to LLT represents another critical barrier to achieving maximum, sustained reductions in LDL-C. Most patients with ASCVD do not adhere to daily LLT and nonadherence increases rates of major adverse CV events and mortality [Citation57,Citation77].
Common lipid-lowering regimens, including statins and ezetimibe, require patients to take daily oral medication. LLT adherence has been studied by using data on medication refill dates to estimate the proportion of days patients could have taken their medication. This is usually reported as a medication possession ratio (MPR) or as proportion of days covered (PDC): both metrics are defined by the number of doses dispensed in relation to the number of days in a dispensing period and typically result in a percentage between 0% and 100%, where 100% represents perfect adherence [Citation78,Citation79].
While these metrics are imperfect, they can be used to roughly estimate whether patients are adhering to prescribed therapies [Citation57,Citation78,Citation80]. For example, one study, which followed 4,015 patients who had experienced an MI for 6 months and measured their adherence to LLT and hypertensive medications, found that 57% of patients had PDC <80% and 26% had PDC <40% [Citation57]. Similar results were reported for patients with coronary, cerebrovascular, or peripheral artery disease or revascularization: over 12 months, 66% of patients had PDC <80% and 28% had PDC <40% [Citation57]. An independent study which tracked statin adherence in Medicare beneficiaries who had experienced an MI found that only 42% were taking a high-intensity statin with high adherence (PDC >80%) 2 years after discharge [Citation80].
In the post-MI cohort described above, fully adherent patients (PDC ≥80%) experienced fewer major adverse CV events over the follow-up period than those who were partially adherent, with similar results in the ASCVD cohort [Citation57]. An independent study in the US Veterans Affairs health system also found that patients who were most adherent to statins (MPR ≥90%) experienced lower rates of all-cause mortality than those who were least adherent [Citation81]. These data suggest that low adherence is a substantial contributor to ASCVD mortality in secondary prevention populations.
Another factor that contributes to increased ASCVD risk in secondary prevention populations is statin intolerance. Among Medicare beneficiaries who started a statin at a moderate- or high-intensity dosage after hospitalization for MI, the incidence of statin intolerance was low (1.7%). However, statin intolerance was associated with a 36% higher rate of recurrent MI and a 43% higher rate of coronary heart disease events compared with patients with high statin adherence [Citation82].
Patients also often discontinue LLT altogether [Citation80,Citation83]. In the cohort of post-MI Medicare beneficiaries described above, 12% had discontinued statin therapy by 6 months after an MI and 19% had discontinued by 2 years after an MI [Citation80]. A similar study used the MarketScan Research databases to track persistence of statin use in a cohort of 7,802 patients following hospitalization for an acute coronary syndrome (94.6% were diagnosed with an MI) [Citation83]. It found that only 77.8% of patients filled any outpatient statin prescription and 26.5% had discontinued filling their statin prescription within 1 year of hospital discharge [Citation83].
3.4 Solutions to increase adherence to LLT
Implementation and maintenance of a healthy lifestyle could be promoted by family and partner involvement, multidisciplinary healthcare professionals support, improved patient-centered communication, programs that offer multimodal behavioral interventions (e.g. physical activity, nutrition, smoking cessation), and the use of digital aids [Citation76].
Several studies across diverse health systems have identified patient groups at highest risk of low adherence to and discontinuation of LLT.
Among patients with ASCVD who are younger (aged 18–64 years), are female, are African American, and have more comorbidities are less likely to be adherent and persistent in using LLT and less likely to achieve LDL-C < 70 mg/dL than other groups [Citation45,Citation50,Citation84–88]. Distrust of clinicians and the healthcare system, related to past abuses, is likely to be a factor in low adherence in Black communities [Citation89–91]. Structural and socioeconomic factors, including poverty, low education, and geographic distance from a treatment center are also associated with reduced adherence to LLT and antihypertensive therapy [Citation87]. Comorbid disease requiring multiple medications, mental health disorders and cognitive impairment, lack of support during changes to medication regimen, first-time statin use, and prescribing by primary care clinics or non-cardiology specialty have also been associated with low adherence, suggesting that patients who take multiple medications may be less adherent because they do not understand the importance of LLT [Citation87,Citation92–94]. These data suggest that the most complex and underserved patients are most likely to be nonadherent to LLT, underscoring the importance of education and support for these groups.
