ABSTRACT
Objectives
To substantiate the clinical efficacy and investigate the real-world effectiveness of the herbal medicinal product BNO 1016 in acute rhinosinusitis (ARS) in the context of antibiotic stewardship.
Methods
We performed a meta-analysis of the clinical trials ARhiSi-1 (EudraCT No. 2008-002794-13) and ARhiSi-2 (EudraCT No. 2009-016682-28) comprising 676 patients, analyzing the reduction of the Major Symptom Score (MSS) and improvement of the Sino-Nasal Outcome Test 20 (SNOT-20) by the herbal medicinal product BNO 1016. In addition, we performed a retrospective cohort study including 203,382 patients, comparing the real-life effectiveness of BNO 1016 in reducing ARS-related adverse outcomes in comparison to antibiotics and several other established therapies.
Results
Treatment with BNO 1016 ameliorated symptoms of ARS by reducing MSS by 1.9 points (p < 0.0001) and improved quality of life (QoL) for patients by improving SNOT-20 by 3.5 points (p = 0.001) in comparison to placebo. In patients with moderate/severe symptoms, the positive effects of BNO 1016 were even more pronounced (MSS: −2.3 points (p < 0.0001); SNOT-20: −4.9 points (p = 0.0158)). In addition, treatment with BNO 1016 was as effective or significantly more effective in reducing the risk for adverse ARS-related outcomes such as follow-up antibiotic prescriptions, sick leave ≥7 days or medical appointments due to ARS, especially when compared to antibiotics.
Conclusion
BNO 1016 is a safe and effective treatment for ARS that can help reduce the overuse of antibiotics.
1. Introduction
Acute rhinosinusitis (ARS) is a common infection of the upper respiratory tract with a 1-year prevalence of 6–15% [Citation1]. The high number of patient visits to physicians in primary care places an economical burden on health-care systems [Citation2]. In Europe, for example, 1–2% of visits to physicians are for suspected ARS [Citation1]. In the United States, ARS accounts for 2–10% of primary care and otolaryngology visits [Citation3] and direct costs related to ARS were estimated to be nearly $6 billion in 2000 [Citation4]. Although usually being the consequence of a viral common cold, ARS is frequently treated with antibiotics [Citation5]. Overuse of antibiotics, especially when not indicated, contributes to the promotion of antibiotic resistance which constitutes a worldwide problem [Citation6]. According to guidelines, ARS should be treated symptomatically with decongestants (<10 days), nonsteroidal anti-inflammatory drugs (NSAIDs)/paracetamol, zinc, vitamin C, saline spray/rinses or herbal medicinal products [Citation1,Citation3,Citation7].
A herbal medicinal product for the treatment of ARS is BNO 1016 (Sinupret® extract), comprising a dry extract of gentian root (Gentiana lutea L.), primula flower (Primula veris L.), sorrel herb (Rumex crispus L.), elder flower (Sambucus nigra L.), and verbena herb (Verbena officinalis L.). BNO 1016 or BNO 1011 (same as BNO 1016, but without excipients) exerted well-documented mucosecretolytic and secretomotoric [Citation8–11], anti-inflammatory [Citation12,Citation13] as well as antiviral [Citation14,Citation15] effects in vitro and in vivo. But what about clinical, human data to evaluate the benefit of a medicinal product?
The British pioneer clinical epidemiologist Archie Cochrane defined three criteria to evaluate the benefit of health-care interventions, i.e. efficacy (‘can it work?’), effectiveness (‘does it work?’) and (cost-)efficiency (‘is it worth it?’) [Citation16,Citation17]. To answer the question ‘can it work?’, randomized controlled clinical trials (RCTs) remain the gold standard for proving efficacy and safety of new medicinal products before gaining marketing authorization and market access. ‘Does it work?’ can be answered by testing the effectiveness under real-world circumstances of health-care practice, e.g. by conducting non-interventional studies (NIS) or by analyzing otherwise recorded real-world data (RWD), e.g. electronic patient records [Citation18,Citation19].
