?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Malaria remains a public health concern with vector control still the vital component of disease prevention, control, and elimination strategies. Recent years has seen a “stalling” in the progress made towards the reduction in the global malaria burden, highlighting the need to develop new, innovative, and safe alternative tools and delivery systems to achieve global malaria elimination. Interventions based on the use of indoor residual spraying (IRS) and long-life insecticidal bed nets (LLINs), i.e. insecticide-containing wall linings (IWLs), can contribute towards the reduction of malaria. Both LLINs and IWLs rely on the presence of insecticides on the fibre or filament surfaces. However, materials directly incorporating the insecticides into the polymer melt during extrusion, allows for effective killing of the mosquitoes when they come into contact with the surface of the material, only if there is insecticide present there. This means that the insecticide must migrate to the surface and precipitate there (bloom). Over time the internal concentration of insecticide will decay. This investigation was done using Fourier transform infrared spectroscopy (FTIR) in both the transmission and attenuated total reflection (ATR) modes to better understand the blooming of three World Health Organization-approved contact insecticides, i.e. alphacypermethrin, fipronil and chlorfenapyr, from mesh or film to better understand the likeliness of insecticides within the materials to migrate to the surface. Film-based samples were prepared in addition to wall lining mesh, because of their easier characterisation than the irregular shaped mesh filaments. FTIR, in ATR and in transmission modes, enabled the tracking of the migration of the three insecticides, over time to the surface of polyethylene mesh or film. This made it possible to estimate the apparent solubility of the insecticides in the polymer matrix. However, scanning electron microscopy (SEM) revealed that a portion of the insecticide is trapped, in a crystalline state, inside the polymer matrix. These results suggest the possibility of developing products-based insecticides for protection against infective mosquito bites in malaria-endemic regions.
1. INTRODUCTION
Malaria is not only a global public health concern, but also a disease of poverty that impacts on productivity and impedes socio-economic progress and development in Africa (Sarma et al., Citation2019, Alonso et al., Citation2019, Birkholtz et al., Citation2012). Vector control is a vital component of malaria disease prevention, control and elimination strategies. The two main vector control methods applied to curtail malaria, as recommended by the World Health Organization (WHO), are the use of indoor residual spraying (IRS) and insecticide treated nets or long-lasting insecticidal nets (ITNs/LLINs) (WHO, Citation2019, Fullman et al., Citation2013, Kruger et al., Citation2015, Zhu et al., Citation2017). The scaling up of these two strategies have contributed to a significant reduction in the global malaria burden during the previous decade, especially in Africa (Zhu et al., Citation2017, Birkholtz et al., Citation2012). Between 2000 and 2014, global malaria incidence rates and mortality dropped by 37% and 60% respectively (Korenromp et al., Citation2016, WHO, Citation2019). Despite the large decrease, the disease remains a devastating source of morbidity and mortality (Fullman et al., Citation2013). According to the WHO, in more recent years the tremendous progress made against the disease appears to have “stalled” and in some regions malaria infections and mortalities are starting to increase again (WHO, Citation2019).
Interventions based on IRS and LLINs play important contributing roles in reducing the malaria disease burden. However, better efficacy and efficiency of existing control interventions, and the development of new, innovative and safe alternative tools and delivery systems are vital if the goal of global malaria elimination is to be realised (Karunamoorthi, Citation2011). In future, insecticidal wall linings (IWLs) may provide a similarly effective malaria vector control strategy. Both LLINs and IWLs rely on the presence of insecticides on the fibre or filament surfaces. The WHO recommends bed nets containing insecticides such as alphacypermethrin and chlorfenapyr because of their low mammalian toxicity, low volatility, and high contact-toxicity against mosquitoes (Smith et al., Citation2018). When the target mosquitoes touch the surface of the net, they pick up toxic amounts of the poison causing them to perish. The polyethylene wall linings, developed at the University of Pretoria, are produced by directly incorporating the insecticides into the polymer melt during the Netlon mesh extrusion process (Kruger et al., Citation2015, Sibanda and Focke, Citation2014). Since the insecticides are contact poisons, only that portion present and exposed at the surface of the filaments is effective at killing the target insects. Therefore, it is necessary for the insecticide to bloom, i.e. to migrate to the surface and precipitate there.
