Abstract
Edoxaban is an oral direct factor Xa inhibitor for prophylaxis and treatment of thromboembolic disorders. The effects on common coagulation assays are clinically valuable information and in certain clinical situations a quick assessment of the anticoagulant is wanted. Our aim was to investigate the effect of edoxaban on routine coagulation methods and evaluate anti-Xa assays, commonly used for other direct factor Xa inhibitors, for estimation of the drug concentration. Edoxaban was spiked to plasma samples from healthy subjects in the concentration range 0–742 µg/L and analyzed using different reagents for activated partial thromboplastin time (APTT) and prothrombin time (PT). Assays for antithrombin, activated protein C resistance, lupus anticoagulant (LA) and chromogenic anti-Xa assays were also included. Edoxaban displayed similar effects in vitro to other oral direct Xa inhibitors. The concentration needed to double the coagulation time varied between assays and reagents; 539–758 µg/L for the APTT and between 329 and 2505 µg/L for the PT. Edoxaban gave false high antithrombin activities in assays based on Xa-inhibition. Two integrated assays for LA, both based on activation with dilute Russell’s viper venom, displayed different results. Chromogenic anti-Xa assays displayed linear dose-response curves with edoxaban up to approximately 500 µg/L. In conclusion, therapeutic concentrations of edoxaban variably affect different coagulation assays, and even different reagents within an assay group. In comparison with other oral Xa-inhibitors, the in vitro effects of edoxaban were more similar to rivaroxaban than apixaban. For measurement of edoxaban concentration in plasma, it is possible to use the chromogenic anti-Xa assays.
Introduction
A selective direct factor X inhibition has become a common approach in the treatment and prophylaxis of venous and arterial thromboembolism in recent years. The latest specific anti-Xa inhibitor is edoxaban (Lixiana®) from Daiichi Sankyo Co. Ltd. (Tokyo, Japan), [Citation1,Citation2], and it is currently approved for stroke prophylaxis in patients with non-valvular atrial fibrillation, treatment of pulmonary embolism and deep venous thrombosis and as the predecessors, for thromboprophylaxis after hip and knee replacements, although so far only in Japan for thromboprophylaxis [Citation2]. These drugs are, at least partially, excreted by the kidneys and/or metabolized by the liver, hence it may be necessary to assess the anticoagulant effect with a specific test to avoid drug accumulation in specific patient groups [Citation3,Citation4]. Other clinical situations, in which testing can be considered, are emergency situations such as major bleeds or preoperative preparation [Citation5].
The true concentration of all DOACs can be measured over a wide range with various assay protocols based on high-performance liquid chromatography linked with mass spectrometry [Citation6]. The manufacturer of edoxaban reports predictable and consistent pharmacokinetic and pharmacodynamics profiles with a direct linear correlation between edoxaban plasma concentrations and the coagulation parameters, prothrombin time (PT), activated partial thromboplastin time (APTT) and anti-factor Xa activity [Citation7–10]. In a direct comparison of the in vitro effect of anti-Xa DOACs on PT and APTT, a variable and regent-dependent dose-response with rivaroxaban and edoxaban was seen but the sensitivity for apixaban was poor [Citation11]. With this knowledge about the inadequate PT and APTT sensitivities for DOACs, it cannot be advised to rely on these assays in the assessment of the anticoagulant effect [Citation12]. However, so far, there are only few reports on the pharmacodynamic profiles of edoxaban [Citation11,Citation13–17] and only three of these present more extensive results based on in vitro effects on routine and more specialized coagulation assays [Citation11,Citation13,Citation15]. All these studies utilized pooled normal plasma spiked with edoxaban. To facilitate comparisons between NOACs, we have in the current study used the same study design as we previously did for other DOACs [Citation18–20], where plasma samples from 10 healthy individuals were spiked with edoxaban to investigate the overall and inter-individual anticoagulant activities over a wide concentration range and for several different PT and APTT reagents. We also studied the effects on assays for antithrombin, activated protein C resistance, lupus anticoagulant (LA) and specific anti-Xa assays for determination of edoxaban concentration in plasma.