For BNO 1016, efficacy and safety were already evaluated in two randomized, placebo-controlled, double-blind, multicenter, good clinical practice (GCP)-compliant clinical trials conducted in Germany. The phase IIb/III dose-finding clinical trial ARhiSi-1 [Citation20] already showed efficacy in several secondary endpoints, e.g. the Major Symptom Score (MSS), a frequently used composite score of five main symptoms of rhinosinusitis, comprising rhinorrhea (anterior discharge), postnasal drip, nasal congestion, headache and facial pain/pressure [Citation4,Citation21]. The subsequent confirmatory phase III clinical trial ARhiSi-2 assessed the MSS as the primary endpoint (MSS at end of treatment, visit 5, day 14). There, BNO 1016 showed superiority over placebo with a mean difference of 1.03 ± 0.24 score points (p = 0.0008) in the MSS at day 14 [Citation22]. A new meta-analysis of the two RCTs has been conducted to provide additional evidence to answer the question ‘can BNO 1016 work?'. A real-world data prospective cohort study gives insight into whether BNO 1016 ‘does work’ and its use might contribute to reducing unnecessary antibiotic prescriptions in the indication ARS.
2. Materials and methods
Detailed methods based on the individual study reports, including inclusion/exclusion criteria and statistical analyses can be found in the supplementary materials. Here, a short description of the scope of the meta-analysis of ARhiSi-1 and ARhiSi-2 and the RWD study is given.
2.1 Meta-analysis
The meta-analysis of the clinical trials ARhiSi-1 (N = 450 (Safety Evaluable Population (SEP))) and ARhiSi-2 (N = 385 (SEP)) was done by Scope International AG (Mannheim, Germany).
The objective of the new meta-analysis was to combine efficacy and safety data from the clinical trials ARhiSi-1 (only the treatment arms placebo and BNO 1016 480 mg, which represents the marketed BNO 1016 dosage) and ARhiSi-2 and to evaluate the influence of ARS baseline severity on improvement of ARS symptoms and health-related quality of life (HRQoL) under BNO 1016 compared to placebo. To assess efficacy and HRQoL, the MSS as well as the 20-item Sino-Nasal Outcome Test German Adapted Version (SNOT-20 GAV) questionnaire were assessed by the investigators or patients of the trials, respectively.
The main analyses from the meta-analysis presented in this manuscript are MSS at visit 5 (day 14) as well as SNOT-20 total scores at day 14. Also, least squares (LS) mean differences between BNO 1016 and placebo were calculated. Moreover, two subgroups within the analysis sets were defined. Subgroup ‘moderate/severe’ comprised patients with assessment moderate or severe for each of the five MSS symptoms at baseline (always resulting in MSS ≥10). Subgroup ‘none/mild’ comprised all patients without assessment moderate or severe for each of the five MSS symptoms at baseline (complementary group to subgroup moderate/severe).
2.2 Real-world data retrospective cohort study
The retrospective cohort study was based on data from the IMS® Disease Analyzer database (IMS®DA) from IQVIA (Frankfurt a. M., Germany), which contains case-based information provided by office-based physicians (both general practitioners (GPs) and ear-nose-throat (ENT) specialists) in Germany [Citation23]. IMS®DA contains data from more than 13 million patients in the time period between 2012 and 2020 and information was provided by nearly 3,000 office-based physicians, representing approximately 3.5% of all German practices (IMS®DA status date: March 2019).
The study included 203,382 patients diagnosed with ARS (ICD-10: J01) by a GP or an ENT in the period between January 2012 and December 2020 and who had a prescription of BNO 1016 or one of the following therapies: antibiotics, topical intranasal corticosteroids (INCS), nasal spray without corticosteroids (CS), antibiotics + BNO 1016, antibiotics + INCS, antibiotics + nasal spray without CS, antibiotics + analgesics (NSAIDs or paracetamol), nasal spray without CS + analgesic (NSAIDs or paracetamol) and BNO 1016 + nasal spray without CS, which comprise the most common treatment prescriptions in daily practice. The first diagnosis of ARS documented during this period was considered the index date and each patient was subject to follow-up for up to 365 days. The covariables used in this study included age, sex, month of the year and Charlson Comorbidity Index (CCI).
The following main analyses were conducted: Percentage of patients with antibiotic prescriptions due to ARS within 4–30 days and 31–365 days after the index date, percentage of patients with sick leave ≥7 days associated with ARS diagnosis within the first 30 days after the index date and number of medical appointments due to ARS within 4–30 days after the index date. All analyses were carried out for GPs and ENTs in combination (GPs + ENTs).