Polyethylene is processed at temperatures ranging from 180°C to 250°C. Solubility usually increases with increasing temperature. It is therefore likely that, at these high processing temperatures, the insecticides are fully dissolved in the polymer melt in particular when added at very low concentrations. However, after the polymer melt has been shaped into fibres, films or filaments, it cools down and partially crystallizes. Guest molecules are excluded from the crystalline domains, being soluble in the amorphous regions of the polymer only. This means that as the polymer cools and crystallizes the portion of the matrix available for dissolution of the additive decreases proportionally. Together with the lowered temperature, this sets up a supersaturated state for the non-volatile insecticide present. This explains the tendency for additives to migrate to the surface where they accumulate (Lewis and Cowsar, Citation1977). This is important as blooming can only occur when the migrating species is present at supersaturated levels in the polymer (Calvert and Billingham, Citation1979). It will continue until the concentration inside the polymer matrix falls to saturation.
Calvert and Billingham (Citation1979) assumed that the active ingredient, in its supersaturated state, is initially homogeneously and uniformly distributed in the polymer matrix. This simplifies the mathematical modelling of the process. The kinetics of the process depends upon the sample geometry, the solubility of the additive and its diffusion coefficient within the bulk of the polymer (Calvert and Billingham, Citation1979). The mechanism of the blooming process entails the diffusion from the supersaturated state inside the polymer to the surface covered by the neat active. This means that the surface concentration is fixed and that the internal concentration will, over time, decay to the solubility limit in the polymer (Calvert and Billingham, Citation1979). A polymer film may be approximated by an infinite flat plate of thickness L. After a sufficiently long time (see below), the average concentration, for the insecticide remaining in the film is given by (Beek et al., Citation1999):
(1)
(1) where
, Co and C∞ correspond to the mean concentration, the initial concentration and solubility of the insecticide in the polymer respectively; D is the diffusion coefficient and t is the time. This expression provides good approximations for blooming times exceeding t > 0.1 L2/D. Spatafore and Pearson (Citation1991) found that the propensity of a phenolic antioxidant to bloom, initially present at high concentrations in polypropylene, could indeed be described simply in terms of such a Fickian diffusion model. Similarly, Möller and Gevert (Citation1994) found that the diffusion of antioxidants in low-density polyethylene (LDPE) is undeniably Fickian with concentration-independent diffusion coefficients and an Arrhenius-type temperature dependence.
The quantification of the low levels of insecticides present on the surface or inside polymer films or filaments is not a simple matter. It requires the use of very sensitive analytical techniques to measure the low levels of additives used in practice (Spatafore and Pearson, Citation1991). Fourier transform infrared spectroscopy (FTIR) is a simple analytical technique that was successfully applied to measure the diffusion coefficients of antioxidants in low-density polyethylene (Möller and Gevert, Citation1994) and to study the blooming of additives from polypropylene films (Spatafore and Pearson, Citation1991). Furthermore, attenuated total reflection infrared spectroscopy (FTIR-ATR) has been employed to estimate the surface concentration of additives that have bloomed from low-density polyethylene (Vitali, Citation2001) and from epoxy coatings (Du et al., Citation2014).
This study employed FTIR in transmission mode in combination with FTIR-ATR to probe the blooming of three WHO-approved contact insecticides (alphacypermethrin, fipronil and chlorfenapyr) from polyethylene mesh and film. Information regarding the chemical and physical characteristics of the insecticides are presented in the Supplementary Material. The objective of the investigation was to gain a better understanding of the portions of insecticides incorporated into the insecticidal mesh that are likely to migrate to the surface. Film-based samples were prepared in addition to mesh used as wall linings. The reason for this was that the films were deemed easier to characterise than the irregular shapes presented by the filaments comprising the mesh.
