ABSTRACT
Objectives
Appraise the evidence for daridorexant 50 mg and 25 mg versus placebo when treating chronic insomnia disorder in terms of number needed to treat (NNT), number needed to harm (NNH), and likelihood to be helped or harmed (LHH).
Methods
NNT, NNH, and LHH were calculated from a 3-month pivotal Phase 3 study (N = 930; randomized 1:1:1 to daridorexant 50 mg, daridorexant 25 mg, or placebo once nightly). Wakefulness after sleep onset, latency to persistent sleep, self-reported total sleep time, Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ), and Insomnia Severity Index were used for the NNT efficacy analysis. NNH safety analysis was performed using rates of adverse events (AEs) occurring in >1% of the participants in any arm. LHH was assessed for all NNT estimates, contrasting them with NNH estimates for somnolence, headache, and fatigue AEs.
Results
NNT estimates for daridorexant 50 mg versus placebo were <10 for clinically meaningful thresholds across all outcomes. NNT estimates for daridorexant 25 mg versus placebo were not as robust as those observed for daridorexant 50 mg, with many values exceeding 10. NNH estimates for daridorexant 50 mg and 25 mg versus placebo did not show a statistically significant treatment difference except for falls, where NNH was negative for the daridorexant 50 mg group (−44 [95% CI −328; −21]; rate of falls was greater with placebo than for daridorexant 50 mg). All LHH ratios at Months 1 and 3 were >1 (except for daridorexant 25 mg for the IDSIQ alert/cognition domain), indicating that patients were more likely to respond to daridorexant 50 mg and 25 mg than to experience an AE of somnolence, headache, or fatigue.
Conclusion
Daridorexant 50 mg and 25 mg have a favorable benefit–risk ratio over 3 months. Daridorexant 50 mg demonstrated more robust (lower) NNT estimates versus placebo than daridorexant 25 mg.
Plain Language Summary
Daridorexant, a dual orexin receptor antagonist, is a new treatment for chronic insomnia disorder. This analysis examined the effect and safety of daridorexant 50 and 25 mg, using data from a 3-month Phase 3 study (NCT03545191) to measure ‘number needed to treat’ (NNT) and ‘number needed to harm’ (NNH).
NNT estimates how many patients need to be treated over a specific period to see one more beneficial response. Estimates versus placebo <10 indicate an effective treatment. Daridorexant 50 mg estimates were <10 for all objective and subjective measurements of insomnia assessed in this analysis, including evaluation of daytime functioning. NNT estimates for daridorexant 25 mg versus placebo were not as robust as daridorexant 50 mg, with values >10.
NNH is calculated in the same way as NNT but estimates harmful outcomes rather than benefits. Estimates versus placebo >10 means the treatment is reasonably well tolerated.
Using NNT and NNH, the ‘likelihood to be helped or harmed’ (LHH) ratio was calculated, determining how more likely a patient is to benefit versus experiencing harm from a treatment (LHH of >1 denotes a positive benefit–risk ratio). Both daridorexant doses had a favorable benefit–risk ratio over 3 months with LHH > 1.
This analysis supports daridorexant 50 mg as the optimal dose to treat insomnia in adults, offering improved effectiveness compared with daridorexant 25 mg, with a similarly good safety profile.
1. Introduction
Chronic insomnia disorder (CID) is defined as difficulty in initiating or maintaining sleep, occurring at least three nights per week for at least 3 months, and associated with daytime impairment [Citation1]. These symptoms occur despite adequate opportunity for sleep. The estimated prevalence of CID ranges from 6% to 10%, while CID symptoms are present in approximately one-third of the general population [Citation2–6]. CID is associated with substantial impairments in an individual’s day-to-day quality of life and is responsible for a significant economic burden, including costs related to loss of productivity [Citation7–13]. In addition, CID is associated with increased risk for morbidity (e.g. psychiatric disorders, diabetes, and cardiovascular disease) [Citation14].
International guidelines recommend cognitive-behavioral therapy for insomnia, both face-to-face and digital, as first-line treatment in adults with CID. Cognitive-behavioral therapy as a CID treatment has demonstrable clinical efficacy but suffers from low accessibility and adherence [Citation4,Citation15–18]. Pharmacotherapies are recommended as second-line treatment, but generally for short-term use due to safety concerns [Citation19,Citation20].
A new class of treatments emerged with the availability of orexin receptor antagonists [Citation17]. Orexin A and B are excitatory neuropeptides with a role in different physiologic functions related to sleep/wake rhythms, and include thermoregulation, control of energy, metabolism, cardiovascular responses, feeding behavior, and spontaneous physical activity via projections from the hypothalamus to other parts of the brain [Citation21]. Daridorexant is a new dual-orexin receptor antagonist (DORA) with dose-dependent sleep-promoting effects without residual next-morning effects in adults and older adults diagnosed with CID [Citation22,Citation23]. Daridorexant has market authorization in Canada, the European Union, Switzerland, the United Kingdom, and the United States for both 25 mg and 50 mg doses [Citation24–27].
