ABSTRACT
Objectives
Sexually transmitted infection (STI) diagnosis is complicated as these infections can present with lower genitourinary tract symptoms (LGUTS) that overlap with other disorders, i.e. urinary tract infections (UTIs). The study's objective was to determine potential missed STI diagnoses from patients presenting with LGUTS in the US between January 2010 and December 2019.
Methods
The de-identified insurance claims data from the IBM® MarketScan® Research Databases were collected from patients (14–64 years old) who presented with LGUTS, which could be caused by an STI. A ‘GAP’ cohort was created, consisting of episodes with potentially delayed STI (Chlamydia trachomatis [CT]/Neisseria gonorrhoeae [NG]) treatment. The intention was to capture episodes where an STI was not initially suspected. Four subgroups were defined depending on the treatment received (fluoroquinolone; azithromycin and/or doxycycline; cephalosporins; gentamicin and azithromycin).
Results
The GAP cohort consisted of 833,574 LGUTS episodes from the original cohort (23,537,812 episodes). Post-index CT/NG testing was carried out for 4.6% and 5.4% of the episodes from men and women, respectively. There were ≥2 return visits for 16.1% and 15.8% of the episodes from men and women, respectively. A substantial percentage of episodes from men (52.1%) and women (68.3%) were diagnosed with a UTI and/or acute cystitis at the index prior to receiving post-index STI treatment. Other top conditions diagnosed at index for men were dysuria (25.8% of the episodes), orchitis/epididymitis (14.3% of the episodes), and acute prostatitis (10.1% of the episodes), and for women were dysuria (24.2% of the episodes), vaginitis/vulvitis/vulvovaginitis (11.7% of the episodes), and cervicitis (3.3% of the episodes).
Conclusion
These findings highlight delayed STI antibiotic treatment and low rates of CT/NG testing, suggesting late STI consideration and suboptimal diagnosis. Additionally, our study illustrates the importance of accurately diagnosing and treating STIs in patients with LGUTS and associated conditions, to avoid antibiotic misuse and complications from delayed administration of appropriate treatment.
1. Introduction
Sexually transmitted infections (STIs) are a significant public health concern [Citation1]. In the United States (US), 2021 surveillance data show that, for the two most common notifiable STIs, 1,644,416 cases of Chlamydia trachomatis (CT) and 710,151 cases of Neisseria gonorrhoeae (NG) were reported to the Centers for Disease Control and Prevention (CDC) [Citation1]. In 2021, the number of people with CT increased by 3.9% compared with 2020. A similar trend was noted for NG, with a 4.6% increase compared with 2020 [Citation1].
Diagnosis of STIs may be missed because STI symptoms are sometimes similar to those reported for other non-sexually transmitted urogenital tract infections (UGTIs), including urinary tract infections (UTIs) [Citation2,Citation3]. Overlapping symptoms in men and women with conditions caused by STIs and other UGTIs include dysuria, frequency, urgency, and discharge [Citation2,Citation3]. STIs and UGTIs can also cause similar conditions, including epididymitis, prostatitis, urethritis, vulvovaginitis, and cervicitis [Citation2]. Thus, distinguishing between these infections can be challenging [Citation3] and can lead to misdiagnosis [Citation2]. An observational study determining the accuracy of clinical diagnoses of UTIs and STIs in adult women in the US presenting to the emergency department (ED) with genitourinary symptoms or infections found that 23% of the women had an STI and 37% of this group did not receive treatment for an STI within 7 days of visit, highlighting missed STI diagnoses [Citation3]. Therefore, a prompt and effective diagnosis is crucial for the prevention and control of STIs [Citation2,Citation4], especially in the setting of potentially obfuscating lower genitourinary tract symptoms (LGUTS).
For the diagnosis of CT and NG, nucleic acid amplification tests (NAATs) are recommended; culture can also be used to detect NG infection, as well as Gram stain in symptomatic males with urethral discharge/urethritis [Citation4,Citation5]. The current CDC-recommended treatment regimen for CT is doxycycline (DOX) and for NG is ceftriaxone (CRO) [Citation4].
We have recently published a study describing US diagnostic testing and antimicrobial treatment trends between 2010 and 2019 using data from the IBM® MarketScan® Research Databases relating to patients presenting with LGUTS that could be indicative of an STI [Citation6]. This study revealed a surprisingly low rate of STI testing – only 17.3% of all LGUTS episodes received CT/NG testing – and high levels of apparently empiric antibiotic treatment prior to confirmation of diagnosis [Citation6]. Among the ~24 million episodes of patients with diagnostic codes indicative of LGUTS that were described in this previous study [Citation6], it was hypothesized that there would be a group that presented with LGUTS secondary or in addition to an STI but were not promptly identified and instead coded or treated as a non-STI disorder at the initial visit.