2. MATERIALS AND METHODS
2.1. Materials
Proprietary insecticide-containing masterbatches were supplied by Xyris Technology. They contained 11 wt-% active insecticide in a polyethylene carrier. The actives were alphacypermethrin, fipronil and chlorfenapyr.
High density polyethylene (HDPE) grade Safrene H 4765 ex Safripol and low-density polyethylene grade LF2103 ex Sasol were used in this study. Both featured a melt flow index of 0.33 g/10 min at 190°C/2.16 kg. According to the suppliers, the densities were 0.958 g·cm−3 and 0.921 g·cm−3 for the HDPE and LDPE respectively.
2.2. Methods
2.2.1. Sample preparation and conditioning
Polyethylene films were blown using a 1:1 blend by mass of HDPE and LDPE in addition to the two neat polymers. Films, nominally 40 μm, were made on a Collin BL 180/400 laboratory film-blowing unit. It featured a single screw extruder with a diameter of 30 mm and L/D = 25. The temperature profile was, from hopper to die, 180/180/185/185/185/185/210/190°C. The extruder screw speed was 50 rpm. The masterbatch addition was done in order that the insecticide contents of the final films were as follows: alphacypermethrin: 0.5 wt-%; chlorfenapyr: 1.4 wt-%, and fipronil: 2.5 wt-%. These concentrations were chosen on the basis of recommendations provided by industry experts.
Commercial samples of insecticidal mesh, weighing ca. 120 g m−2, were produced on a Netlon extrusion line at the Huhthamaki factory in Springs, Gauteng Province, South Africa. The mesh polymer was a 1:1 HDPE-LDPE blend. These production runs employed the same masterbatches, but slightly different addition levels were used.
2.2.2. Field trial
Mesh samples containing 0.29 and 0.47 wt-% alphacypermethrin insecticide, as well as untreated linings, were evaluated in a field trial over a period of five years (Kruger et al., Citation2015). The field trial commenced in October 2012. The linings were randomly installed on the inner walls of traditional mud huts and modern brick houses in a community village in Vhembe District, Limpopo Province, as part of a short field trial. The linings were exposed to conditions within these dwellings over a six-month period. Data were collected monthly through questionnaires (to determine user acceptance and perceived effectiveness), and entomological residual efficacy analysis of ITWL, as part of durability testing, was done bimonthly using WHO prescribed bioassays. The efficacy testing continued for a further 54 months, with sampling occurring on an annual basis. Simultaneously, the residual efficacy of the insecticide was also determined. Kruger et al. (Citation2015) provide comprehensive details of this field trial, including the performance of the mesh six months after installation. Following this interim report, further samples were retrieved by cutting pieces off the installed mesh to continue with residual efficacy testing. This was done annually for five years after installation ().
2.2.3. Bioassay
The residual efficacy of mesh samples against mosquitoes was evaluated using Standard WHO bioassays tube tests (WHO, Citation2006). In these tests, typically twenty-five non-blood-fed female Anopheles arabiensis mosquitoes (KGB colony housed at the National Institute for Communicable Diseases, Johannesburg, South Africa), 3–5 days old, were exposed for 30 min to mesh samples. Knockdown was recorded 30 min after the end of exposure and mortality was recorded 24 h after initial exposure. During the 24 h waiting period, mosquitoes were given a sugar solution as nourishment. shows such a WHO bioassay tube test in progress.
2.2.3. Film ageing
The film thicknesses were measured with a Mitutoyo Digital RHS769 micrometer. The films were suspended vertically in a forced convection oven and aged at 40°C for up to 38 days. Small pieces were cut from the film samples at regular intervals and the FTIR spectra were recorded. Films aged for 38 days or more were also characterised using other techniques mentioned in the characterisation section.
2.3. Characterisation
2.3.1. Optical microscopy
Optical micrographs of the extruded film containing alphacypermethrin were obtained using a Leica DMRX microscope, operated in transmission mode. The films were oriented in the microscope such that the thickness direction was parallel to the light path. Images were taken with the sample placed between crossed polarisers, or in plain transmission. Additional images were taken on a WiTec-alpha 300R microscope through a numerical aperture of 0.4 at 100× magnification.