Understanding patients’ self-reported reasons for discontinuing LLT is critical to creating effective interventions to support adherence and persistence. Among 617 patients in the PALM registry who had declined or discontinued a statin, the most common reasons were fear of side effects (37%) and experiencing perceived side effects (60%) [Citation60]. Patients who had declined or discontinued a statin were less likely than current statin users to believe that statins were safe (70% of current users versus 36% and 37% of patients who had declined or discontinued a statin, respectively) [Citation60]. Other common reasons for discontinuing statins reported by secondary prevention patients included a preference for diet and exercise (13%) or natural remedies (6%), a dislike of taking medication (7%), and a belief that the statin is not needed at all (22%) or is no longer needed (14%) [Citation60]. Likewise, a survey of US primary care clinicians found that side effects, especially symptoms related to muscles and joints, were the most common reason patients reported for discontinuing statins (47% of votes) [Citation61]. Similar results were found in an analysis of data from the IMPROVE-IT trial, comparing ezetimibe with placebo in secondary prevention patients also taking simvastatin: 13.9% of patients across both arms discontinued treatment within 1 year and perceived statin-related muscle symptoms (11.1%), along with drug-related AEs (19%) and non-drug-related AEs (16.2%), were the most commonly cited reasons for discontinuation [Citation95].
Importantly, objectively confirmed serious muscle-related events are exceedingly rare in statin clinical trials: only 0.6% of patients in the IMPROVE-IT trial experienced a serious muscle-related event, including rhabdomyolysis, myopathy, or myalgia with creatine kinase elevation, within 30 days of taking medication [Citation95]. A recent study challenged patients who had discontinued statin therapy due to perceived side effects with a random sequence of statin, placebo, and no treatment: it found that 90% of the symptom burden elicited by a statin challenge was also elicited by placebo [Citation96]. These data strongly suggest that the majority of patient-reported muscle symptoms are not related to statin therapy (the so-called ‘nocebo effect’), and a large majority of patients who discontinue LLT could and should be safely re-challenged with a statin at a lower dose or a different statin.
One strategy to improve adherence is to improve patient education by offering plain-language, patient-centric communications, such as follow-up motivational interviews, on the urgency to achieve recommended LDL-C thresholds in order to prevent future ASCVD events [Citation97]. Patient education could serve to reduce patient resistance to intensifying LLT and might also correct clinical inertia by prompting patients to initiate conversations about lowering LDL-C and therapy options.
Another simple and potentially highly effective approach to improve adherence to LLT is to ensure secondary prevention patients receive regular lipid monitoring, as recommended by guidelines, and to initiate discussions about adherence to LLT with patients whose LDL-C has increased or remains elevated [Citation10,Citation62,Citation63]. Approximately 50% of patients who have discontinued a statin report willingness to reinitiate, so identification of these patients via LDL-C testing, and provider education on perceived side effects and the importance of LLT, may make a substantial difference in outcomes for many patients [Citation60,Citation96].
Other strategies to promote adherence to LLT have been made possible by the advent of health information technology and electronic medical records (EMRs), which have enabled the creation of automated systems that can track individual patients and identify gaps in care, such as elevated lipid levels and unfilled prescriptions for LLT [Citation98]. Once nonadherent patients have been identified, alerts to intervene can be embedded in the EMR [Citation71]. Interventions can also be undertaken in which patients are contacted by providers, potentially including nurses or pharmacists, in order to better understand any barriers to adherence, educate patients, and encourage improved adherence to LLT. A recent study tracked medication adherence (measured by PDC) in a cohort of Medicare Advantage patients: nonadherent patients were randomized to receive phone-based motivational interviews or no intervention [Citation97]. Interviews significantly improved PDC and reduced discontinuation [Citation97]. Such approaches could begin to rebuild trusting provider–patient relationships and could be a springboard to improve multiple aspects of health. This approach would be time- and resource-intensive to implement at scale. However, given that fully adherent patients experience dramatically lower rates of major adverse CV events and concomitant healthcare costs, intensive interventions to improve adherence are likely justified in terms of improving patient outcomes and reducing the use of healthcare resources [Citation57].
Importantly, other strategies to promote adherence, including financial incentives, do not appear to improve LDL-C levels in patients with ASCVD. These data suggest that adherence may not be the most important target in clinical trials or practice in this patient population [Citation99].