Regulatory approval of interventions generally requires the demonstration of statistically significant treatment effects when comparing the test medication versus placebo and is assessed by p values. However, clinical trial results may be clinically irrelevant if the treatment effect size is small, and thus there is a need to calculate effect size [Citation28]. Number needed to treat (NNT) for benefits and number needed to harm (NNH) for harms are effect sizes that are relatively simple to calculate and easy to understand [Citation29]. Likelihood to be helped or harmed (LHH), calculated by dividing NNH by NNT, can assist in appraising benefit–risk ratio [Citation30]. NNT, NNH, and LHH can be calculated from the binary outcomes reported in randomized clinical trials [Citation31] and provide a useful means for healthcare providers to interpret the clinical relevance of statistically significant trial results [Citation32].
NNT is an estimate of the number of patients ‘needed to be treated’ with treatment A versus treatment B to expect one more positive response. Likewise, NNH is an estimate of the number of patients needed to be treated with treatment A versus treatment B to expect one more event of a harm (such as an adverse event [AE]). Broadly speaking, for efficacy endpoints, NNT estimates versus placebo <10 indicate a clinically relevant signal for efficacy, and an NNH versus placebo >10 denotes reasonable tolerability (subject to interpretation depending on the safety outcome being measured) [Citation32,Citation33]. LHH contrasts the potential benefit of an intervention to its risk, with LHH > 1 denoting that the likelihood of encountering a benefit is greater than that for encountering a harm [Citation30]. NNT, NNH, and LHH have been used to characterize other DORAs [Citation33,Citation34].
The efficacy and favorable safety profile of daridorexant has been demonstrated in adult and older adult populations diagnosed with CID [Citation22,Citation23]. The aim of this study was to estimate the NNT, NNH, and LHH of daridorexant (25 mg and 50 mg) in comparison with placebo, calculated using data from a Phase 3 pivotal study (NCT03545191) of daridorexant in adults with CID [Citation22]
2. Methods
2.1. Data source
This post hoc analysis was performed using data from a 3-month Phase 3 clinical trial (NCT03545191: ‘Study to assess the efficacy and safety of ACT-541468 (daridorexant) in adult and elderly subjects with insomnia disorder’), which included the two approved daridorexant doses (25 mg and 50 mg) and placebo [Citation22]. Full inclusion criteria, study design, details of ethics approval and study conduct, and results of the Phase 3 trial have been reported by Mignot et al. [Citation22]. Among other inclusion and exclusion criteria, the study recruited participants with a latency to persistent sleep (LPS) ≥20 minutes, wakefulness after sleep onset (WASO) ≥30 minutes, and mean total sleep time (TST) of <7 hours, as measured by polysomnography recording during two consecutive nights. Overall, 930 patients were included (all randomized participants; 310 received daridorexant 25 mg, 310 received daridorexant 50 mg, and 310 received placebo). Mean age was 55.4 years and 364 (39%) of the participants were ≥65 years old. The majority of patients were female (67% among the treatment arms).
2.2. Outcome measures
The efficacy endpoints were assessed at Months 1 and 3 following treatment initiation (). The objective sleep measures (primary endpoints in the pivotal study) were the change from baseline in WASO and LPS, measured by polysomnography. The subjective endpoints were the change from baseline in self-reported TST (sTST, a secondary endpoint in the pivotal study) and total and domain scores of the Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ; the sleepiness domain was a secondary endpoint in the pivotal study). In addition, the Insomnia Severity Index (ISI) was assessed.
Table 1. Responder thresholds for the NNT analysis.
2.3. Definition of clinically meaningful thresholds
describes the thresholds for the dichotomization of the continuous efficacy outcomes and the rationale for their selection. Due to the absence of validated minimal clinical improvement (MiCI) thresholds for the within-patient change from baseline in objective WASO and LPS endpoints, a reduction of ≥20 minutes for WASO and ≥15 minutes for LPS were used as thresholds in this analysis. These were determined visually using the empirical cumulative distribution function of the change from baseline in WASO and LPS, to identify the value at which the two curves for daridorexant and placebo become distinct and are approximately parallel (the minimum improvement; see Supplementary Figures S1 and S2).
The clinically meaningful within-patient change for sTST was estimated utilizing anchor-based analyses on two interventional clinical trials in adults with CID, including the Phase 3 pivotal study of daridorexant (NCT03545191) [Citation35,Citation36]. These analyses supported a clinically meaningful within-patient change threshold of 55 minutes. Therefore, an increase in sleep time of 55 minutes (excluding perceived awakenings) was determined to be the MiCI responder threshold for sTST analysis.
For the IDSIQ sleepiness domain score, an anchor-based analysis on change from baseline to Month 3 showed a meaningful within-patient decrease of ≥4 points for the sleepiness domain. For the other IDSIQ domain scores, the same anchor-based analyses identified an MiCI responder threshold of 9 points for the IDSIQ alert/cognition domain and 4 points for the IDSIQ mood domain, which align with previous findings [Citation37]. Finally, an MiCI within-patient change threshold of at least 20 points for the IDSIQ total score was found ().