2.3.2. Confocal fluorescence microscopy (CFM)
Small filaments were cut from the insecticidal Netlon mesh and mounted on a microscope cover slip. The samples were viewed with a Zeiss LSM 510 META Laser scanning microscope (Carl Zeiss, Jena, Germany) fitted with a Plan Apochromat 20× objective. The exciting laser wavelength was 405 nm and the pinhole setting was 59.8 nm. The detector gain was set at 850 and the amplitude gain and offset were 1 and 0.045, respectively. The band pass filter setting was LP420. The top surface of the samples was imaged. They are shown superimposed on the optical shadow of the Netlon filaments that were imaged.
2.3.3. Scanning electron microscopy and energy dispersive X-Ray spectroscopy (EDS)
Samples of the blown films were fixed on an aluminium sample tray and the surfaces were coated with carbon. The measurements were carried, out at different positions, at an acceleration voltage of 6 keV using a Zeiss UltraPlus equipped with a Bruker XFlash Quad 5060F.
2.3.4. FTIR spectroscopy
FTIR spectra were recorded on a Perkin-Elmer Spectrum 100 instrument in both the transmission and ATR modes. The spectra represent averages of 16 scans, scanned at a resolution of 4 cm−1. The samples evaluated included the neat insecticide powders (using ATR only), the neat polyethylene films and the insecticide-containing films before and after oven ageing at 40°C for different times, up to 38 days. The same insecticide film samples were re-analysed in transmission and ATR mode after removal of all surface deposits by wiping with tissue paper wetted with acetone. Following this treatment, the ATR spectra featured HDPE absorption bands only. This confirmed that all of the insecticide that was present on the surface at that point in time, was effectively removed by this procedure.
2.3.5. Wide-angle X-ray scattering (WAXS)
X-ray scattering experiments were performed by means of a Bruker X-Ray diffractometer D8 Discovery with Cu Kα radiation (monochromatic beam, λ = 0.1542 nm). The experiments were done in transmission mode perpendicular to the machine direction. Scattering intensities were measured by a two-dimensional (2D) Vantec500 detector. 2D scattering patterns and radial intensity scattering curves were evaluated to analyse the structure and its orientation.
3. RESULTS AND DISCUSSION
3.1. Insecticidal mesh
reports the outcome of the bioassay results obtained on samples that were collected 60 months after installation. Surprisingly, even the mesh samples that contained just 0.29 wt-% alphacypermethrin still fully complied with the WHO requirements for bed nets.
shows confocal fluorescence microscope generated images of small portions of insecticidal meshes. The fluorescence observed in the fipronil and chlorfenapyr samples suggest virtually complete insecticide coverage of the filament surfaces. However, only isolated bright spots are visible on the surface of the sample containing alphacypermethrin. The results presented in suggest that such a discontinuous distribution on the mesh surface can be sufficient for effective mosquito control. More detailed investigations, not shown here, suggested that the insecticides were present in the form of needle-shaped crystals. In some cases, notably for fipronil and chlorfenapyr, these crystals protruded away from the polymer surface. Unfortunately, the irregular surface structure of the mesh filaments made it difficult to image the true state of the insecticides on the polymer surface. This prompted the laboratory investigation that considered the blooming from film samples. The flat surfaces of the film geometry make it more amenable for investigating the morphology of the insecticides that migrated to the surface.
Figure 3. Fluorescence images recorded on filaments of insecticidal Netlon mesh samples. (a) alphacypermethrin; (b) fipronil, and (c) chlorfenapyr.
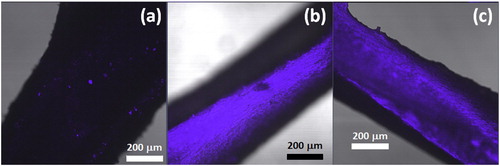
Table 1. WHO tube bioassay results for insecticidal mesh impregnated with alphacypermethrin, 60 months after installation in the field trial.