3.5 Barrier #3: limited access
Patients may be unable to achieve guideline-recommended LDL-C because of financial barriers to access add-on therapies (i.e. PCSK9i) and specialized care. Patients seen by cardiologists and lipidologists are more likely to be prescribed intensified statins [Citation100] and receive payer approval for PCSK9i [Citation101]; therefore, access to these specialists is critical for many patients in order to achieve guideline-recommended LDL-C. A nationally representative survey of 22,521 US adults with ASCVD in the Medical Expenditure Panel Survey from the Agency of Healthcare Research and Quality found that the mean individual out-of-pocket health expenditures of patients with ASCVD were $2,227 per year, including $722 in insurance premiums and $1,505 in other health expenditures, with medication costs representing 45% of direct spending [Citation102]. Among families with a member who has ASCVD, 13.7% experienced high financial burden (medical expenses exceeding 20% of post-subsistence income), especially among low-income families (7% versus 25% experiencing high financial burden; odds ratio 4.42) [Citation102]. In the US National Health Interview Survey, 12.6% of adults with ASCVD experienced cost-related nonadherence, including missing doses (8.6%), taking lower than prescribed doses (8.8%), and delaying a medication refill in order to save money (10.5%) [Citation103]. Therefore, lack of access to care may also underlie issues with adherence and clinical inertia.
The approval of LLT-targeting PCSK9i has enabled patients with far from optimal baseline LDL-C to reach guideline-recommended thresholds but has also highlighted problems with ensuring access to optimal LLT. In CVD outcome trials, use of PCSK9i has resulted in substantial reductions in a composite of CV death and major adverse CV events [Citation22,Citation104,Citation105]. Wide access to these treatments has the potential to transform ASCVD management, allowing up to 99% of patients to cross guideline-recommended lipid thresholds and producing corresponding reductions in CV events and mortality [Citation47].
However, despite regulatory approval and the incorporation of PCSK9i into treatment guidelines as an adjunct to diet and maximally tolerated statin therapy, real-world uptake has been poor, with a recent analysis of a large EMR database indicating that 99.5% of patients with LDL-C ≥ 70 mg/dL and eligibility for PCSK9i are not receiving a prescription [Citation106]. The initial list price of PCSK9i was approximately $14,000 per year, and pharmacy claims datasets suggest that large insurers responded to limit drug costs by denying up to 80% of on-label claims for PCSK9i, with final approval rates between 25% and 65% [Citation101,Citation107,Citation108]. In practice, this has resulted in reservation of PCSK9i prescriptions for small numbers of patients in the highest CV risk categories and with higher baseline LDL-C [Citation106]. A recent study using a large insurance claims dataset found that patients with denied or abandoned PCSK9i claims experienced an increased risk of CV events, demonstrating that lack of access to PCSK9i is adversely affecting real-world outcomes [Citation109].
3.6 Solutions to limited access
Overcoming access barriers will require collaboration between clinicians, systems of care, payers, professional and advocacy organizations, and pharmaceutical companies. Pharmaceutical companies, professional organizations, and clinicians can collaborate to develop robust real-world health economics and outcomes evidence demonstrating the value of advanced LLTs in terms of reduced hospitalization and healthcare expenditures. In turn, streamlined payer drug authorization and eliminating barriers such as clinician processing fees for appealing claim decisions, which disincentivize the on-label guideline-directed prescription of needed therapies to high-risk patients, will improve access [Citation107]. Pharmaceutical companies, payers, and systems of care should collaborate on pricing and access models that reward innovation and are sustainable for payers and systems, providing maximum value in terms of improved outcomes for patients. By using this process and sustainably maximizing access to existing therapies, we can enable up to 99% of patients to achieve recommended lipid thresholds and dramatically reduce the incidence of ASCVD events [Citation57].
4. Evolving treatment landscape in ASCVD
New pharmacologic interventions may help to address barriers to maximum, sustained reductions in LDL-C in patients with ASCVD. Monoclonal antibodies that block PCSK9, for example, can reduce LDL-C and the incidence of major adverse CV events [Citation22,Citation29,Citation104,Citation110]. Small-interfering RNAs, such as inclisiran, may build on the LDL-C-lowering effects of PCSK9i by halting transcription of PCSK9 and increasing LDL receptor density in hepatocytes [Citation111]. In phase 3 randomized controlled trials, inclisiran, when administered subcutaneously every 6 months, significantly reduced LDL-C compared to placebo. AEs were similar between the inclisiran and placebo groups, but injection-site reactions were more frequent with inclisiran [Citation112]. Inclisiran was therefore recently approved by the United States Food and Drug Administration as an adjunct to diet and maximally tolerated statin therapy for the treatment of heterozygous familial hypercholesterolemia or ASCVD in adults who require additional lowering of LDL-C [Citation113]. Along with demonstrating efficacy and safety in these patient populations, inclisiran may offer a convenient patient adherence advantage over other LLTs, as it has a simple twice-yearly (versus daily) dosing regimen [Citation111]. Studies (NCT05030428; NCT03705234) to determine the effects of inclisiran on CV morbidity and mortality are ongoing [Citation111,Citation113]. Access barriers to inclisiran remain unknown.