Here, we describe the identification of this group, referred to as the GAP cohort (indicating potentially missed STIs as a result of overlapping urogenital symptoms), and describe the testing rates and treatment patterns for this group.
2. Materials and methods
2.1. Study design and population
This is a retrospective, observational study using de-identified insurance claim data from the IBM MarketScan Research Databases between January 2010 and December 2019, as originally reported in Lillis et al., 2023 [Citation6]. All the patients were 14–64 years of age and presented with International Classification of Diseases (ICD) codes indicative of LGUTS, which could be caused by an STI. The inclusion and exclusion criteria for this group, as well as the definitions for index date, episode and visit, the diagnosis and procedure codes, and the antimicrobial drugs used are described in Lillis et al., 2023 [Citation6]. Briefly, the index date is the first date that any ICD code of interest has been captured. An episode is defined as any period after the index visit in which ICD codes of interest occurs one or more times, including the index visit, sequentially with no more than 21 days elapsing between each code. This criterion was chosen based on the rationale that passage of more than 21 days without a relevant ICD code strongly suggests resolution of the symptoms or concerns presented at the index visit. The first visit is the index date plus two more days to capture events related to the first visit [Citation6].
All data complied with the Health Insurance Portability and Accountability Act (HIPAA) of 1996. Research was performed per the Declaration of Helsinki. Institutional review board approval was not required for this secondary data analysis as the identity of the human subjects could not be ascertained directly or through identifiers linked to the subjects, and the subjects were not contacted or reidentified. There is no identifiable information from individuals within these datasets. Consent to participate was not necessary.
2.2. Identification of the GAP population
From the parent group of LGUTS episodes [Citation6], a sequence of exclusion steps was taken to determine the GAP cohort. This consisted of eliminating episodes in which there was a high level of confidence that an STI was the cause at the first visit (as exemplified by provision of antibiotics appropriate for STI treatment, especially when STI diagnostic testing was performed), as well as other episodes where treatments were indicative of other discrete non-STI diagnoses (e.g. cystitis, as exemplified by the administration of antibiotics such as nitrofurantoin [Citation7]). The intention was to capture episodes where an STI was not suspected at the index date (as evidenced by a lack of diagnostic tests for an STI and/or provision of STI-appropriate antibiotics), but STI treatments were prescribed after the index date. The series of exclusion steps are described below and illustrated in .
Figure 1. Flowchart of exclusion steps to determine final GAP cohort.
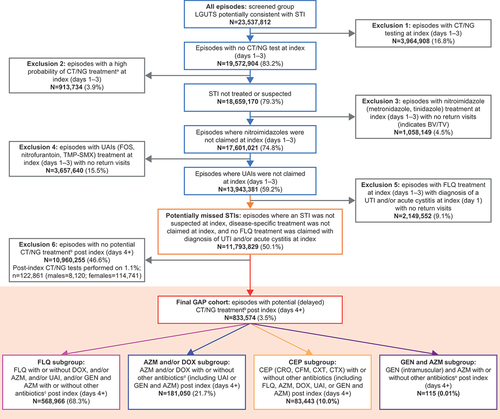
The first group excluded comprised episodes where diagnostic tests for STIs (CT/NG) were ordered at the index visit (days 1–3). The second group excluded comprised episodes where STI-specific (CT/NG) treatment was prescribed at index (days 1–3). The rationale for these first two exclusions was that clinicians demonstrated sufficient suspicion of an STI at the initial encounter, which led to ordering of CT/NG diagnostic testing or treatment. The third group excluded comprised episodes treated with nitroimidazole antimicrobials (e.g. metronidazole) at index (days 1–3) and had no return visits. Administration of a nitroimidazole at initial contact is indicative of only a few likely diagnoses, most commonly bacterial vaginosis (BV) or Trichomonas vaginalis (TV) [Citation4]. The fourth group excluded comprised episodes prescribed urinary anti-infectives (e.g. fosfomycin [FOS], nitrofurantoin, trimethoprim-sulfamethoxazole [TMP-SMX]) [Citation7] at index (days 1–3) with no return visits. These episodes were likely related to common lower UTIs, and there are no other commonly recognized diagnoses for which nitrofurantoin is routinely prescribed. The fifth group excluded comprised episodes treated with fluoroquinolone (FLQ) at index (days 1–3), with diagnosis of a UTI and/or acute cystitis at index (day 1) with no return visits. The provision of FLQ with an appropriately corresponding diagnosis (e.g. UTI) combined with the absence of additional follow-up treatment is suggestive of an appropriate non-STI diagnosis. The sixth group excluded comprised episodes that did not receive any antibiotics that could be considered potential treatment for STIs (CT/NG/Mycoplasma genitalium [MG]) post index (days 4+). Among this group, a small minority (122,861; 1.1%) underwent post-index testing, but since there was no prescription for antibiotic treatment for an STI, it was assumed that these post-index tests were not indicative of an STI.