3.2. Insecticidal films
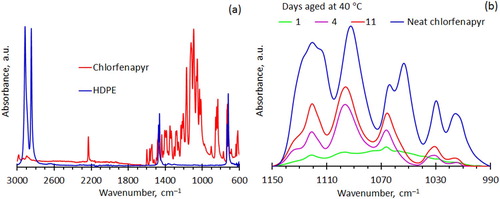
FTIR-ATR spectra for virgin HDPE and neat chlorfenapyr powder are shown in . The bands located between 990 and 1150 cm−1 are unique to chlorfenapyr while the two bands located at 1472 and 1462 cm−1 are characteristic of HDPE. The relative chlorfenapyr content of the films was estimated from the ratio of the two areas defined by the two wavenumber regions. The acetone wipes removed all the insecticide present on the film surface. Therefore, the transmission spectrum recorded after this cleaning action enabled the estimation of the insecticide remaining inside the film at that point in time. The FTIR-ATR spectra for the insecticide-containing films were normalised with respect to the spectrum obtained for the neat chlorfenapyr powder. A similar procedure was used for the other insecticide-containing films and further details are presented in the Supplementary Material.
Figure 4. FTIR-ATR spectra. (a) Comparing the spectra for the neat chlorfenapyr powder to that for virgin HDPE. (b) Change in the FTIR-ATR spectra with ageing time at 40°C for the chlorfenapyr-containing HDPE film compared to the neat spectrum obtained for the neat insecticide powder.
shows the normalised insecticide contents of the polyethylene films as they changed over time on ageing at 40°C. The plotted composition data obtained from the transmission spectra, after rinsing with acetone, indicate the amount of insecticide remaining at any specific point in time. For example, right after the film was made, the chlorfenapyr inside the film amounted to 84%. This means that 16% had already migrated to the surface. However, the data for day 4 to day 22 are approximately the same and they indicate that 22.8 ± 0.9% of chlorfenapyr appears to remain indefinitely in the polymer matrix.
Figure 5. Normalised insecticide contents of films as a function of ageing time at 40°C. The transmission data indicate compositions estimated from spectra recorded after rinsing the films with acetone. The FTIR-ATR data provide a relative indication of the accumulation of the insecticides on the surface of the films. The dotted lines indicate error bands corresponding to one standard deviation above and below the mean.
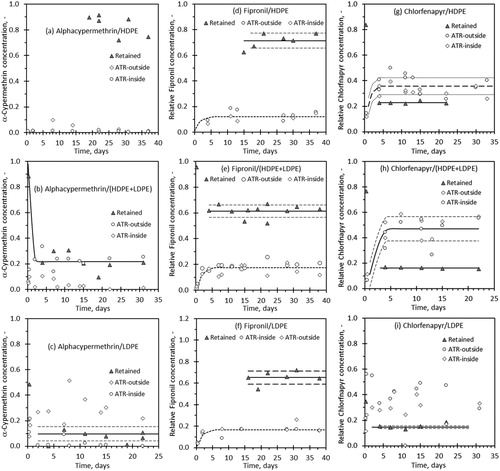
indicates that similar observations hold true for the other insecticides in that a plateau value, in the amount of insecticide that is retained, is reached after a certain time. The exception is alphacypermethrin in HDPE. In this system the data scatter and the relatively limited degree migration prevented the drawing of definitive conclusions. The most striking result is the fact that, irrespective of the insecticide, a greater proportion is retained in HDPE than in LDPE. As expected, the results for the 1:1 HDPE-LDPE blend are intermediate except for fipronil. As mentioned before, the insecticides can only dissolve in amorphous regions of the polymer. Since HDPE is highly crystalline when compared to LDPE, the observed retention cannot be attributed to solubility of the insecticide in the polymer matrices. An alternative hypothesis is that the insecticides became trapped as solid inclusions when the films were formed and polymer matrix crystallised. This either prevented migration or slowed it down significantly.