2.3 Institutional review board statement
The studies ARhiSi-1 and ARhiSi-2 were conducted in accordance with the Declaration of Helsinki and the ICH Harmonised Tripartite Guideline for Good Clinical Practice (CPMP/ICH/135/95). Both studies were approved by the respective ethic committees and registered at the European Medicines Agency (ARhiSi-1 EudraCT number: 2008–002794–13, ARhiSi-2 EudraCT number: 2009–016682–28).
The retrospective cohort study database includes only anonymized data in compliance with the regulations set forth in the applicable data protection laws. German law allows the use of anonymous electronic medical records for research purposes under certain conditions. In accordance with this legislation, it is not necessary to obtain informed consent from patients or approval from a medical ethics committee for this type of observational study which contains no directly identifiable data. Because patients were only queried as aggregates and no protected health information was available for queries, no Institutional Review Board approval was required for the use of this database or the completion of this study.
3. Results
3.1. Meta-analysis of BNO 1016 on symptom relief and health-related quality of life in ARS
The meta-analysis presented here comprises 676 patients altogether from both the ARhiSi-1 and ARhiSi-2 trials that were treated with 480 mg BNO 1016 vs. placebo.
Patients treated with BNO 1016 improved significantly according to MSS at day 14 compared to placebo-treated patients in the overall population (reduction to 2.5 vs. 3.5 score points). The LS mean difference in MSS between BNO 1016-treated and placebo-treated patients was −1.9 (p < 0.0001; ). The subgroup of patients with moderate/severe symptoms according to MSS assessment at baseline (MSS ≥10), i.e. more severely ill patients, exhibited an even stronger improvement with a mean MSS of 2.4 vs. 4.1 score points in the placebo-treated group and a significant LS mean difference of −2.3 (p < 0.0001) at day 14. The subgroup of patients with none/mild symptoms according to baseline MSS assessment improved less pronounced, but still statistically superior to placebo treatment (LS mean difference in MSS of −0.7, p = 0.016) ().
Figure 1. Meta-analysis: MSS LS mean difference of BNO 1016 treatment vs. placebo in the full analysis data set (FAS). MSS LS mean difference at day 14 is depicted for the overall pooled population of ARhiSi-1 (placebo and 480 mg BNO 1016 treatment arm) and ARhiSi-2 as well as for the subgroups with moderate/severe (MSS ≥10) and none/mild symptoms at baseline. MSS LS mean difference from the ARhiSi-2 trial is shown as reference. MSS: Major Symptom Score.
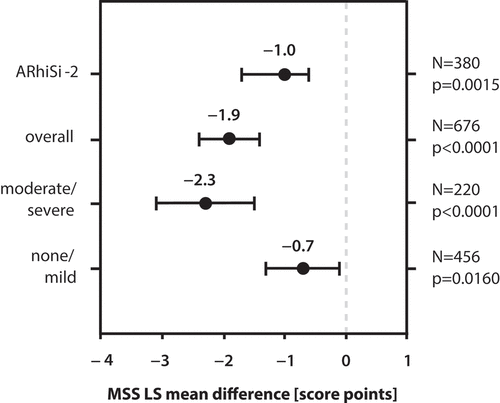
Table 1. Meta-analysis: Mean MSS and mean SNOT-20 as well as least squares (LS) mean differences at day 14 between BNO 1016 and placebo, full analysis data set (FAS).
Regarding HRQoL, the SNOT-20 questionnaire, as at the time of study conduct effective SNOT-version in German language, was evaluated by the patients. SNOT-20 total score was significantly lower in the BNO 1016 treatment group compared to placebo at day 14 (12.3 vs. 15.8 score points; ). The LS mean difference (−3.5) between the two groups was statistically significant (p = 0.001). In the subgroup of patients with moderate/severe symptoms according to baseline MSS assessment (MSS ≥10), improvement of the SNOT-20 score was also more pronounced, with a SNOT-20 score of 12.3 vs. 18.7 for placebo at day 14 and a LS mean difference of −4.9 (p = 0.0158) between the two groups. Again, patients with none or mild symptoms according to MSS baseline assessment also improved, but less pronounced (12.3 vs. 14.5 score points, LS mean difference of −2.1 (p = 0.0932)).
Regarding safety, the percentage of patients reporting at least one adverse event (AE) in the meta-analysis was higher in the placebo group than in the BNO 1016 treatment group (10% vs. 8.5%). In general, the frequency of AEs was low.