5. Conclusion
In summary, achieving maximum, sustained reductions in LDL-C is central to reducing CV events and mortality in secondary prevention patients with ASCVD. Achieving LDL-C < 70 mg/dL provides reduction in CV risk without offsetting AEs, with no known threshold below which further LDL-C reduction is unsafe or ineffective. However, in real-world practice, achieving maximum, sustained reductions in LDL-C is hampered by clinical inertia, low medication adherence, and lack of access. To overcome these three barriers, we propose the following strategies:
Clinical inertia can be reduced by routine LDL-C monitoring and embracing new developments in health information technology, such as clinical decision support systems.
Adherence can be improved through patient education, rechallenging patients who discontinue therapy, health information technology-assisted monitoring of adherence, and the development of interventions to support adherence and rebuild trusting patient–provider relationships for high-risk patients.
Access issues can be overcome through collaboration between healthcare delivery stakeholders to generate evidence on the value of advanced treatments and develop sustainable models for medical access.
Although optimizing current LLT could enable the majority of patients to achieve LDL-C thresholds, there are still unmet needs in the lipid-lowering space that could be addressed by new therapies. Highly effective LLT, which does not require repeated dose changes, and add-on therapies could be excellent tools to combat inertia. Likewise, LLT with infrequent, in-office dosing could overcome issues with adherence, and highly effective therapies with a sustainable model for ensuring access could overcome existing issues with access to advanced LLT.
6. Summary of key concepts
Elevated LDL-C levels are a root cause of ASCVD events
The greatest reduction in ASCVD risk is associated with the greatest absolute reduction in LDL-C, and there is no known level below which further LDL-C reduction is not safe and effective
In secondary prevention, lowering LDL-C as far as possible produces the strongest benefits
Extensive studies have found no major safety issues with very low LDL-C
Aggressive treatment following guideline-recommended treatment options improves patient outcomes, regardless of how close or far away patients are to recommended thresholds
Barriers to LDL-C achievement include inertia, adherence, and access
Inertia could be overcome through clinician education
Nonadherence could be overcome through patient follow-up and intervention
Access barriers could be overcome through outcomes research and payer reform
Optimized, personalized LDL-C treatment based on appropriate risk assessment can achieve overwhelming improvements in prevention and community health
Abbreviation list
Acknowledgments
Joseph Kruempel, PhD, Brittany Y. Jarrett, PhD, and Rachel Fairbanks, BA (Hons), of Complete HealthVizion, a McCann Health Medical Communications company, provided medical writing and submission support with this manuscript, based on detailed discussion and feedback from all the authors and funded by Novartis Pharmaceuticals Corporation.
Disclosure statement
J. Underberg: Consulting fees - Amgen; Advisory Boards- Amgen, Novartis, Pfizer; Speaker Fees- Amgen, Regeneron, Esperion, Novartis , Ambry; Contracted Research- Amryt
P.P. Toth: speakers bureau for Amarin, Amgen, Esperion, and Novo Nordisk; consultant for Amarin, Amgen, bio89, Kowa, Resverlogix, and Theravance.
F. Rodriguez: consulting fees from Amgen (advisory board), HealthPals, Novo Nordisk (CEC), and Novartis.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep. 2019;68:1–77.
- Herrington W, Lacey B, Sherliker P, et al. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118(4):535–546.
- Nelson S, Whitsel L, Khavjou O, et al. Projections of cardiovascular disease prevalence and costs: 2015–2035. Technical report. 2016.
- Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472.
- Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808.
- Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5(1):56.
- Bentzon JF, Otsuka F, Virmani R, et al. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–1866.
- Ference BA, Graham I, Tokgozoglu L, et al. Reprint of: impact of lipids on cardiovascular health: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(23, Part B):2980–2995.