The results presented in suggest that, except for alphacypermethrin in HDPE film, that the migration to the surfaces reached a steady state value by day five. reports theses steady state values. Unlike the FTIR transmission results, the FTIR-ATR results are only indicative assessments of the amount of insecticide that has accumulated at the film surfaces. The reason is that these measurements were normalised using the FTIR-ATR spectra recorded using the neat insecticide powders. Nevertheless, for fipronil and chlorfenapyr, the estimated insecticide fractions present on the surfaces and internally retained do sum up approximately to unity. However, this is not the case for the alphacypermethrin-containing films at all.
Table 2. Summary of the estimates of insecticides accumulated at the surface and retained internally in the polyethylene film samples.
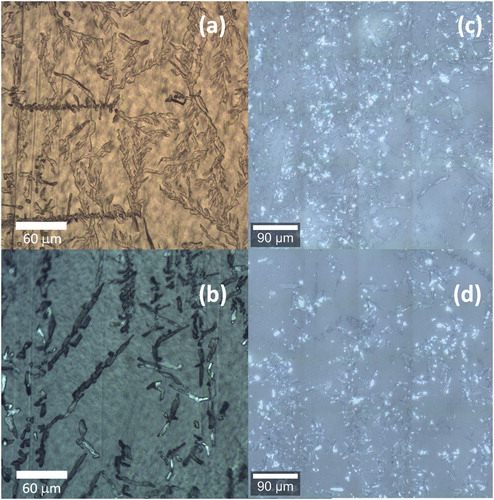
shows images of the insecticide deposits observed on the surface of films after long-term ageing. (a,b) are images of needle-shaped deposits of alphacypermethrin present in optical micrographs taken on 1:1 m/m LDPE/HDPE and LDPE films, respectively. The image in (b) was captured with the sample placed between crossed polarisers, showing a rather dark/greyish appearing polymer matrix due to its semi-crystallinity and orientation of the macromolecules (Nase et al., Citation2010). Individual polymer crystals are too small to be detected in the distinct spherulitic crystalline-amorphous superstructure, typical for semi-crystalline PE crystallized from the quiescent melt, and is not seen due to crystallization during film blowing. However, most important is the observation of phase-separated, needle-like objects with a length of up to around 50 µm and width of 5 µm. Such morphology is untypical for PE crystals and is unambiguously associated to the presence of alphacypermethrin. The image in (a) was collected in plain transmission mode, revealing similar observation of insecticide crystals, which here even grew dendritically. Worth noting, insecticide crystals of a large variety of shapes have been detected in case of all investigated samples. Examples are shown in (c,d) which show the appearance of aged LDPE films with chlorfenapyr and fipronil respectively. The latter two optical images were taken with the confocal Raman microscope.
Figure 6. Optical images of the surfaces of polymer films after long-term ageing. (a) alphacypermethrin in the 1:1 m/m LDPE/HDPE film. LDPE films that contained (b) alphacypermethrin, (c) chlorfenapyr and (d) fipronil.
Further evidence for the migration and consequently precipitation of insecticide crystals on the surface of the polyethylene films was confirmed by SEM-EDX investigations ( and ). Chlorine, as the element that occurs in the insecticides, has been detected in all samples containing insecticides. In the sum spectra, collected from surface regions measuring 172 µm × 129 µm, the typical peak of Kα chlorine appears at 2.61 keV in all samples with exception of the reference sample (neat HDPE without insecticides). on top shows the sum spectra taken from HDPE films with different insecticides as an example for all investigated polyethylene films.
Figure 7. Sum spectra and SEM-EDX images of HDPE film surfaces. (a) neat HDPE film; (b) alphacypermethrin; (c) fipronil, and (d) chlorfenapyr. The false green colour indicates elemental distribution of chlorine detected by EDX.