3.2. Real-world data analysis of BNO 1016 in ARS patients
To determine real-world effectiveness of BNO 1016 in ARS, a retrospective cohort study with data from the IMS® Disease Analyzer database was analyzed. A total of 203,382 patients diagnosed with ARS by a GP (120,178 patients) or an ENT specialist (83,204 patients) in the period between January 2012 and December 2020 were included (). Several ARS-related outcomes of BNO 1016 therapy were compared over the course of 365 days to 9 other established therapies for ARS (mainly antibiotics in mono- or combination therapy), which comprise the most common treatment regimen in daily practice for ARS, although partly not indicated. As main outcomes, antibiotic prescriptions within the first 30 days and in the period between 31-365 days after index date, sick leave ≥7 days as well as number of medical appointments within the first 30 days were analyzed.
Figure 2. Retrospective cohort study: Selection of study patients from the IMS® Disease Analyzer database.
The baseline characteristics of the study patients were comparable among all treatment groups (see ). Mean age of the study patients was between 32.5 and 42.2 years, and more patients were female in all groups, with around 60% of all patients. Due to young age, most patients had a very low Charlson Comorbidity Index. As expected, incidence of ARS was highest in the winter months (December–February) and lowest in the summer months (June–August).
Table 2. Retrospective cohort study: Baseline characteristics of the study patients.
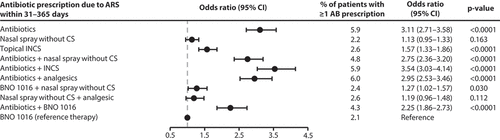
Firstly, (further) antibiotic prescription due to ARS within the first 30 days and in the period 31–365 days after initiation of therapy was analyzed. Surprisingly, the majority of patients (94,756 patients) still received antibiotics in monotherapy as initial ARS treatment plus 49,213 patients antibiotics in combination with other medicinal products (see ). Nearly all patients receiving antibiotics (monotherapy or as combination therapy) at the beginning of the study period were at a significantly higher risk for further antibiotic prescription within the first 30 days after therapy begin compared with BNO 1016. Moreover, all patients receiving antibiotics in mono- or combination therapy were at a significantly higher risk for a further antibiotic prescription in the follow-up time period of days 31–365 (see ). Thus, odds ratios (ORs) for further antibiotic prescriptions were quite high, ranging from 2.25 to 3.54. Also, treatment with topical INCS was associated with a significantly higher risk of antibiotic prescription (OR: 1.57). In contrast, treatment with BNO 1016 led in only 2.1% of cases to a prescription of an antibiotic for ARS within the follow-up period. In summary, no therapy performed better (OR < 1) than BNO 1016 as monotherapy in preventing subsequent antibiotic prescriptions within the follow-up period.
Figure 3. Retrospective cohort study: Prescription of antibiotics due to ARS within 31–365 days after start of therapy. Multivariable Cox proportional hazard regression adjusted for sex, age, insurance status, month and CCI. AB: Antibiotics; CI: Confidence interval; CS: Corticosteroids; INCS: Intranasal corticosteroids.
Secondly, sick leave with a duration of ≥7 days within the first 30 days was analyzed (see ). In contrast to the other endpoints, sick leave data was analyzed only for the patients diagnosed by a GP. This is due to the fact that GPs are usually responsible for sick notes. Sick notes from ENTs are less common and usually short in duration. Also, only patients diagnosed by GPs aged 18–65 years receiving sick notes were considered for this analysis, as this population is considered to be of working age. Compared to BNO 1016, the majority of therapies exhibited a significantly higher OR for sick leave ≥7 days, ranging between 1.09 and 1.55. However, topical INCS were associated with a significantly and strongly decreased risk for sick leave ≥7 days (OR: 0.68). In summary, therapy with BNO 1016 was associated with a significantly lower or equal risk for sick leave ≥7 days compared to most of the other therapies.
Figure 4. Retrospective cohort study: Sick leave ≥7 days within first 30 days after therapy begin (a) and medical appointments due to ARS within 4–30 days after therapy begin (b). For (a) and (b): Multivariable logistic regression; All adjusted for sex, age, insurance status, month and CCI. CI: Confidence interval; CS: Corticosteroids; INCS: Intranasal corticosteroids.