- Abdullah SM, Defina LF, Leonard D, et al. Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease: results from the Cooper Center Longitudinal Study. Circulation. 2018;138(21):2315–2325.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143.
- Di Angelantonio E, Gao P, Pennells L, Emerging Risk Factors Collaboration. Lipid-related markers and cardiovascular disease prediction. JAMA. 2012;307(23):2499–2506.
- Prospective Studies Collaboration. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet. 2007;370(9602):1829–1839.
- Cohen JC, Boerwinkle E, Mosley TH, et al. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–1272.
- Nikpay N, Goel A, Won HH, et al. CARDIoGRAMplusC4D Consortium. A comprehensive 1000 Genomes–based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47(10):1121–1130. https://www.nature.com/articles/ng.3396#cite
- Ference BA, Yoo W, Alesh I, et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol. 2012;60(25):2631–2639.
- Linsel-Nitschke P, Götz A, Erdmann J, et al. Lifelong reduction of LDL-cholesterol related to a common variant in the LDL-receptor gene decreases the risk of coronary artery disease—a Mendelian randomisation study. PLoS One. 2008;3(8):e2986.
- Holmes MV, Asselbergs FW, Palmer TM, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36(9):539–550.
- Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681.
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–2397.
- Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192.
- Sabatine MS, Giugliano RP, Keech A, et al. Rationale and design of the further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk trial. Am Heart J. 2016;173:94–101.
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722.
- Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289–1297.
- Boekholdt SM, Arsenault BJ, Mora S, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA. 2012;307(12):1302–1309.
- Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64(5):485–494.
- Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295(13):1556–1565.
- Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078–2087.
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316(22):2373–2384.
- Sabatine MS, Wiviott SD, Im K, et al. Efficacy and safety of further lowering of low-density lipoprotein cholesterol in patients starting with very low levels: a meta-analysis. JAMA Cardiol. 2018;3(9):823–828.
- Robinson JG, Rosenson RS, Farnier M, et al. Safety of very low low-density lipoprotein cholesterol levels with alirocumab: pooled data from randomized trials. J Am Coll Cardiol. 2017;69(5):471–482.
- Giugliano RP, Wiviott SD, Blazing MA, et al. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT trial. JAMA Cardiol. 2017;2(5):547–555.
- Giugliano RP, Pedersen TR, Park JG, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the Fourier trial. Lancet. 2017;390(10106):1962–1971.
- Gliozzi M, Musolino V, Bosco F, et al. Cholesterol homeostasis: researching a dialogue between the brain and peripheral tissues. Pharmacol Res. 2021;163:105215.
- Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377(7):633–643.
- O’Keefe JH Jr., Cordain L, Harris WH, et al. Optimal low-density lipoprotein is 50 to 70 mg/dl: lower is better and physiologically normal. J Am Coll Cardiol. 2004;43(11):2142–2146.
- Samaras K, Makkar SR, Crawford JD, et al. Effects of statins on memory, cognition, and brain volume in the elderly. J Am Coll Cardiol. 2019;74(21):2554–2568.
- Gencer B, Marston NA, Im K, et al. Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2020;396(10263):1637–1643.
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):1376–1414.
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020;42(14):1289–1367.
- Handelsman Y, Jellinger PS, Guerin CK, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the management of dyslipidemia and prevention of cardiovascular disease algorithm - 2020 Executive Summary. Endocr Pract. 2020;26(10):1196–1224.
- Linton MF, Yancey PG, Davies SS, et al. The role of lipids and lipoproteins in atherosclerosis. In: Feingold KR , editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc; 2000.
- Dominiczak MH, Caslake MJ. Apolipoproteins: metabolic role and clinical biochemistry applications. Ann Clin Biochem. 2011;48(Pt 6):498–515.
- Olsson AG, Angelin B, Assmann G, et al. Can LDL cholesterol be too low? Possible risks of extremely low levels. J Intern Med. 2017;281(6):534–553.
- Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21(4):225–245.
- Allen JM, Arnold SV, Lohr NL, et al. Assessing low-density lipoprotein cholesterol risk in secondary prevention patients within the PINNACLE national outpatient registry. Circulation. 2019;140:A12904.
- Menzin J, Aggarwal J, Boatman B, et al. Ezetimibe use and LDL-C goal achievement: a retrospective database analysis of patients with clinical atherosclerotic cardiovascular disease or probable heterozygous familial hypercholesterolemia. J Manag Care Spec Pharm. 2017;23(12):1270–1276.