For the ISI, three responder thresholds for the within-subject change from baseline in total ISI score were previously reported [Citation38,Citation39] and used in the present NNT analysis: a reduction of ≥6 points for MiCI, ≥7 points for moderate clinical improvement (MoCI), and ≥8 points for marked clinical improvement (MaCI).
To describe multiple clinically meaningful thresholds (CMTs) in the context of NNT assessment, threshold responder evolution curve (TREC) figures were developed, displaying NNT estimates according to a range of responder thresholds. TRECs were developed for all efficacy endpoints, beginning with the least robust threshold defined by the value preceding MiCI and ending with the threshold where the last significant estimate is NNT ≤20 regardless of dose, unless specified.
The NNH analysis included treatment-emergent AEs occurring in >1% of the participants in at least one treatment arm over the 3-month trial period plus the 30 days of the placebo run-out period.
2.4. Statistics
NNT efficacy analyses for daridorexant 25 mg and daridorexant 50 mg at Months 1 and 3 were performed on all participants randomized in the pivotal study, i.e. the full analysis set. NNH safety analyses were performed on all participants who received at least one dose of study treatment, i.e. the safety set.
When calculating NNT, a responder (n) is defined as a participant with an improvement equal to or exceeding a pre-defined within-patient change threshold. The denominator (N) to calculate responder rates is the number of participants with information available at a given timepoint. The absolute adjusted difference (AD) in responder rates between daridorexant and placebo for each threshold was computed using Mantel–Haenszel common risk (proportion) difference stratified by age group (<65 years and ≥65 years) with corresponding 95% confidence intervals (CIs). A sensitivity analysis for NNT for daridorexant 25 mg and daridorexant 50 mg at Months 1 and 3 was also conducted using the total number of patients in the full analysis set as the denominator; participants with missing data were counted as non-responders.
The NNT and NNH were estimated by taking the reciprocal of the AD in responder rates and the absolute difference in AE incidences, respectively, with corresponding 95% CIs. If the 95% CI of the absolute difference between daridorexant and placebo included 0, reflecting no difference between treatment groups, the corresponding NNT or NNH 95% CI would include ‘infinity’ and so the NNT or NNH would thus be considered not significant [Citation40].
In the TREC figures, the upper bound of the graph was limited to NNT = 50, which equals a difference in responder rates of 2%. All NNT and NNH estimates were rounded up.
The LHH ratio was calculated for each efficacy variable across the AEs of headache (the most frequent AE in the daridorexant 50 mg arm), fatigue, and somnolence (nervous system-related AEs). LHH ratios cannot be calculated with negative NNH estimates; therefore, negative NNH estimates were redefined (imputed) as having a value of 1000 (representing an absolute risk increase of 0.001, and with an incalculable 95% CI) when determining the LHH ratio, as has been done in other reports of a similar nature [Citation41,Citation42].
The numerators and denominators used in the calculation for the NNT and NNH estimates are reported in the supplementary material.
3. Results
3.1. Efficacy: NNT results
All NNT results for polysomnographic and self-reported efficacy endpoints with regard to the MiCI thresholds are summarized in .
Table 2. NNT estimates for the WASO, LPS, sTST, IDSIQ, and ISI at month 1 and month 3 based on MiCI thresholda
At Month 1, NNT estimates for daridorexant 50 mg compared with placebo were <10 for all efficacy endpoints at MiCI thresholds. NNT estimates for the nighttime sleep parameters WASO, LPS, and sTST were 5 (95% CI 4 to 8; AD 21% [95% CI 13.1 to 28.6]), 7 (95% CI 5 to 15; AD 14.6% [95% CI 6.8 to 22.2]), and 6 (95% CI 4 to 9; AD 19.6% [95% CI 12.3 to 26.6]), respectively. For daytime functioning, NNT estimates for IDSIQ domain (sleepiness, alert/cognition, and mood) or total score were achieved with NNT estimates in the range of 6–8 for daridorexant 50 mg compared with placebo. Therefore, on average, eight patients needed to be treated with daridorexant 50 mg versus placebo to obtain one additional responder for all domains of the IDSIQ. NNT estimates for ISI score for the three CMTs ranged from 8 to 9 for daridorexant 50 mg versus placebo.
At Month 3, NNT estimates for daridorexant 50 mg compared with placebo ranged from 6 to 12 for all efficacy endpoints at MiCI thresholds. For the nighttime sleep parameters WASO, LPS, and sTST, NNT estimates were, respectively, 6 (95% CI 4 to 10; AD 18.9% [10.7 to 26.7]), 11 (95% CI 6 to 74; AD 9.1% [1.4 to 16.7]), and 9 (95% CI 6 to 28; AD 11.6% [3.6 to 19.5]). For daytime functioning, NNT estimates for IDSIQ domain (sleepiness, alert/cognition, and mood) or total score were achieved within a range of 6 to 12 patients treated with daridorexant 50 mg compared with placebo. NNT estimates for ISI score were more robust from 11, 8, and 6 for the MiCI, MoCI, and MaCI thresholds, respectively.