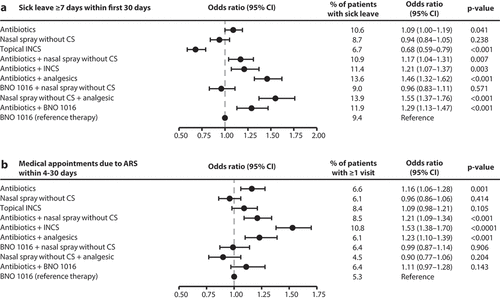
Besides sick leave, the number of medical appointments due to ARS within the first 30 days of the study was analyzed (see ). With ORs ranging from 1.11 to 1.53, all patients receiving antibiotics as monotherapy or as combination therapy at the beginning of the study had a higher risk than the reference therapy BNO 1016 for ≥1 additional medical appointments within the first 30 days. Except for the treatment group ‘antibiotics + BNO 1016’ (OR: 1.11), the higher risks were significant for all other therapies involving antibiotics. The three therapies ‘nasal spray without CS’, ‘nasal spray without CS + analgesic’ and ‘BNO 1016 + nasal spray without CS’ showed similar, slightly lower but non-significant risks for ≥1 additional medical appointments within the first 30 days (ORs: 0.96, 0.90 and 0.99). In summary, therapy with BNO 1016 was associated with a significantly lower or equal risk for ≥1 additional medical appointments due to ARS within the first 30 days.
Regarding safety, the occurrence of epistaxis as adverse reaction was studied within the complete study period of 4–365 days. The concomitant diagnosis of epistaxis was very low (0.13–0.29% of patients) in general and there were no significant differences between all treatment groups.
In summary, ARS treatment with BNO 1016 was as effective or significantly more effective in preventing adverse outcomes in ARS when compared to other established ARS therapies (first of all antibiotics), with the sole exception of topical INCS, which were more effective lowering the risk for sick leaves ≥7 days within the first 30 days after start of therapy, but with a significantly higher risk for the need of antibiotic prescription in the follow-up period of days 31–365.
4. Discussion
The clinical efficacy and safety of BNO 1016 have convincingly been shown in clinical trials [Citation22], leading to the recommendation of the herbal medicinal product in the European Position Paper on Rhinosinusitis and Nasal Polyps, as well as in national guidelines for ARS [Citation1,Citation7].
The data presented in this manuscript confirm the clinical efficacy (‘can it work?’) by a new meta-analysis and show the real-life effectiveness (‘does it work?’) of the herbal medicinal product BNO 1016 in the treatment of ARS.
ARS is still widely treated with antibiotics although the disease is usually caused by viruses including rhinovirus, adenovirus, influenza, and parainfluenza virus. Acute bacterial rhinosinusitis is rare, with an incidence of 0.5–2% of viral ARS in adults and 5–10% in children [Citation1,Citation3,Citation24–26]. Nevertheless, the prevalence of antibiotic prescription for ARS was found to be around 50% in Germany without any clinically relevant difference between patients attending GPs and those treated by ENT specialists [Citation27]. Studies from Spain [Citation5], the United Kingdom [Citation28], Canada [Citation29], and the USA [Citation30] show a similar picture, with even higher trends (62–82%) for potentially inappropriate antibiotic prescriptions. In the UK, 82% of the patients with ARS received antibiotic prescriptions in primary care, whereas only 11% of these prescriptions were identified as appropriate by experts [Citation28]. In Canada, over 60% of the patients received antibiotics for ARS, although only 18% of the patients were in need of an antibiotic therapy [Citation29]. Often, there is no difference in the proportion of antibiotic prescription between patients who meet the criteria for the prescription of an antibiotic and those who do not [Citation27]. In a chart review study from the USA, 92% of the patients who did not fulfil the criteria for acute bacterial sinusitis (e.g. fever >38°C, double sickening) received antibiotics [Citation31]. It is likely that patients often have misconceptions about the indications and effectiveness of antibiotics for ARS triggering the high antibiotic prescription rates [Citation25,Citation32].
Our retrospective cohort study in a large population of 203,382 German ARS patients confirms that antibiotics as monotherapy or in combination are still the most frequently prescribed therapy for ARS. Their prescription is associated with an elevated risk of a further antibiotic prescription in the follow-up period of up to 365 days. In addition, the data indicate a higher probability of longer sick leaves ≥7 days and more frequent visits to a GP or ENT specialist in the first 30 days after antibiotic prescription compared to a reference therapy with BNO 1016.