After exclusion criteria were applied, the GAP cohort consisted of episodes with potential delayed STI (CT/NG) treatment post index (days 4+). These episodes include patients presenting with LGUTS who were not initially identified and/or treated as having CT/NG and then were treated several days (4+) after the initial presentation with antimicrobials suggestive of these STIs. The GAP cohort was split into four GAP subgroups based on antimicrobials prescribed.
2.3. GAP subgroups
The FLQ subgroup consisted of episodes treated with FLQ with or without DOX, and/or azithromycin (AZM), and/or urinary anti-infectives (UAI), and/or gentamicin (GEN) with AZM with or without other antibiotics post index (days 4+). The rationale for defining this subgroup was that the aforementioned exclusion groups 4 and 5 would have eliminated those treated with antibiotics specific for UTIs or FLQ at index who did not return for further visits (likely indicating successful symptomatic resolution); thus, this subgroup may contain episodes receiving delayed STI treatment as certain FLQs can be alternative treatments for CT [Citation8]. Notably, 61% to 67% of NG infection in the US may be sensitive to ciprofloxacin [Citation9], although FLQs have not been a recommended treatment since 2007 [Citation10].
The AZM and/or DOX subgroup consisted of episodes treated with AZM and/or DOX with or without other antibiotics (including UAI or GEN and AZM) post index (days 4+). Treatment with these could be indicative of a suspected STI as DOX can be used as treatment for cervicitis, non-gonococcal urethritis, pelvic inflammatory disease (PID), epididymitis, prostatitis, CT, and MG, and AZM is commonly prescribed for CT and has demonstrated activity against NG [Citation4,Citation8].
The cephalosporins (CEP) subgroup consisted of episodes treated with CEP (CRO, cefixime [CFM], cefoxitin [CXT], cefotaxime [CTX]) with or without other antibiotics (including FLQ, AZM, DOX, UAI, or GEN and AZM) post index (days 4+). These antibiotics are used to treat STIs; the most common indication for CRO is NG [Citation4]. While there are other indications for CEP, these are largely outside the boundaries of LGUTS [Citation11].
The GEN and AZM subgroup consisted of episodes treated with GEN (intramuscular) and AZM with or without other antibiotics post index (days 4+). The rationale for inclusion of this subgroup was due to the recommendation to treat NG with GEN and AZM in cases of CEP allergy [Citation12].
2.4. Data analysis
For the GAP cohort, the distribution of gender, age, number of visits, rates of CT/NG or non-CT/NG testing, time between index and STI treatment, and numbers of episodes with UTI/cystitis at index were described. Continuous variables were summarized with mean, standard deviation, median, and interquartile range (IQR) (25%, 75%). Categorical variables were summarized using frequency counts and the percentage of subjects within each category. The analysis was performed using SAS Studio, version 3.8.
3. Results
3.1. Patient characteristics of the GAP cohort
In total, the GAP cohort consisted of 833,574 LGUTS episodes from the original screened LGUTS group of 23,537,812 episodes [Citation6]. Of these 833,574 episodes, 82.2% were from women and 17.8% were from men. The median age of patients at index was 45.0 years (IQR 32.0–56.0). Further patient demographics are shown in ().
Table 1. Patient characteristics of the GAP cohort (episode-level).
3.2. STI testing patterns and return visits within the GAP cohort and subgroups
For the entire GAP cohort, 4.6% of men and 5.4% of women received the CT/NG testing post index (days 4+). Of the episodes from men, 64.9% did not receive any testing, compared with 54.1% for women ().
Table 2. STI testing rates and number of return visits for the GAP cohort post index (days +4).