Materials and methods
Study design
A working group, representing the coagulation laboratories at three Swedish University Hospitals (Gothenburg, Linköping and Malmö) and one county hospital (Halmstad, Sweden), undertook the task to investigate the effects of edoxaban on common coagulation assays in the Nordic countries. The study design was similar to the previously used by the same authors to investigate the in vitro effects of dabigatran [Citation18], rivaroxaban [Citation19] and apixaban [Citation20]. In brief, plasma samples from 10 different healthy individuals were spiked with edoxaban at 10 different concentrations and aliquots were investigated at the four participating laboratories. One lab (Malmö) investigated the effects on the different APTT reagents, another lab (Linköping) ran all PT assays and one lab (Gothenburg) performed the antithrombin and functional APC resistance assays. Lupus anticoagulant assays were done in two laboratories (Gothenburg and Halmstad) and two laboratories also performed the chromogenic Xa-assays (Halmstad and Linköping).
Preparation of plasma samples and edoxaban calibrator
Edoxaban was a kind gift from Daiichi-Sankyo but also purchased from Selleckchem.com (Munich, Germany). Stock preparations of 0.1 mg/mL monohydrate tosylate edoxaban salt were made by dissolving the drug in 100% dimethyl sulphoxide (DMSO, Merck, Darmstadt, Germany). The concentration of the stock preparations was calculated using the molecular weight of the monohydrate tosylate edoxaban salt (Mr 738.27) but the final sample concentrations are presented as the edoxaban concentrations (Mr 548.06). The stock solutions were diluted 1:100 with each of the donor plasmas to obtain 10 sets of working solutions of 742 µg/L edoxaban. The working solutions was further diluted with each donor plasma to obtain 10 concentrations in the range between 0 and 742 µg/L. The samples were stored at –70 °C until they were transported on dry ice to the participating laboratories for analysis. The highest spiked sample, with an edoxaban concentration of 742 µg/L, was analyzed at the Stago QC department using their reference method based on high-performance liquid chromatography linked with mass spectrometry. The mean concentration of four independent measurements was 730 µg/L with a standard deviation of 1.3 µg/L, indicating a pure material with 98% recovery.
A set of calibrator samples, used for the Coamatic anti-Xa assay, was made in the Linköping laboratory by weighing pure edoxaban on an analytical scale and dissolved in 100% DMSO and thereafter used to spike pooled normal plasma (n = 20 healthy donors; Affinity Biologicals, Ancaster, Canada). The concentration was checked by liquid chromatography and tandem mass-spectrometry by Stago.
Routine coagulation analyses
The APTT was assayed using five different reagents; Actin FSL from Siemens Healthcare Diagnostics (Marburg, Germany); PTT-Automate from Stago (Asnieres, France); APTT-SP IL test liquid from Instrumentation Laboratory SpA (Milano, Italy); TriniCLOT aPTT HS from Trinity Biotech (Bray, Ireland); and APTT-DG from Grifols (Barcelona, Spain). The APTT assays were performed on a BCS-XP instrument from Siemens Healthcare Diagnostics (Marburg, Germany) according to the reagent manufacturers’ instructions, i.e. equal volumes of sample and APTT reagent were mixed and incubated (3 or 5 minutes depending on reagent) at 37 °C and then one volume of 25 mM calcium chloride solution was added to the mixture and the clotting time determined.
The PT was performed with both plain (Quick type) and combined (Owren type) PT reagents. Five common Quick type PT assays were tested: Dade Innovin and Thromborel S (Siemens Healthcare Diagnostics, Marburg, Germany); STA-Neoplastine CI Plus (Stago, Asnieres, France); RecombiPlastTin 2G (Instrumentation Laboratory, Milano, Italy); and Technoplastin HIS (Technoclone GmbH, Vienna, Austria). Two different Owren type PT assays were also investigated: Stago prothrombin complex assay, SPA+, from (Stago, Asnieres, France) and PT Owren from MediRox AB (Nyköping, Sweden). The Quick PT assays were calibrated using local determination of ISI and mean normal PT for each reagent. The Owren PT assays were all calibrated using the national INR calibrators certified by the Swedish external quality assessment organization EQUALIS (Uppsala, Sweden) traceable to a WHO reference thromboplastin [Citation21]. The PT assays were performed on an ACLTop (Instrumentation Laboratory SpA, Milano, Italy) coagulation instrument.