Efficacy for daridorexant 25 mg versus placebo was not as robust as for the 50 mg dose versus placebo, reflecting a dose–response relationship.
The results of the NNT sensitivity analysis using the total number of patients in the full analysis set (n = 310) as the denominator did not differ from those of the primary analysis (Supplementary Material, Table S2).
3.2. Threshold responder evolution curves
For WASO, daridorexant 50 mg versus placebo demonstrated NNT estimates ≤10 for CMTs ranging from ≤20 to ≤59 minutes at Month 1, and ≤20 to ≤44 minutes at Month 3 (Supplementary Material, Figure S3). NNT estimates >10 but <20 were observed for CMTs ranging from ≤ 45 to ≤54 minutes at Month 3.
For LPS, NNT estimates with daridorexant 50 mg versus placebo were ≤10 for all the CMTs ranging from ≤15 to ≤30 minutes at Month 1, with the exception of the CMT ≤22 minutes. At Month 3, for the same dose, NNT values were ≤10 for CMTs from ≤24 to ≤26 minutes (Supplementary Material, Figure S4).
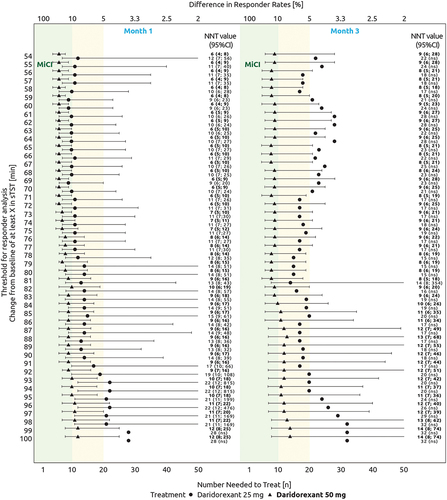
For sTST, daridorexant 50 mg versus placebo demonstrated NNT estimates ≤10 for all CMTs ranging from ≥55 to ≥95 minutes at Month 1, and ≥55 to ≥84 minutes at Month 3 (). NNT estimates >10 but <20 were observed for CMTs ranging from ≥96 to ≥100 minutes at Month 1, and ≥85 to ≥100 minutes at Month 3.
Figure 1. Threshold responder evolution curve across a range of estimated values for change from baseline in sTST (minutes). The green area indicates NNT estimates ≤10. The yellow area indicates NNT estimates between 10 and 20. The lower x-axis focuses on NNT estimates between 1 and 50. NNT estimates >50 are not shown in the figure and the 95% CI values are only shown if the comparison with placebo is significant. NNT and 95% CI values (in or outside the figure) are reported in the left side of the figure. The absolute adjusted difference in responder rates is displayed on the upper x-axis.
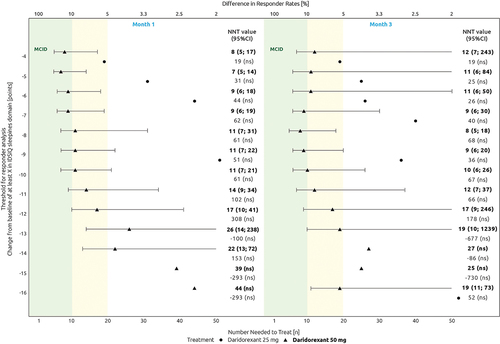
For the IDSIQ sleepiness domain, NNT estimates for daridorexant 50 mg versus placebo ranged from 7 to 12 for CMTs between ≤4 (MiCI) and ≤10 points at Months 1 and 3 ().
Figure 2. Threshold responder evolution curve across a range of estimated values for change from baseline in IDSIQ sleepiness domain (scale of 0 to 28 points). The green area indicates NNT estimates ≤10. The yellow area indicates NNT estimates between 10 and 20. The lower x-axis focuses on NNT estimates between 1 and 50. NNT estimates >50 are not shown in the figure and the 95% CI values are only shown if the comparison with placebo is significant. NNT and 95% CI values (in or outside the figure) are reported in the left side of the figure. The absolute adjusted difference in responder rates is displayed in the top x-axis.
The TREC figures for the two other IDSIQ domains (alert/cognition and mood) and the IDSIQ total score are shown in the Supplementary Material (Figures S5 to S7) and demonstrated similar results to those observed for the IDSIQ sleepiness domain.
For ISI, NNT estimates with daridorexant 50 mg versus placebo ranged between 8 and 11 for CMTs from ≤6 to ≤11 points and ≤6 to ≤14 points, at Months 1 and 3, respectively. At Month 3, NNT values declined gradually from 10 to 20 for CMTs ranging from −14 to −18 points (Supplementary Material, Figure S8).