The FLQ subgroup was the largest. Of those episodes in this subgroup, 2.2% of men and 3.1% of women received CT/NG testing (days 4+ post index) (). The majority of this subgroup received no further testing post index (men: 65.2%; women: 51.2%). Of the episodes in the FLQ subgroup, 16.1% of men and 16.4% of women incurred two or more return visits in relation to their LGUTS (). For the AZM and/or DOX subgroup, 7.8% of men and 7.7% of women in this subgroup went on to have testing for CT/NG post index (). The CEP subgroup had the highest frequency of post-index CT/NG STI testing for both women (15.3%) and men (16.5%) (). Of the episodes in the CEP subgroup, 28.2% and 27.5% of men and women, respectively, had two or more return visits (). The GEN and AZM subgroup consisted of 115 LGUTS episodes (), which is only 0.01% of the GAP cohort ().
3.3. Other conditions diagnosed at index and post index (days 4+) within the GAP cohort
Over half of the GAP cohort (52.1% of men; 68.3% of women) was diagnosed with a UTI and/or acute cystitis at index (day 1) (). Among the men and women who had a UTI and/or acute cystitis at index, administration of post-index (days 4+) treatment with antimicrobials appropriate for STIs occurred at a median of 7–14 days post index across all subgroups ().
Table 3. Time to post-index STI treatment among patients within the GAP cohort who were diagnosed with a UTI and/or acute cystitis at index.
The most common diagnosis at index (day 1) for men and women across all subgroups was a UTI; 48.6% of episodes in men and 61.7% of episodes in women from the entire GAP cohort were diagnosed with a UTI at index (). Across the subgroups, the other top conditions diagnosed at index for men were dysuria, orchitis/epididymitis, acute prostatitis, urethritis, and acute cystitis, and for women were dysuria, acute cystitis, vaginitis/vulvitis/vulvovaginitis, and cervicitis ().
Table 4. Conditions diagnosed at index (day 1) within the GAP cohort.
For all subgroups, the most common condition diagnosed post index (days 4+) was a UTI, ranging from 11.8% (men in AZM and/or DOX subgroup) to 56.5% (men in GEN and AZM subgroup) ().
Table 5. Conditions diagnosed post index (days 4+) within the GAP cohort.
4. Discussion
The incidence of STIs in the US shows no sign of stabilizing, with overall reported STIs increasing from 2.4 million to 2.5 million, a 7% increase, from 2017 to 2021 [Citation13]. This is despite the US Preventive Services Task Force and CDC testing guidelines recommending that screening for CT and NG is carried out for certain populations, such as sexually active women <25 years old, men who have sex with men, and persons living with HIV [Citation4,Citation14,Citation15]. There is a need for de-stigmatization and casting a wider net for STI prevention screening/care services, so that they are available to everyone that is sexually active. In Canada, recommendations for using opportunistic STI screening (offered in primary care settings during visits that may or may not be for sexual health-related concerns) [Citation16] have the potential to increase diagnostic yield and reduce stigma through normalization of sexual health conversations [Citation17]. The data from our study and the current screening guidelines [Citation4,Citation14,Citation15] underscore the importance of STI testing in those with LGUTS, even when an initial STI diagnosis is not considered.
Previous literature in the US has indicated that a sizable minority of persons presenting to the ED with LGUTS have an STI, either instead of or in addition to another diagnosis [Citation3,Citation18–21]. Another study evaluating adolescent men and women presenting with LGUTS to the ED in the US found a lack of STI testing in 49% of the patients, representing missed opportunities for STI diagnosis [Citation22]. In the dataset we analyzed, prescriptions or diagnostic tests for STIs were inadequately provided, such that we are confident a meaningful number of STI cases were missed. When clinicians encounter patients presenting with LGUTS, greater consideration should be given to the potential for the presence of an STI.
In our study, from the identification of the GAP cohort, we have made the following observations that indicate missed opportunities for STI testing and accurate treatment. The median age of women and men in the GAP cohort (women: 45.0 years old, men: 51.0 years old) were older than their counterparts in the original LGUTS study (women: 37 years old, men: 45 years old) [Citation6]. This suggests that clinicians are less likely to consider an STI diagnosis in older adults. Similarly, a previous integrative review observed that, despite reported cases of STIs increasing among older adults, there are still missed opportunities to identify STIs in this population due to infrequent testing [Citation23].