Of course, retrospective cohort database studies like the one presented here exhibit some limitations. First, they can only display correlations and some outcomes therefore may appear inconsistent. Moreover, the inclusion criterion ARS (ICD-10: J01) does not clearly differ between viral, post-viral and bacterial rhinosinusitis resulting in a mixed population potentially having an impact on prescription practices. In addition, selection bias cannot be entirely excluded. One might argue that antibiotics were most likely given to more severely ill patients and that this would also explain the generally high re-prescription of antibiotics or more frequent medical appointments in this group. Even if more severely ill ARS patients are more likely to consult a physician, a rate of >70% of antibiotic prescriptions in mono- or combination therapy in our study is inappropriate, especially when seen in the light that only up to 2% of all adult ARS cases are of bacterial origin [Citation1,Citation3]. Nevertheless, 92% of the patients without bacterial sinusitis still receive antibiotics although not being indicated [Citation31].
Further limitations of the retrospective cohort study are that data on socioeconomic status and lifestyle-related factors (e.g. smoking, alcohol, physical activity) are not available. Therefore, the possibility of residual confounding cannot be excluded. Besides that, patients within this study were only observed in a single practice; if they received a diagnosis or prescription from another physician, these prescriptions were not documented. Also, the database and analysis only included data on prescriptions for the different medications. As BNO 1016, nasal sprays and analgesics are also available over-the-counter (OTC), their (additional) usage was not documented.
In contrast, the strength of the retrospective cohort study is the large study population of 203,382 ARS patients under real-world conditions over a period of 9 years. The study provides important data on the use of BNO 1016 within its target population.
First findings on the effectiveness of BNO 1016 have already been published by Martin et al. in 2020. The authors found positive results approaching significance for BNO 1016 treatment in the reduction of sick leave >7 days in acute upper and lower respiratory tract infections (OR 0.84 [0.7–1.0], p = 0.054) [Citation33]. A recent review comparing the existing evidence of different herbal medicinal products in relation to N-acetylcysteine and mometasone furoate nasal spray highlighted the evidence for BNO 1016 in the treatment of ARS [Citation34].
For BNO 1016, our new meta-analysis found an elevated symptom relief and improvement of HRQoL (SNOT-20) compared to placebo than the individual trials alone. Although there is, to our knowledge, no minimum clinical important difference (MCID) for MSS in ARS, a difference of 1 score point in the mean MSS at day 14 (LS mean difference of −1.9 score points) is in line or even exceeds treatment effects seen for other therapies in this indication, like other herbals or the intranasal corticosteroid mometasone furoate [Citation21,Citation35]. More severely ill patients exhibited even stronger improvements (LS mean difference of −2.3 score points).
To promote the reasonable use of antibiotics and limit unnecessary overuse, the European Commission has issued guidelines on the prudent use of antimicrobials in human health [Citation36]. Overuse of antibiotics can be highly problematic. It leads to an increase in antimicrobial resistances [Citation37], which are, as recently published, associated with or the direct cause of nearly 700,000 deaths in Europe in 2019 [Citation38]. In addition, frequent use of antibiotics can also be directly detrimental to patients by compromising the gut microbiota [Citation39], which may promote the development and aggravation of disease [Citation40]. Our retrospective cohort study indeed showed that treatment with antibiotics often led to higher risks for adverse outcomes in ARS. With the new results, BNO 1016 as a herbal treatment alternative has the potential to further reduce inappropriate antibiotic use in ARS.
In summary, BNO 1016 was as effective or significantly more effective in preventing adverse outcomes of ARS such as antibiotic prescriptions, sick leave ≥7 days and number of medical appointments due to ARS, when compared to the most frequently prescribed ARS treatments such as antibiotics, topical INCS, nasal sprays or combinations thereof. Apart from topical INCS, which reduced the risk for sick leaves ≥7 days stronger than BNO 1016, no other treatment performed significantly better in any outcome measure than BNO 1016. Moreover, BNO 1016 treatment led to a significantly lower risk for adverse outcomes, especially when compared to antibiotics as mono- or combination therapy.
5. Conclusion
The data presented in this manuscript confirm the clinical efficacy by a new meta-analysis and show the real-life effectiveness of the herbal medicinal product BNO 1016. It is therefore a valuable, evidence-based option for the treatment of ARS when antibiotics are clearly not indicated.