For daridorexant 25 mg versus placebo, TREC figures showed NNT estimates ≤10 in WASO for CMTs from ≤20 to ≤28 minutes, at Months 1 and 3 (Supplementary Material, Figure S3). In LPS, NNT estimates with daridorexant 25 mg versus placebo were ≤10 for CMTs ranging from ≤15 to ≤29 minutes at Month 1 (Supplementary Material, Figure S4). At Month 3, for the same dose, NNT estimates were ≤10 for CMTs ≤21 to ≤24 and ≤30 to ≤33 minutes (Supplementary Material, Figure S4). In sTST, at Month 1, NNT estimates were between 9 and 12 for CMTs ranging from ≥54 to ≥78 (). At Month 3, approximately half of the NNT values for daridorexant 25 mg values were between 10 and 20. In IDSIQ sleepiness domain and ISI, NNT estimates were above 10 for all CMTs ( and Supplementary Material, Figure S8).
3.3. Safety: NNH results
During the study, the rates of treatment discontinuation due to an AE were low, and higher in the placebo group (10 [3%] of 309 participants) than in those on active treatment: 7 (2%) of 310 participants for daridorexant 25 mg and 3 (1%) of 308 participants for daridorexant 50 mg. provides the NNH estimates for each treatment-emergent AE occurring in >1% of the participants in at least one treatment arm at Month 3, plus the placebo run-out plus safety follow-up period (30 days). All risk differences (except one) for daridorexant and placebo were not significant, with absolute NNH estimates >39 for both daridorexant doses. Only the NNH estimates with daridorexant 50 mg versus placebo at Month 3 for falls (NNH −44 [95% CI −328 to −21]; absolute difference −2.3% [−4.7 to −0.3]) was significant and negative, indicating this event occurred more often with placebo than with daridorexant 50 mg.
Table 3. NNH estimates for daridorexant 25 mg and daridorexant 50 mg compared with placebo related to the treatment-emergent AEs occurring in >1% of subjects at least in one treatment arm at Month 3 + placebo run-out period (30 days).
3.4. Benefit–risk ratio: LHH results
Regarding the LHH ratio, at Months 1 and 3, a patient was 83.3 to 200.0 times more likely to respond to daridorexant 50 mg on all efficacy endpoints than to experience a somnolence-related AE (). For fatigue-related AEs, the LHH ratios of daridorexant 50 mg for all efficacy endpoints were between 4.3 and 10.2 at Months 1 and 3. For headache, the most common AE in the daridorexant 50 mg arm, the LHH ratios for all efficacy endpoints were between 3.2 and 7.6 at Months 1 and 3.
Table 4. LHH ratio with daridorexant 25 mg and daridorexant 50 mg compared with placebo at Months 1 and 3 for all efficacy endpoints and AEs of fatigue, somnolence, and headache.
LHH ratios for daridorexant 25 mg were lower than for the 50 mg dose. At Months 1 and 3, a patient was up to 11.1 times more likely to respond to daridorexant 25 mg on all efficacy endpoints than to experience a somnolence-related AE (). The only efficacy endpoint of daridorexant 25 mg with an LHH ratio <1 was the IDSIQ alert/cognition domain score, contrasting with the occurrence of the AEs of somnolence, fatigue, and headache.
4. Discussion
In patients with CID, daridorexant demonstrated clinically relevant beneficial effects for a broad range of outcomes, as measured by NNT versus placebo, with more robust efficacy reported for the daridorexant 50 mg dose and NNT <10 for all efficacy endpoints at Month 1. Analysis of AEs and NNH for daridorexant 50 mg or daridorexant 25 mg versus placebo demonstrated a good tolerability profile. The overall benefit–risk ratio was favorable for both daridorexant 25 mg and daridorexant 50 mg doses (LHH ratio >1). Efficacy was maintained between Month 1 and Month 3. Overall, the present analysis supports the use of daridorexant 50 mg as the optimal dose to treat CID in adults, offering improved efficacy compared with daridorexant 25 mg, with a similarly good safety profile.
CMTs exist on a spectrum, from MiCI, MoCI, to MaCI [Citation43]. To avoid focusing on only one threshold when assessing NNT, in this study we described a novel approach (TREC) to display NNT estimates as a function of a large range of CMTs. With TREC, it is possible to visually demonstrate if a response is maintained over different responder thresholds. Using TREC in the present analysis, daridorexant 50 mg had at least one NNT estimate <10 versus placebo for CMTs across all efficacy endpoints. Thus, daridorexant 50 mg demonstrated ‘good’ and stable NNT estimates for the efficacy endpoints over a large range of CMTs.
Published estimates of NNT and NNH versus placebo can serve as a source to make indirect comparisons among similar treatments tested under similar conditions, as reported with other hypnotics [Citation33]. A caveat is that the categorical efficacy outcomes being assessed generally differ from study to study. An exception is the availability of responder rates as defined by a ≥ 6-point improvement in ISI total score; this is available for the commercially available DORAs with overlap in the 95% CIs. In this analysis, the NNT estimates with daridorexant 50 mg versus placebo for this outcome were aligned with previously published values for DORAs.