FLQs are not recommended by the US Food and Drug Administration for management of uncomplicated UTIs [Citation24], and levofloxacin and ofloxacin are alternative CT treatment options recommended in the CDC 2015 guidelines [Citation8]. More than 50% of the FLQ subgroup were diagnosed with UTIs at index, which could indicate inappropriate FLQ treatment if these were uncomplicated UTIs. It is possible that treating clinicians were covering multiple possible diagnoses (UTI and STI) with the use of FLQ, while not testing for STIs in these patients. However, the lack of post-index STI testing in this subgroup is surprising, particularly as a thorough work-up evaluation is required for complicated UTIs that often occur in men [Citation25]. Additionally, the observed high rates of return visits in this subgroup may reflect diagnostic uncertainty, unresolved symptoms, or both. Although FLQ is not recommended for NG, most cases would have been effectively treated with ciprofloxacin [Citation10,Citation26], and there is ongoing research into using molecular testing to predict ciprofloxacin resistance, which could potentially enable this treatment option to be made available for those with ciprofloxacin-susceptible NG infections [Citation27]. Nevertheless, inappropriate use of treatment and suboptimal diagnosis could contribute to increasing antimicrobial resistance (AMR) in NG [Citation28], as ciprofloxacin resistance occurs in around 35% of NG isolates in the US [Citation9].
DOX and AZM are not recommended treatments for UTIs, dysuria, and cystitis, yet over half of the men and women in the AZM and/or DOX subgroup were diagnosed with a UTI or dysuria at index, suggesting a delayed consideration of a possible STI. Around a third of men in this subgroup were diagnosed with orchitis/epididymitis, acute prostatitis, or urethritis at index. STIs are potential causes of these conditions [Citation2]; DOX is the recommended treatment for CT, and AZM is a component of an alternative treatment for NG [Citation4]. However, diagnostic CT/NG testing rates post index were still very low (<10%). Women in this subgroup who were diagnosed with a UTI and/or acute cystitis at index had the longest median time to treatment post index (9 days), which is concerning as a delay in treatment could increase the risk of STI transmission and development of complications, such as PID [Citation4]. Given the treatment administered to this subgroup, most of the episodes probably represented an STI, and therefore the entirety of this subgroup should have been tested for CT/NG. Empirical treatment of STIs without testing hampers accurate STI surveillance and, potentially, partner referrals if the index patient was not clearly informed of the potential for an STI to be the cause of their symptoms.
Due to CEPs (specifically CRO) being the recommended treatment for NG [Citation4], all episodes in the CEP subgroup are likely to be delayed suspected NG infections. This subgroup had the highest post-index CT/NG testing rates; however, these rates were still low (<20%), suggesting that treatment in most cases was provided empirically. This subgroup was among those with the highest number of return visits, which could indicate patients needing to return for parenteral treatment or test of cure.
A GEN treatment is recommended alongside AZM for the treatment of NG in the case of CEP allergy [Citation12]. As around 8–10% of the US population has reported beta-lactam allergy, this treatment recommendation would be suitable for uncomplicated NG [Citation29,Citation30]. However, only a small group of episodes were in the GEN and AZM subgroup. This could indicate underutilization of GEN to treat STIs after this recommendation was incorporated in 2015 and subsequent CDC STI treatment guidelines.
Our analysis highlights the importance of accurate testing to avoid missing diagnosis of STIs and use of inappropriate treatment in patients presenting with LGUTS. The current NAAT tests used to diagnose CT and NG can be costly and require laboratory infrastructure, which limits accessibility [Citation31]. However, point-of-care (POC) tests for rapid STI diagnosis are now available or in development, which have the potential to expand access to testing and reduce the time to treatment [Citation31–33]. Additionally, using POC testing could avoid overuse and misuse of antimicrobials and reduce transmission of AMR NG strains [Citation32]. Conventional laboratory-based testing for STIs can also involve multiple clinic visits, which can result in loss to follow-up [Citation34]; instead, technologies that are able to produce same-day results are preferred by patients [Citation34]. Other studies have investigated the performance of POC tests using self-collected swabs for the detection of CT and NG [Citation35,Citation36]. A CT/NG POC test using self-obtained vaginal swab samples had equivalent performance to laboratory-based molecular assays, and the use of this test facilitated testing and treatment of these STIs in a single patient visit [Citation35]. Another study using a CT POC test found that the majority of women preferred to self-collect vaginal swabs and were willing to wait up to 20 min for results [Citation36]. Further advances in POC testing will hopefully improve the speed and accuracy of STI diagnoses and enable earlier and appropriate treatment in patients presenting with LGUTS.