- Cannon CP, Khan I, Klimchak AC, et al. Simulation of lipid-lowering therapy intensification in a population with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2(9):959–966.
- Gitt AK, Lautsch D, Ferrières J, et al. Contemporary data on treatment practices for low-density lipoprotein cholesterol in 6794 patients with stable coronary heart disease across the world. Data Brief. 2018;18:1937–1940.
- Morales C, Plana N, Arnau A, et al. Causes of failure to achieve the low density lipoprotein cholesterol therapeutic target in patients with high and very high vascular risk controlled in lipid and vascular risk units. EROMOT study. Clin Investig Arterioscler. 2018;30(1):1–9.
- Bruckert E, Parhofer KG, Gonzalez-Juanatey JR, et al. Proportion of high-risk/very high-risk patients in Europe with low-density lipoprotein cholesterol at target according to European guidelines: a systematic review. Adv Ther. 2020;37:1724–1736.
- Wong ND, et al. Prevalence of the American college of Cardiology/American Heart Association statin eligibility groups, statin use, and low-density lipoprotein cholesterol control in US adults using the National Health and Nutrition Examination Survey 2011–2012. J Clin Lipidol. 2016;10(5):1109–1118.
- Cannon CP, De Lemos JA, Rosenson RS, et al. Use of lipid-lowering therapies over 2 years in GOULD, a registry of patients with atherosclerotic cardiovascular disease in the US. JAMA Cardiol. 2021;6(9):1–9.
- Nelson AJ, Haynes K, Shambhu S, et al. High-intensity statin use among patients with atherosclerosis in the U.S. J Am Coll Cardiol. 2022;79(18):1802–1813.
- Lowenstern AM, Li S, Navar AM, et al. Measurement of low-density lipoprotein cholesterol levels in primary and secondary prevention patients: insights from the PALM registry. J Am Heart Assoc. 2018;7(18):e009251.
- Rodriguez F, Lin S, Maron DJ, et al. Use of high-intensity statins for patients with atherosclerotic cardiovascular disease in the Veterans Affairs Health System: practice impact of the new cholesterol guidelines. Am Heart J. 2016;182:97–102.
- Navar AM, Wang TY, Li S, et al. Lipid management in contemporary community practice: results from the Provider assessment of lipid management (PALM) registry. Am Heart J. 2017;193:84–92.
- Bansilal S, Castellano JM, Garrido E, et al. Assessing the impact of medication adherence on long-term cardiovascular outcomes. J Am Coll Cardiol. 2016;68(8):789–801.
- Toth PP, Granowitz C, Hull M, et al. Long-term statin persistence is poor among high-risk patients with dyslipidemia: a real-world administrative claims analysis. Lipids Health Dis. 2019;18(1):175.
- Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–834.
- Bradley CK, Wang TY, Li S, et al. Patient‐reported reasons for declining or discontinuing statin therapy: insights from the PALM registry. J Am Heart Assoc. 2019;8(7):e011765.
- Tanner RM, Safford MM, Monda KL, et al. Primary care physician perspectives on barriers to statin treatment. Cardiovasc Drugs Ther. 2017;31(3):303–309.
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41(1):111–188.
- Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J. 2020;41(2):255–323.
- Parchman ML, Pugh JA, Romero RL, et al. Competing demands or clinical inertia: the case of elevated glycosylated hemoglobin. Ann Fam Med. 2007;5(3):196–201.
- Spencer-Bonilla G, Chung S, Sarraju A, et al. Statin use in older adults with stable atherosclerotic cardiovascular disease. J Am Geriatr Soc. 2021;69(4):979–985.
- Zullig LL, Egbuonu-Davis L, Trasy A, et al. Countering clinical inertia in lipid management: expert workshop summary. Am Heart J. 2018;206:24–29.
- Yao X, Shah ND, Gersh BJ, et al. Assessment of trends in statin therapy for secondary prevention of atherosclerotic cardiovascular disease in US adults from 2007 to 2016. JAMA Network Open. 2020;3(11):e2025505.
- Cannon CP, de Lemos JA, Rosenson RS, et al. Getting to an improved understanding of low-density lipoprotein-cholesterol and dyslipidemia management (GOULD): methods and baseline data of a registry of high cardiovascular risk patients in the United States. Am Heart J. 2020;219:70–77.