Declaration of financial/other relationships
CB Bittner, H Steindl, D Abramov-Sommariva, M Plach and C Abels are employees of Bionorica SE, Neumarkt, Germany. C Bachert has received honoraria from Bionorica SE, Neumarkt, Germany for scientific services. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download Zip (50.7 KB)Acknowledgments
Statistical assistance was provided by Björn Bosse, Scope International AG, Mannheim, Germany. RWD analysis was conducted by Karel Kostev, IQVIA, Frankfurt a. M., Germany. Medical writing assistance was provided by co.medical, Berlin, Germany.
Data availability statement
The data that support the findings of this study are available from the corresponding author, C Bachert, upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00325481.2023.2234274
Additional information
Funding
References
- Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020 Feb 20;58(Suppl S29):1–464.
- Jaume F, Alobid I, Mullol J, et al. Direct costs of acute rhinosinusitis in Spain: a Prospective and Observational Study (PROSINUS). J Investig Allergol Clin Immunol. 2021 Dec 21;31(6):481–488.
- Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021 Mar;11(3):213–739.
- Stjarne P, Odeback P, Stallberg B, et al. High costs and burden of illness in acute rhinosinusitis: real-life treatment patterns and outcomes in Swedish primary care. Prim Care Respir J. 2012 Jun;21(2):174–179.
- Jaume F, Quinto L, Alobid I, et al. Overuse of diagnostic tools and medications in acute rhinosinusitis in Spain: a population-based study (the PROSINUS study). BMJ Open. 2018 Jan 31;8(1):e018788.
- Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015 Apr;40(4):277–283.
- Deutsche Gesellschaft für Hals-Nasen-Ohren-Heilkunde, Kopf- und Hals-Chirurgie e.V., Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin e.V. Rhinosinusitis, 2k-Leitlinie, AWMF-Register-Nr. 017/049 und p. 053–012. 2017.
- Kreindler JL, Chen B, Kreitman Y, et al. The novel dry extract BNO 1011 stimulates chloride transport and ciliary beat frequency in human respiratory epithelial cultures. Am J Rhinol Allergy. 2012;26(6):439–443. doi: 10.2500/ajra.2012.26.3821
- Zhang S, Skinner D, Hicks SB, et al. Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PLoS One. 2014;9(8):e104090. doi: 10.1371/journal.pone.0104090
- Cho DY, Skinner D, Mackey C, et al. Herbal dry extract BNO 1011 improves clinical and mucociliary parameters in a rabbit model of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019 Jun;9(6):629–637.
- Workman AD, Maina IW, Triantafillou V, et al. Effects of BNO 1016 on ciliary transport velocity and cell culture surface liquid height of sinonasal epithelial cultures. Clin Phytosci. 2021;7(1): doi: 10.1186/s40816-021-00276-2
- Rossi A, Dehm F, Kiesselbach C, et al. The novel Sinupret® dry extract exhibits anti-inflammatory effectiveness in vivo. Fitoterapia. 2012 Jun;83(4):715–720.
- Zupanets IA, Shebeko SK, Popovych VI, et al. Study of the anti-inflammatory effect of the combined extract BNO 1016 in a leukotriene-dependent in vivo inflammation model. Clin Phytosci. 2020;6(1). doi: 10.1186/s40816-020-0153-8
- Glatthaar-Saalmüller B, Rauchhaus U, Rode S, et al. Antiviral activity in vitro of two preparations of the herbal medicinal product Sinupret® against viruses causing respiratory infections. Phytomedicine. 2011;19(1):1–7. doi: 10.1016/j.phymed.2011.10.010
- Tran HTT, Peterburs P, Seibel J, et al. In vitro screening of herbal medicinal products for their supportive curing potential in the context of SARS-CoV-2. Evid Based Complement Alternat Med. 2022;2022:1–9. doi: 10.1155/2022/8038195
- Cochrane A. Effectiveness and efficiency: random reflections on health services. The Nuffield Provincial Hospitals Trust, London, UK; 1972.