Our study has some limitations. Because the individual patient-level data come from insurance claims and do not provide all clinical details (e.g. reasons for prescribing certain treatments), a number of assumptions have to be made to derive clinically pertinent conclusions. First, we assume that when treatments are provided within 2 weeks of the index visit, this treatment is directed at the problem identified at the index. While it is possible that a prescription issued 1 week after an index LGUTS visit was for an unrelated issue, we feel that is generally unlikely. Second, the absence of evidence of prescriptions for STIs within our database does not exclude the potential that some patients may have received treatment that was not captured in the database, for example, due to the treatment not being reimbursed or being provided at the clinic without billing. Finally, provision of antibiotic treatment without a return visit was presumed to indicate that the patient’s problem was satisfactorily resolved, as indicated by the failure to have a return visit. However, some patients may not have come back for a return visit, even if their problem was not resolved. Only testing codes for CT/NG were used in this study; MG does not have an MG-specific Current Procedural Terminology (CPT) code, and therefore MG testing is billed using a generic CPT code. Additionally, due to a lack of awareness and no standardized testing or guidelines for MG during the study period, we could not make any firm conclusions regarding testing and treatment rates for MG in this study. It should be acknowledged that within the excluded groups, particularly exclusion group 6 (), there will be other misdiagnosed/undiagnosed STI infections. This exclusion group comprised episodes in which there was no evidence of having an STI at index, nor any of the prototypic diagnoses related to specific antibiotics (e.g. TV and UTIs), and only 1.1% underwent post-index CT/NG testing. Therefore, for 99% of this group, the STI status of these episodes is unknown, and as previous literature has highlighted misdiagnosis of STIs in patients with UTI symptoms [Citation3,Citation18–21], it could be speculated that a substantial number of the episodes in this exclusion group contained undiagnosed STIs. However, this cannot be confirmed in the absence of confirmatory tests or antibiotic prescriptions.
5. Conclusions
In conclusion, our study analyzed 833,574 LGUTS episodes, identified from the IBM MarketScan Research Databases between 2010 and 2019, that were coded or treated as a non-STI disorder at the first visit but were potential missed STIs as a result of LGUTS that overlap with other disorders, such as UTIs. We found delayed treatment with all antibiotics in the four GAP subgroups used to treat CT and NG and low rates of CT/NG testing, indicating delayed consideration of CT/NG, possible inappropriate antibiotic use, and suboptimal diagnosis. These findings emphasize the importance of prompt and accurate STI diagnosis and treatment in patients presenting with LGUTS. Delays in diagnosis or treatment could lead to various complications in the patient, as well as result in the spread of infection and negative consequences to those in contact with undiagnosed or inappropriately treated individuals.
Abbreviations
| AMR | = | antimicrobial resistance |
| AZM | = | azithromycin |
| BV | = | bacterial vaginosis |
| CDC | = | Centers for Disease Control and Prevention |
| CEP | = | cephalosporins |
| CFM | = | cefixime |
| CPT | = | Current Procedural Terminology |
| CRO | = | ceftriaxone |
| CT | = | Chlamydia trachomatis |
| CTX | = | cefotaxime |
| CXT | = | cefoxitin |
| DOX | = | doxycycline |
| ED | = | emergency department |
| FLQ | = | fluoroquinolone |
| FOS | = | fosfomycin |
| GEN | = | gentamicin |
| HIPAA | = | Health Insurance Portability and Accountability Act |
| IBM | = | The International Business Machines Corporation |
| ICD | = | International Classification of Diseases |
| IQR | = | interquartile range |
| LGUTS | = | lower genitourinary tract symptoms |
| MG | = | Mycoplasma genitalium |
| NAATs | = | nucleic acid amplification tests |
| NG | = | Neisseria gonorrhoeae |
| PID | = | pelvic inflammatory disease |
| POC | = | point-of-care |
| STI | = | sexually transmitted infections |
| TMP-SMX | = | trimethoprim-sulfamethoxazole |
| TV | = | Trichomonas vaginalis |
| UAI | = | urinary anti-infectives |
| UGTIs | = | urogenital tract infections |
| US | = | United States |
| UTIs | = | urinary tract infections |
Declaration of financial/other relationships
Louis Kuritzky reports receiving consulting fees for this study from Roche Diagnostics Solutions, as well as receipt of previous consulting fees and medical writing support from Roche Molecular Systems. Zune Huynh, Rodney Arcenas, Avneet Hansra, Roma Shah, and Baiyu Yang are employees of Roche Molecular Systems and report receiving stocks and stock options from Roche. Rebecca Lillis reports receiving funding for this study from Roche, receipt of previous grants for clinical trials from Abbot, Hologic, Visby, OrthoClinical Diagnostics, Becton Dickinson, BioFire, Chembio, Cue, Cepheid, Merck, LabCorp, Roche, and Gilead, as well as a previous speakers’ bureau payment from Cepheid, and reports participation in Roche and Abbot scientific advisory boards. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Louis Kuritzky, Zune Huynh, and Rodney Arcenas were involved in the conceptualization of the study. Louis Kuritzky, Zune Huynh, Roma Shah, and Baiyu Yang participated in the design of the methodology. Louis Kuritzky, Zune Huynh, Avneet Hansra, Roma Shah, Baiyu Yang, and Rebecca Lillis contributed to the data analysis and interpretation. Zune Huynh, Rodney Arcenas, Avneet Hansra, and Baiyu Yang were involved in project administration. All authors reviewed and edited the draft for important intellectual content and all have read and agreed to the final version of the manuscript.