Regarding safety, in the present analysis, no significant NNH estimates were reported for either daridorexant dose versus placebo for AEs, except for a negative NNH estimate for falls (indicating a higher incidence of falls in the placebo arm than in the daridorexant arm). These results confirm the good safety profile of daridorexant. The low incidence of somnolence with daridorexant is likely due to the optimal pharmacokinetic profile of this molecule, including a half-life of 8 hours [Citation22,Citation44,Citation45]. In contrast, the NNH for somnolence for lemborexant 5 mg and 10 mg compared with placebo at Week 4 was 28 (95% CI 18 to 61) and 15 (95% CI 11 to 22), respectively [Citation33]. Regarding suvorexant, NNH versus placebo for somnolence at Month 3 for 15 mg/day or 20 mg/day was 28 (95% CI 17–82) [Citation34].
Limitations of this study include its post hoc nature and the limited sample size using data from one of the two pivotal clinical trials (daridorexant 50 mg n = 310, daridorexant 25 mg n = 310, and placebo n = 310) resulting in larger 95% CIs than if the second pivotal trial had been included in a pooled analysis (which was not done because the second study did not include a daridorexant 50 mg treatment arm). The results may not be generalizable to patients outside the confines of a clinical trial; this is always a concern for results of randomized controlled trials because of the strict inclusion/exclusion criteria that these studies require. Factors influencing outcomes and the observed effect sizes include the baseline characteristics of the participants, study time frame, and a responder threshold definition [Citation46]. Limitations of NNT as a measurement have been discussed previously [Citation47]. NNH analysis is best suited for common tolerability challenges because rare events may not necessarily be observable within the restricted time frame of a trial or detectable in relatively small samples. Moreover, when applying results from clinical trials to clinical practice, there is substantial patient heterogeneity in therapeutic response and tolerability. For example, there may be some patients who are more likely to experience specific AEs than other patients (e.g. somnolence). Decisions on prescribing should therefore be individualized based on the patient’s past history regarding medication side effects. Finally, due to heterogeneity in study design and available outcome measures, caution must be exercised when conducting any indirect comparisons of NNT, NNH, and LHH with those calculated for other treatments in other studies.
Future research with daridorexant with larger sample sizes (allowing subgroup analyses and more precise determinations of NNT), particularly with real-world data (e.g., compared with standard of care), and over a longer observation period (e.g. 1 year) would enable confirmation of the results reported here and contribute to a greater understanding of daridorexant as a treatment for CID.
5. Conclusion
In this study, NNT, NNH, and LHH were calculated with data from a 3-month pivotal Phase 3 placebo-controlled study of daridorexant 50 mg and daridorexant 25 mg [Citation22]. The results confirm that both daridorexant 50 mg and daridorexant 25 mg have a favorable benefit–risk ratio. Daridorexant 50 mg consistently demonstrated efficacy for CMTs for efficacy endpoints at Months 1 and 3, in contrast to the 25 mg dose group, with a similar good safety profile for both doses. Thus, for most patients, 50 mg is likely the optimal daridorexant dose for clinicians to prescribe.
Author contributions
Conceptualization: François-Xavier Chalet, Cristina Rabasa, Pierre-Philippe Luyet, Cédric Vaillant. Methodology: François-Xavier Chalet, Cristina Rabasa, Pierre-Philippe Luyet, Leslie Citrome. Formal Analysis: François-Xavier Chalet, Cristina Rabasa, Pierre-Philippe Luyet. Writing – Original Draft Preparation: François-Xavier Chalet, Cristina Rabasa, Pierre-Philippe Luyet. Writing – Review & Editing: François-Xavier Chalet, Cristina Rabasa, Pierre-Philippe Luyet, Cédric Vaillant, Paul Saskin, Ajay Ahuja, Leslie Citrome. Visualization: François-Xavier Chalet, Pierre-Philippe Luyet. Supervision: Leslie Citrome.
Declaration of financial/other relationships
François-Xavier Chalet, Pierre-Philippe Luyet, Cristina Rabasa, Cédric Vaillant, Paul Saskin, and Ajay Ahuja are employees and shareholders at Idorsia Pharmaceuticals Ltd. Leslie Citrome serves as a consultant for AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, Biogen, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Cerevel, Clinilabs, COMPASS, Delpor, Eisai, Enteris BioPharma, HLS Therapeutics, Idorsia, INmune Bio, Impel, Intra-Cellular Therapies, Janssen, Karuna, Lundbeck, Luye, Lyndra, MapLight, Marvin, Medavante-ProPhase, Merck, Mitsubishi-Tanabe Pharma, Neumora, Neurocrine, Neurelis, Noema, Novartis, Noven, Otsuka, Ovid, Praxis, Recordati, Relmada, Reviva, Sage, Sumitomo/Sunovion, Supernus, Teva, University of Arizona, Vanda, Wells Fargo, and one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research; speaker for AbbVie/Allergan, Acadia, Alkermes, Angelini, Axsome, BioXcel, Eisai, Idorsia, Intra-Cellular Therapies, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Recordati, Sage, Sunovion, Takeda, Teva, and CME activities organized by medical education companies such as Medscape, NACCME, NEI, Vindico, and Universities and Professional Organizations/Societies; owns stocks in Bristol-Myers Squibb, Eli Lilly, J&J, Merck, and Pfizer; and owns stock options in Reviva. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Informed consent statement
Informed consent was obtained from patients in the 3-month Phase 3 pivotal study (NCT03545191).