- Ramsaran E, Preusse P, Sundaresan D, et al. Adherence to blood cholesterol treatment guidelines among physicians managing patients with atherosclerotic cardiovascular disease. Am J Cardiol. 2019;124(2):169–175.
- Toth PP, Foody JM, Tomassini JE, et al. Therapeutic practice patterns related to statin potency and ezetimibe/simvastatin combination therapies in lowering LDL-C in patients with high-risk cardiovascular disease. J Clin Lipidol. 2014;8(1):107–116.
- Adusumalli S, Westover JE, Jacoby DS, et al. Effect of passive choice and active choice interventions in the electronic health record to cardiologists on statin prescribing: a cluster randomized clinical trial. JAMA Cardiol. 2021;6(1):40–48.
- Maddox TM. Clinical decision support in statin prescription—what we can learn from a negative outcome. JAMA Cardiol. 2021;6(1):48–49.
- Langer A, Tan M, Goodman SG, et al. GOAL Canada: physician education and support can improve patient management. CJC Open. 2020;2(2):49–54.
- Muñoz D, Uzoije P, Reynolds C, et al. Polypill for cardiovascular disease prevention in an underserved population. N Engl J Med. 2019;381(12):1114–1123.
- Bramlage P, Sims H, Minguet J, et al. The polypill: an effective approach to increasing adherence and reducing cardiovascular event risk. Eur J Prev Cardiol. 2017;24(3):297–310.
- van Trier TJ, Mohammadnia N, Snaterse M, et al. Lifestyle management to prevent atherosclerotic cardiovascular disease: evidence and challenges. Neth Heart J. 2022;30(1):3–14.
- Guglielmi V, Bellia A, Pecchioli S, et al. Effectiveness of adherence to lipid lowering therapy on LDL-cholesterol in patients with very high cardiovascular risk: a real-world evidence study in primary care. Atherosclerosis. 2017;263:36–41.
- De Vera MA, Bhole V, Burns LC, et al. Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol. 2014;78(4):684–698.
- Maningat P, Gordon BR, Breslow JL. How do we improve patient compliance and adherence to long-term statin therapy? Curr Atheroscler Rep. 2013;15(1):291.
- Colantonio LD, Huang L, Monda KL, et al. Adherence to high-intensity statins following a myocardial infarction hospitalization among Medicare beneficiaries. JAMA Cardiol. 2017;2(8):890–895.
- Rodriguez F, Maron DJ, Knowles JW, et al. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4(3):206–213.
- Serban M-C, Colantonio LD, Manthripragada AD, et al. Statin intolerance and risk of coronary heart events and all-cause mortality following myocardial infarction. J Am Coll Cardiol. 2017;69(11):1386–1395.
- Boklage SH, Malangone-Monaco E, Lopez-Gonzalez L, et al. Statin utilization patterns and outcomes for patients with acute coronary syndrome during and following inpatient admissions. Cardiovasc Drugs Ther. 2018;32(3):273–280.
- Newman JD, Alexander KP, Gu X, et al. Baseline predictors of low-density lipoprotein cholesterol and systolic blood pressure goal attainment after 1 year in the ISCHEMIA trial. Circ Cardiovasc Qual Outcomes. 2019;12(11):e006002.
- Rodriguez F, Olufade TO, Ramey DR, et al. Gender disparities in lipid-lowering therapy in cardiovascular disease: insights from a managed care population. J Womens Health. 2016;25(7):697–706.
- Chang AY, Abou-Arraj NE, Rodriguez F. Interventions to reduce ethnic and racial disparities in dyslipidemia management. Curr Treat Options Cardiovasc Med. 2019;21(5):24.
- Ferdinand KC, Senatore FF, Clayton-Jeter H, et al. Improving medication adherence in cardiometabolic disease: practical and regulatory implications. J Am Coll Cardiol. 2017;69(4):437–451.
- Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137(20):2166–2178.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133(1):407–455.
- Nanna MG, Navar AM, Zakroysky P, et al. Association of patient perceptions of cardiovascular risk and beliefs on statin drugs with racial differences in statin use: insights from the Patient and Provider Assessment of Lipid Management registry. JAMA Cardiol. 2018;3(8):739–748.