Acknowledgments
The authors would like to thank Joanna Sickler (Roche Molecular Systems) who supported with the study conceptualization and protocol development, Stephanie N. Taylor (Louisiana State University Health Sciences Center) who supported with the study conceptualization and data interpretation, and Yutong Liu (Genesis Research) for her help with the data analysis for this study. Medical writing support was provided by Katie Farrant at Elements Communications Ltd, London, UK, and was funded by Roche Molecular Systems. IBM Watson Health and MarketScan are trademarks of IBM Corporation in the US, other countries, or both. The opinions expressed in this publication do not reflect or express the opinions of the University of Florida or its employees. These data have not been previously presented elsewhere.
Data availability statement
The patient data that support the findings of this study are available from the IBM (now Merative™) MarketScan Research Databases (Commercial Database and Multi-State Medicaid Database), but restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. The databases are available for secondary use on a commercial basis, and requests for access to the data should be sent to IBM Watson Health and not the corresponding author. All data generated from the analysis of the databases are included in this published article.
Additional information
Funding
References
- Centers for Disease Control and Prevention. National overview of STDs, 2021. 2023 [updated May 16, 2023; cited Jun 12, 2023]. Available from: https://www.cdc.gov/std/statistics/2021/overview.htm
- Behzadi P, Behzadi E, Pawlak-Adamska EA. Urinary tract infections (UTIs) or genital tract infections (GTIs)? it’s the diagnostics that count. GMS Hyg Infect Control. 2019;14:Doc14. doi: 10.3205/dgkh000320
- Tomas ME, Getman D, Donskey CJ, et al. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol. 2015;53(8):2686–2692.
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187.
- Tuddenham S, Hamill MM, Ghanem KG. Diagnosis and treatment of sexually transmitted infections: a review. JAMA. 2022;327(2):161–172. doi: 10.1001/jama.2021.23487
- Lillis R, Kuritzky L, Huynh Z, et al. Outpatient sexually transmitted infection testing and treatment patterns in the United States: a real-world database study. BMC Infect Dis. 2023;23(1):469.
- Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. 2017 [updated 2017 Oct 3; cited 2023 Jan 3]. Available from: https://www.cdc.gov/antibiotic-use/clinicians/adult-treatment-rec.html
- Workowski KA, Bolan GA, Centers for Disease C, et al. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR–03):1–137.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020: gonococcal isolate surveillance project profile. 2022 [cited 2023 Jun 12]. Available from: https://www.cdc.gov/std/statistics/gisp-profiles/gisp_profiles_national_final.pdf
- Centers for Disease Control and Prevention. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56(14):332–336 .
- Bui T, Preuss CV. Cephalosporins. StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2022, StatPearls Publishing LLC; 2022.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1911–1916.
- Centers for Disease Control and Prevention. U.S. STI epidemic showed no signs of slowing in 2021. 2023 [updated 2023 Apr 11; cited 2023 Apr 11]. Available from: https://www.cdc.gov/nchhstp/newsroom/2023/2021-STD-surveillance-report.html
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. JAMA. 2021;326(10):949–956.
- Centers for Disease Control and Prevention. Screening recommendations and considerations referenced in treatment guidelines and original sources. 2021 [updated 2022 Jun 6; cited 2023 Jun 6]. Available from: https://www.cdc.gov/std/treatment-guidelines/screening-recommendations.htm
- Moore A, Traversy G, Reynolds DL, et al. Recommendation on screening for chlamydia and gonorrhea in primary care for individuals not known to be at high risk. CMAJ. 2021;193(16):E549–E559.