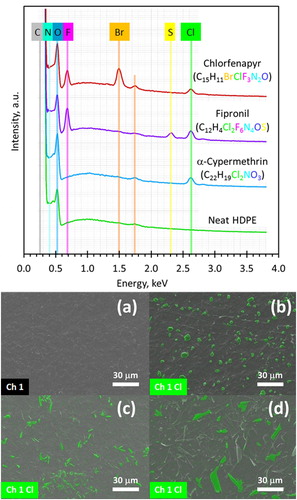
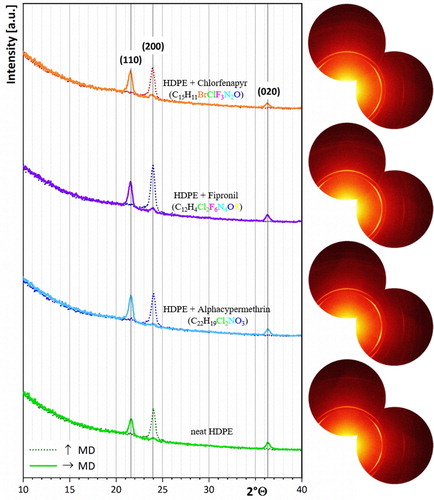
Figure 8. Radial scattering curves (left) and 2D-WAXS patterns (right) of HDPE films containing insecticides.
The size, shape and distribution of insecticides, which have accumulated on the surface of HDPE films is shown in the bottom portion of . In this image the elemental map of chlorine detected with EDX (false colour image) is superimposed on the topographical contrast SEM image. It confirms that there is indeed some alphacypermethrin, which managed to migrate to the surface from inside the HDPE films. (c,d) suggest flake-shaped crystalline deposits of fipronil and chlorofenapyr on the surface of HDPE films. Similar observations hold for the LDPE films shown in . The images reproduced in were obtained on samples that were aged for more than forty days at 40°C.
Interestingly, it was not possible to detect the insecticide crystals by WAXS. As shown by the radial intensity curves (, left), only the typical reflections of HDPE can be seen. One reason might be the minute amounts of insecticide present on the surface of the films. However, a more likely reason could be the crystallinity of the insecticides. Since no additional peaks were observed in the scattering curves in neither the machine direction nor perpendicular to the machine direction, it can be assumed that the migrated insecticides have an amorphous structure. Furthermore, it can be concluded that the insecticides do not affect the crystallization process of HDPE. Both, the position as well as the scattering intensity of HDPE films containing insecticides are identical to those of neat HDPE.
4. CONCLUSIONS
The insecticides alphacypermethrin, chlorfenapyr and fipronil were successfully incorporated into polyethylene films at a concentration of approximately 0.5, 1.4 and 2.5 wt-% respectively. The migration of the insecticide to the surface was tracked, over time, using FTIR-ATR. The portion remaining inside the film was estimated from FTIR in transmission mode after removal of surface deposits with acetone rinses. The blooming process was complete in less than four days of oven ageing at 40°C. Irrespective of the insecticide, the quantity that remained trapped in the polymer matrix was greater for HDPE than LDPE. Optical and SEM imaging of the film surfaces revealed the presence of insecticides as discontinuous flake-shaped deposits, in some cases in dendritic formations. The insecticides do not affect the crystallization process of HDPE. Netlon mesh containing either 0.29 or 0.45 wt-% alphacypermethrin retained bioactivity against mosquitoes at a level required for WHO bed net specifications even five years after indoor installation. These results suggest the possibility of developing products containing insecticides for protection against infective mosquito bites in malaria-endemic regions.
ACKNOWLEDGEMENTS
The authors also acknowledge Robert Tewo and Joseph for help during compounding of the insecticide masterbatches.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
SUPPLEMENTAL DATA
Supplemental data for this article can be accessed at https://doi.org/10.1080/0035919X.2021.1900950.
Additional information
Funding
REFERENCES
- Alonso, S., Chaccour, C.J., Elobolobo, E., Nacima, A., Candrinho, B., Saifodine, A., Saute, F., Robertson, M. & Zulliger, R. 2019. The economic burden of malaria on households and the health system in a high transmission district of Mozambique. Malaria Journal, 18, 360.
- Beek, W.J., Mutzall, K.M.K. & van Heuven, J.W. 1999. Transport Phenomena, Chichester, John Wiley & Sons Ltd.
- Birkholtz, L.M., Bornman, R., Focke, W., Mutero, C. & de Jager, C. 2012. Sustainable malaria control: Transdisciplinary approaches for translational applications. Malaria Journal, 11, 431.