Supplemental Material
Download MS Word (577.3 KB)Acknowledgments
The authors would like to acknowledge Hortense Nanoux for her contribution to the NNT and NNH analysis during her internship and Nicolas Guerro for programming the analyses at Idorsia Pharmaceuticals Ltd. Medical writing support was provided by LATITUDE (Powered by AXON). This work was previously presented in abstract/poster format at SLEEP 2023, the 37th Annual Meeting of the Associated Professional Sleep Societies, LLC (APSS), held June 3–7, Indianapolis, IN, USA.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00325481.2024.2359891.
Additional information
Funding
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edition (DSM-5). Vol. 10. Washington (DC): American Psychiatric Association; 2013.
- Morphy H, Dunn KM, Lewis M, et al. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30(3):274–280.
- Ree M, Junge M, Cunnington D. Australasian Sleep Association position statement regarding the use of psychological/behavioral treatments in the management of insomnia in adults. Sleep Med. 2017;36(Suppl 1):S43–S47. doi: 10.1016/j.sleep.2017.03.017
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307–349. doi: 10.5664/jcsm.6470
- Davidson JR, Dickson C, Han H. Cognitive behavioural treatment for insomnia in primary care: a systematic review of sleep outcomes. Br J Gen Pract. 2019;69(686):e657–e664. doi: 10.3399/bjgp19X705065
- Wilson S, Anderson K, Baldwin D, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: An update. J Psychopharmacol. 2019;33(8):923–947. doi: 10.1177/0269881119855343
- Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30(3):263–273. doi: 10.1093/sleep/30.3.263
- Pollack M, Seal B, Joish VN, et al. Insomnia-related comorbidities and economic costs among a commercially insured population in the United States. Curr Med Res Opin. 2009;25(8):1901–1911. doi: 10.1185/03007990903035505
- Kleinman NL, Brook RA, Doan JF, et al. Health benefit costs and absenteeism due to insomnia from the employer’s perspective: a retrospective, case-control, database study. J Clin Psychiatry. 2009;70(8):1098–1104. doi: 10.4088/JCP.08m04264
- Daley M, Morin CM, LeBlanc M, et al. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32(1):55–64.
- Sarsour K, Kalsekar A, Swindle R, et al. The association between insomnia severity and healthcare and productivity costs in a health plan sample. Sleep. 2011;34(4):443–450. doi: 10.1093/sleep/34.4.443
- Shahly V, Berglund PA, Coulouvrat C, et al. The associations of insomnia with costly workplace accidents and errors: results from the America Insomnia Survey. Arch Gen Psychiatry. 2012;69(10):1054–1063. doi: 10.1001/archgenpsychiatry.2011.2188
- DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLoS One. 2015;10(10):e0137117. doi: 10.1371/journal.pone.0137117
- Fernandez-Mendoza J, Vgontzas AN. Insomnia and its impact on physical and mental health. Curr Psychiatry Rep. 2013;15(12):418. doi: 10.1007/s11920-013-0418-8
- Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. doi: 10.1111/jsr.12594
- Feng G, Han M, Li X, et al. The clinical effectiveness of cognitive behavioral therapy for patients with insomnia and depression: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:8071821. doi: 10.1155/2020/8071821
- Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: A narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021;17:2549–2566. doi: 10.2147/NDT.S297504
- Matthews EE, Arnedt JT, McCarthy MS, et al. Adherence to cognitive behavioral therapy for insomnia: a systematic review. Sleep Med Rev. 2013;17(6):453–464. doi: 10.1016/j.smrv.2013.01.001
- Lie JD, Tu KN, Shen DD, et al. Pharmacological treatment of insomnia. P T. 2015;40(11):759–771. doi: 10.1176/appi.books.9780890425596.dsm12. PMID: 26609210; PMCID: PMC4634348.
- Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22(9):1335–1350. doi: 10.1007/s11606-007-0251-z
- Kaufmann CN, Spira AP, Depp CA, et al. Long-term use of benzodiazepines and nonbenzodiazepine hypnotics, 1999–2014. Psychiatric Serv. 2018;69(2):235–238. doi: 10.1176/appi.ps.201700095
- Mignot E, Mayleben D, Fietze I, et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol. 2022;21(2):125–139. doi: 10.1016/S1474-4422(21)00436-1
- Fietze I, Bassetti CL, Mayleben DW, et al. Efficacy and safety of daridorexant in older and younger adults with insomnia disorder: A secondary analysis of a randomised placebo-controlled trial. Drugs Aging. 2022;39(10):795–810. doi: 10.1007/s40266-022-00977-4
- QUVIVIQTM daridorexant tablets. Product monograph [Internet]. Health Canada; 2023 [cited 2023 Jun 12. Available from: https://www.idorsia.com/documents/com/label/quviviq-product-monograph.pdf
- Quviviq (daridorexant) market authorisation [Internet]. European Medicines Agency; 2023 [cited 2023 Nov 13]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/quviviq
- QUVIVIQ (daridorexant) tablets, for oral use [Internet]. United States Food and Drug Administration; 2022 [cited 2023 Nov 13]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214985s000lbl.pdf
- Final draft guidance: Daridorexant for treating long-term insomnia. September [Internet]. National Institute for Health and Care Excellence; 2023 [cited 2023 Oct 10]. Available from: https://www.nice.org.uk/guidance/gid-ta10888/documents/674
- Kraemer HC, Frank E, Kupfer DJ. How to assess the clinical impact of treatments on patients, rather than the statistical impact of treatments on measures. Int J Methods Psychiatr Res. 2011;20(2):63–72. doi: 10.1002/mpr.340
- Citrome L. Number needed to treat and number needed to harm: What are they good for? Eur Neuropsychopharmacol. 2023;68:105–107. doi: 10.1016/j.euroneuro.2022.12.006
- Straus SE. Individualizing treatment decisions. The likelihood of being helped or harmed. Eval Health Prof. 2002;25(2):210–224. doi: 10.1177/016327870202500206
- Vancak V, Goldberg Y, Levine SZ. Guidelines to understand and compute the number needed to treat. Evid Based Ment Health. 2021;24(4):131–136. doi: 10.1136/ebmental-2020-300232
- Citrome L, Ketter T. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Practice. 2013;67(5):407–411. doi: 10.1111/ijcp.12142
- Citrome L, Juday T, Frech F, et al. Lemborexant for the treatment of insomnia: direct and indirect comparisons with other hypnotics using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2021;82(4):20m13795. doi: 10.4088/JCP.20m13795
- Citrome L. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic–what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(12):1429–1441. doi: 10.1111/ijcp.12568
- Phillips-Beyer A, Kawata A, Kleinman L, et al. 0458 the sleep diary questionnaire: An analysis of meaningful change in subjective total sleep time using phase 2 and phase 3 clinical trial data. Sleep. 2022;45(Supplement_1):A203. doi: 10.1093/sleep/zsac079.455
- Phillips-Beyer A, Kawata AK, Kleinman L, et al. Meaningful within-patient change in subjective total sleep time in patients with insomnia disorder: An analysis of the Sleep Diary Questionnaire using data from open-label and phase III clinical trials. Pharmaceut Med. 2024;38(2):133–144. doi: 10.1007/s40290-023-00512-9
- Phillips-Beyer A, Kawata AK, Kleinman L, et al. Meaningful within-patient change on the Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ): Analysis of phase III clinical trial data of daridorexant. Pharmaceut Med. 2023;37(4):291–303. doi: 10.1007/s40290-023-00484-w
- Morin CM, Belleville G, Bélanger L, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601
- Yang M, Morin CM, Schaefer K, et al. Interpreting score differences in the Insomnia Severity Index: using health-related outcomes to define the minimally important difference. Curr Med Res Opin. 2009;25(10):2487–2494. doi: 10.1185/03007990903167415
- Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317(7168):1309–1312. doi: 10.1136/bmj.317.7168.1309
- Citrome L, Sanchez Del Rio M, Dong Y, et al. Benefit-risk assessment of galcanezumab versus placebo for the treatment of episodic and chronic migraine using the metrics of number needed to treat and number needed to harm. Adv Ther. 2021;38(8):4442–4460. doi: 10.1007/s12325-021-01848-x
- Vo P, Wen S, Martel MJ, et al. Benefit-risk assessment of erenumab and current migraine prophylactic treatments using the likelihood of being helped or harmed. Cephalalgia. 2019;39(5):608–616. doi: 10.1177/0333102418801579
- Ogura T, Ackermann J, Barbieri Mestriner A, et al. Minimal clinically important differences and substantial clinical benefit in patient-reported outcome measures after autologous chondrocyte implantation. Cartilage. 2020;11(4):412–422. doi: 10.1177/1947603518799839
- Citrome L, Juday TR, Lundwall C. Lemborexant and daridorexant for the treatment of insomnia: An indirect comparison using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2023;84(6):23m14851. doi: 10.4088/JCP.23m14851
- Schilling U, Henrich A, Muehlan C, et al. Impact of daridorexant, a dual orexin receptor antagonist, on cardiac repolarization following bedtime dosing: results from a thorough QT study using concentration-QT analysis. Clin Drug Investig. 2021;41(8):711–721. doi: 10.1007/s40261-021-01062-1
- McAlister FA. The “number needed to treat” turns 20—and continues to be used and misused. CMAJ. 2008;179(6):549–553. doi: 10.1503/cmaj.080484
- Hutton JL. Number needed to treat: properties and problems. J R Stat Soc Ser A Stat Soc. 2000;163(3):403–419. doi: 10.1111/1467-985X.00175