- Grennan T, Tan DHS. Benefits of opportunistic screening for sexually transmitted infections in primary care. CMAJ. 2021;193(16):E566–E567. doi: 10.1503/cmaj.210604
- Huppert JS, Biro F, Lan D, et al. Urinary symptoms in adolescent females: STI or UTI? J Adolesc Health. 2007;40(5):418–424.
- Shapiro T, Dalton M, Hammock J, et al. The prevalence of urinary tract infections and sexually transmitted disease in women with symptoms of a simple urinary tract infection stratified by low colony count criteria. Acad Emerg Med. 2005;12(1):38–44.
- Prentiss KA, Newby PK, Vinci RJ. Adolescent female with urinary symptoms: a diagnostic challenge for the pediatrician. Pediatr Emerg Care. 2011;27(9):789–794. doi: 10.1097/PEC.0b013e31822c10f6
- Wilbanks MD, Galbraith JW, Geisler WM. Dysuria in the emergency department: missed diagnosis of Chlamydia trachomatis. West J Emerg Med. 2014;15(2):227–230. doi: 10.5811/westjem.2013.12.18989
- Musacchio NS, Gehani S, Garofalo R. Emergency department management of adolescents with urinary complaints: missed opportunities. J Adolesc Health. 2009;44(1):81–83. doi: 10.1016/j.jadohealth.2008.05.011
- Tillman JL, Mark HD. HIV and STI testing in older adults: an integrative review. J Clin Nurs. 2015;24(15–16):2074–2095. doi: 10.1111/jocn.12797
- U.S. Food and Drug Administration. FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. 2016 [updated 2018 Sep 25; cited 2023 Jun6]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-advises-restricting-fluoroquinolone-antibiotic-use-certain
- Michels TC, Sands JE. Dysuria: evaluation and differential diagnosis in adults. Am Fam Physician. 2015;92(9):778–786.
- Melendez JH, Hsieh YH, Barnes M, et al. Can ciprofloxacin be used for precision treatment of gonorrhea in public STD clinics? Assessment of ciprofloxacin susceptibility and an opportunity for point-of-care testing. Pathogens. 2019;8(4):189.
- Golparian D, Unemo M. Antimicrobial resistance prediction in Neisseria gonorrhoeae: current status and future prospects. Expert Rev Mol Diagn. 2022;22(1):29–48. doi: 10.1080/14737159.2022.2015329
- Unemo M, Seifert HS, Hook EW 3rd, et al. Gonorrhoea. Nat Rev Dis Primers. 2019;5(1):79.
- Plager J, Judd A, Blumenthal K. Role of clinical history in beta-lactam hypersensitivity. Curr Opin Allergy Clin Immunol. 2021;21(4):320–326. doi: 10.1097/ACI.0000000000000758
- Mitchell AB, Ness RA, Bennett JG, et al. Implementation and impact of a β-lactam allergy assessment protocol in a veteran population. Fed Pract. 2021;38:420–425. doi: 10.12788/fp.0172
- Adamson PC, Loeffelholz MJ, Klausner JD. Point-of-care testing for sexually transmitted infections: a review of recent developments. Arch Pathol Lab Med. 2020;144(11):1344–1351. doi: 10.5858/arpa.2020-0118-RA
- Toskin I, Govender V, Blondeel K, et al. Call to action for health systems integration of point-of-care testing to mitigate the transmission and burden of sexually transmitted infections. Sex Transm Infect. 2020;96(5):342–347.
- Binx IO CLIA-waived, point-of-care testing. That’s the binx io. 2023 [cited Jun 6, 2023]. Available from: https://mybinxhealth.com/point-of-care
- Naeem F, Karellis A, Nair S, et al. Multiplexed technologies for sexually transmitted infections: global evidence on patient-centered and clinical health outcomes. BMJ Glob Health. 2021;6(7):e005670.
- Van Der Pol B, Taylor SN, Mena L et al. Evaluation of the performance of a point-of-care test for chlamydia and gonorrhea. JAMA Netw Open. 2020;3:e204819. doi: 10.1001/jamanetworkopen.2020.4819
- Widdice LE, Hsieh YH, Silver B, et al. Performance of the atlas genetics rapid test for Chlamydia trachomatis and women’s attitudes toward point-of-care testing. Sex Transm Dis. 2018;45:723–727. doi: 10.1097/OLQ.0000000000000865