- Haynes B. Can it work? Does it work? Is it worth it? BMJ. 1999;319(7211):652–653. doi: 10.1136/bmj.319.7211.652
- Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence — what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–2297. doi: 10.1056/NEJMsb1609216
- Concato J, Corrigan-Curay J. Real-World Evidence — Where are we now? N Engl J Med. 2022;386(18):1680–1682. doi: 10.1056/NEJMp2200089
- Jund R, Mondigler M, Stammer H, et al. Herbal drug BNO 1016 is safe and effective in the treatment of acute viral rhinosinusitis. Acta Otolaryngol. 2015;135(1):42–50. doi: 10.3109/00016489.2014.952047
- Meltzer EO, Bachert C, Staudinger H. Treating acute rhinosinusitis: comparing efficacy and safety of mometasone furoate nasal spray, amoxicillin, and placebo. J Allergy Clin Immunol. 2005 Dec;116(6):1289–1295. doi: 10.1016/j.jaci.2005.08.044
- Jund R, Mondigler M, Steindl H, et al. Clinical efficacy of a dry extract of five herbal drugs in acute viral rhinosinusitis. Rhinology. 2012 Dec;50(4):417–426.
- Rathmann W, Bongaerts B, Carius H-J, et al. Basic characteristics and representativeness of the German disease analyzer database. Int J Clin Pharm Therap. 2018;56(10):459–466. doi: 10.5414/CP203320
- DeBoer DL, Kwon E. Acute sinusitis. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright© 2022. Florida, USA: StatPearls Publishing LLC; 2022.
- Hopkins C, Surda P, Walker A, et al. EPOS 4 Patients. Rhinology. 2021 Oct 29:1–57. doi: 10.4193/Rhin20.950
- Wiebringhaus R, Haubner F. Acute Rhinosinusitis - an update on therapy. MMW Fortschr Med. 2022 Mar;164(5):40–43. doi: 10.1007/s15006-022-0777-3
- Bittner CB, Plach M, Steindl H, et al. Prevalence of antibiotic prescription in patients with acute rhinosinusitis treated by general practitioners and otolaryngologists in Germany- A retrospective cohort study. Antibiotics (Basel). 2022 Nov 9;11(11):1576.
- Pouwels KB, Dolk FCK, Smith DRM, et al. Actual versus ‘ideal’ antibiotic prescribing for common conditions in English primary care. J Antimicrob Chemother. 2018 Feb 1;73(suppl_2):19–26.
- Schwartz KL, Langford BJ, Daneman N, et al. Unnecessary antibiotic prescribing in a Canadian primary care setting: a descriptive analysis using routinely collected electronic medical record data. CMAJ Open. 2020 Apr;8(2):E360–e369.
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Netw Open. 2018 Jun 1;1(2):e180243.
- Truitt KN, Brown T, Lee JY, et al. Appropriateness of antibiotic prescribing for acute sinusitis in primary care: a cross-sectional study. Clin Infect Dis. 2021 Jan 27;72(2):311–314.
- Smith SS, Caliendo A, Cheng BT, et al. Patient perspectives on the drivers and deterrents of antibiotic treatment of acute rhinosinusitis: a qualitative study. J Gen Intern Med. 2023 Feb;38(3):683–690.
- Martin D, Konrad M, Adarkwah CC, et al. Reduced antibiotic use after initial treatment of acute respiratory infections with phytopharmaceuticals- a retrospective cohort study. Postgrad Med. 2020 Jun;132(5):412–418.
- Bachert C. Evidence-based management of acute rhinosinusitis with herbal products. Clin Phytosci. 2020;6(1). doi: 10.1186/s40816-020-00231-7
- Dejaco D, Bocian-Sobkowska J, Szymanski W, et al. Tavipec in acute rhinosinusitis: a multi-centre, doubleblind, randomized, placebo-controlled, clinical trial. Rhinology. 2019 Oct 1;57(5):367–374.
- European Commission. EU Guidelines for the prudent use of antimicrobials in human health. Off J Eur Union 2017 [1 Jul 2017];C212:1–12.
- Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74(3):417–433. doi: 10.1128/MMBR.00016-10
- Collaborators EAR. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: a cross-country systematic analysis. Lancet Public Health. 2022 Nov;7(11):e897–e913. doi: 10.1016/S2468-2667(22)00225-0
- Nausch B, Bittner CB, Höller M, et al. Contribution of symptomatic, herbal treatment options to antibiotic stewardship and microbiotic health. Antibiotics (Basel). 2022 Sep 29;11(10). doi: 10.3390/antibiotics11101331
- Lange K, Buerger M, Stallmach A, et al. Effects of antibiotics on gut microbiota. Dig Dis. 2016;34(3):260–268. doi: 10.1159/000443360