- Subherwal S, Patel MR, Tang F, et al. Socioeconomic disparities in the use of cardioprotective medications among patients with peripheral artery disease: an analysis of the American College of Cardiology’s NCDR PINNACLE registry. J Am Coll Cardiol. 2013;62(1):51–57.
- Casula M, Tragni E, Catapano AL. Adherence to lipid-lowering treatment: the patient perspective. Patient Prefer Adherence. 2012;6:805–814.
- Koh JJK, Cheng RX, Yap YC, et al. Access and adherence to medications for the primary and secondary prevention of atherosclerotic cardiovascular disease in Singapore: a qualitative study. Patient Prefer Adherence. 2018;12:2481–2498.
- Chen ST, Huang S-T, Shau W-Y, et al. Long-term statin adherence in patients after hospital discharge for new onset of atherosclerotic cardiovascular disease: a population-based study of real world prescriptions in Taiwan. BMC Cardiovasc Disord. 2019;19(1):62.
- Navar AM, Roe MT, White JA, et al. Medication discontinuation in the IMPROVE-IT trial. Circ Cardiovasc Qual Outcomes. 2019;12(1):e005041.
- Wood FA, Howard JP, Finegold JA, et al. N-of-1 trial of a statin, placebo, or no treatment to assess side effects. N Engl J Med. 2020;383(22):2182–2184.
- Abughosh SM, Vadhariya A, Johnson ML, et al. Enhancing statin adherence using a motivational interviewing intervention and past adherence trajectories in patients with suboptimal adherence. J Manag Care Spec Pharm. 2019;25(10):1053–1062.
- Cohen JD, Aspry KE, Brown AS, et al. Use of health information technology (HIT) to improve statin adherence and low-density lipoprotein cholesterol goal attainment in high-risk patients: proceedings from a workshop. J Clin Lipidol. 2013;7(6):573–609.
- Barankay I, Reese PP, Putt ME, et al. Effect of patient financial incentives on statin adherence and lipid control: a randomized clinical trial. JAMA Network Open. 2020;3(10):e2019429.
- Nanna MG, Navar AM, Wang TY, et al. Practice-level variation in statin use and low-density lipoprotein cholesterol control in the United States: results from the Patient and Provider Assessment of Lipid Management (PALM) registry. Am Heart J. 2019;214:113–124.
- Hess GP, Natarajan P, Faridi KF, et al. Proprotein convertase subtilisin/kexin type 9 inhibitor therapy: payer approvals and rejections, and patient characteristics for successful prescribing. Circulation. 2017;136(23):2210–2219.
- Khera R, Valero-Elizondo J, Okunrintemi V, et al. Association of out-of-pocket annual health expenditures with financial hardship in low-income adults with atherosclerotic cardiovascular disease in the United States. JAMA Cardiol. 2018;3(8):729–738.
- Khera R, Valero-Elizondo J, Das SR, et al. Cost-related medication nonadherence in adults with atherosclerotic cardiovascular disease in the United States, 2013 to 2017. Circulation. 2019;140(25):2067–2075.
- Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107.
- Ray KK, Colhoun HM, Szarek M, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(8):618–628.
- Karalis DG, Mallya UG, Ghannam AF, et al. Prescribing patterns of proprotein convertase subtilisin-kexin type 9 inhibitors in eligible patients with clinical atherosclerotic cardiovascular disease or heterozygous familial hypercholesterolemia. Am J Cardiol. 2018;121(10):1155–1161.
- Baum SJ, Toth PP, Underberg JA, et al. PCSK9 inhibitor access barriers—issues and recommendations: improving the access process for patients, clinicians and payers. Clin Cardiol. 2017;40(4):243–254.
- Knowles JW, Howard WB, Karayan L, et al. Access to nonstatin lipid-lowering therapies in patients at high risk of atherosclerotic cardiovascular disease. Circulation. 2017;135(22):2204–2206.
- Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404.
- Cordero A, Rodríguez-Mañero M, Fácila L, et al. Prevention of myocardial infarction and stroke with PCSK9 inhibitors treatment: a metanalysis of recent randomized clinical trials. J Diabet Metab Disord. 2020;19:759–765.
- Kosmas CE, Muñoz Estrella A, Skavdis A, et al. Inclisiran for the treatment of cardiovascular disease: a short review on the emerging data and therapeutic potential. Ther Clin Risk Manag. 2020;16:1031–1037.
- Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–1519.
- Novartis Pharmaceuticals Corp. LEQVIO [prescribing information]. 2021.