- Calvert, P.D. & Billingham, N.C. 1979. Loss of additives from polymers: a theoretical model. Journal of Applied Polymer Science, 24, 357–370.
- Du, K., Yang, G., Yuan, Z., Xu, W. & Liang, X. 2014. Migration of additives toward the surface during aging of epoxy coating by infrared spectroscopy. Journal of Applied Polymer Science, 131, 40051.
- Fullman, N., Burstein, R., Lim, S.S., Medlin, C. & Gakidou, E. 2013. Nets, spray or both? The effectiveness of insecticide-treated nets and indoor residual spraying in reducing malaria morbidity and child mortality in sub-Saharan Africa. Malaria Journal, 12, 62.
- Karunamoorthi, K. 2011. Vector control: a cornerstone in the malaria elimination campaign. Clinical Microbiology and Infection, 17, 1608–1616.
- Korenromp, E., Mahiané, G., Hamilton, M., Pretorius, C., Cibulskis, R., Lauer, J., Smith, T.A. & Briët, O.J.T. 2016. Malaria intervention scale-up in Africa: effectiveness predictions for health programme planning tools, based on dynamic transmission modelling. Malaria Journal, 15.
- Kruger, T., Sibanda, M.M., Focke, W.W., Bornman, M.S. & de Jager, C. 2015. Acceptability and effectiveness of a monofilament, polyethylene insecticide-treated wall lining for malaria control after six months in dwellings in Vhembe District, Limpopo Province, South Africa. Malaria Journal, 14.
- Lewis, D.H. & Cowsar, D.R. 1977. Principles of controlled release pesticides. In Scher, H.B. (ed.) ACS Symposium Series 53: Controlled Release Pesticides. Washington, DC: American Chemical Society, chap 1, 1–16.
- Möller, K. & Gevert, T. 1994. An FTIR solid-state analysis of the diffusion of hindered phenols in low-density polyethylene (LDPE): the effect of molecular size on the diffusion coefficient. Journal of Applied Polymer Science, 51, 895–903.
- Nase, M., Funari, S.S., Michler, G.H., Langer, B., Grellmann, W. & Androsch, R. 2010. Structure of blown films of polyethylene/polybutene-1 blends. Polymer Engineering & Science, 50, 249–256.
- Sarma, N., Patouillard, E., Cibulskis, R.E. & Arcand, J.L. 2019. The economic burden of Malaria: revisiting the evidence. American Journal of Tropical Medicine and Hygiene, 101, 1405–1415.
- Sibanda, M. & Focke, W. 2014. Development of an insecticide impregnated polymer wall lining for malaria vector control. Malaria Journal, 13, P80–P80.
- Smith, S.C.Z.C., Stevie, F.A., Garcia, R. 2018. Imaging and quantitative analysis of insecticide in mosquito net fibers using Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS). PLoS ONE, 13, e0209119.
- Spatafore, R. & Pearson, L.T. 1991. Migration and blooming of stabilizing antioxidants in polypropylene. Polymer Engineering & Science, 31, 1610–1617.
- Vitali, M. 2001. Development of a semi-quantitative attenuated total reflection infrared spectroscopy analysis method for the study of compatibility of hindered amine stabilizers in polyethylene films. Polymer Testing, 20, 741–748.
- WHO. 2006. Guidelines for testing mosquito adulticides for indoor residual spraying and treatment of mosquito nets. In WHO (Ed.) Control of Neglected Tropical Diseases. WHO Pesticide Evaluation Scheme. Geneva: World Health Organisation, 1–30. https://apps.who.int/iris/handle/10665/69296
- WHO. 2019. World Malaria Report 2019. World Health Organization: Geneva, 1–232.
- Zhu, L., Müller, G.C., Marshall, J.M., Arheart, K.L., Qualls, W.A., Hlaing, W.M., Schlein, Y., Traore, S.F., Doumbia, S. & Beier, J.C. 2017. Is outdoor vector control needed for malaria elimination? An individual-based modelling study. Malaria Journal, 16, 266. https://doi.org/10.1186/s12936-017-1920-y.