Two chromogenic reagents for antithrombin activity were evaluated; Innovance Antithrombin, based on Xa inhibition and Berichrom Antithrombin III based on thrombin inhibition, both from Siemens Healthcare Diagnostics (Marburg, Germany). The antithrombin assays were performed on the Sysmex CS-5100 instrument from Siemens Healthcare Diagnostics (Marburg, Germany) according to the manufacturer’s instructions and the locally established reference interval was 0.80–1.20 kIU/L for both assays.
A functional assay for activated protein C resistance, Pefakit APC-R Factor V Leiden, was obtained from Pentapharm (Basel, Switzerland) and run on the BCS-XP instrument (Siemens Healthcare Diagnostics, Marburg, Germany).
Lupus anticoagulant testing was performed with two integrated assays based on a screen reagent and a confirm reagent that utilizes the dilute Russell’s viper venom time (dRVVT) test. One test, Technoclot LA Screen and Technoclot LA Confirm (Technoclone Gmbh, Vienna, Austria) was performed on the BCS-XCP and the other test, STA-Staclot DRVV Screen and STA-Staclot DRVV Confirm from Stago was performed on the STAR Max instrument (Stago, Asnieres, France). Both tests were performed according to the manufacturer’s instructions. A ratio between screen and confirm assays of 1.2 and 1.5 was considered weakly positive or border line, a ratio between 1.5 and 2.0 was classified as moderately positive, and a ratio above 2.0 indicated a strongly positive result as previously described [Citation20].
Anti-Xa assays used for quantitation of edoxaban in plasma
One laboratory used the Coamatic Heparin reagent from Chromogenix on Sysmex CS2000i instrument using the same programming as for rivaroxaban according to the reagent manufacturer’s recommendations. Another laboratory used the first specific edoxaban assay released from the diagnostic industry, based on the STA-Liquid anti-Xa reagent (Stago, Asnieres, France), and was performed according to the manufacturer’s instruction on the STAR Max instrument (Stago, Asnieres, France). Both assays are without addition of exogenous antithrombin, which has been described to compromise the results [Citation22]. The anti-Xa assay based on the Coamatic Heparin reagent was calibrated with locally produced calibrator set whereas the specific Stago assay was calibrated with the STA-Edoxaban calibrator (Stago, Asnieres, France). According to the manufacturer, the measuring range for the certified edoxaban assay is between 20 and 400 µg/L.
Statistics
Results are presented as mean ± standard deviation (SD). The data sets were checked for Gaussian distribution using Shapiro–Wilk’s test. Concentrations required to double the PT or APTT clotting time (CT2) and linear regression equations for anti-Xa activity assays were calculated using Excel 97 software (Microsoft®).
Results
Effects on APTT
The dose-response curves for edoxaban effect on the five investigated APTT reagents () were similar and close to linear. The effect on the APTT, expressed as the edoxaban concentration required for doubling the APTT (CT2), was calculated to be between 539 and 758 µg/L (), which is a relative 1.4-fold difference. The DG-APTT was the most sensitive reagent with a mean increase in the clotting time of 4.7 seconds per 100 µg/L edoxaban. At the assumed therapeutic peak concentration of approx. 200–300 µg/L [Citation7,Citation14], all APTT results were above the mean baseline clotting time for that reagent +2SD. However, at the expected mean trough concentration, which has been reported to be somewhere between 14 and 48 µg/L [Citation7,Citation9,Citation14], only very few samples were above mean baseline clotting time for that reagent +2SD. There was a substantial inter-individual variation (; ) but the individual dose-response curves were parallel, meaning that the donor with the shortest or the longest APTT were the same for each edoxaban concentration (not shown).
Figure 1. Effect of edoxaban on the APTT. The obtained APTTs using five different reagents were plotted against the edoxaban concentration in plasma. TriniCLOT aPTT HS (▴), Actin FSL (●), PTT-Automate (●), APTT-SP (■) and DG-APTT (▼). Results are shown as seconds (mean ± SD) of 10 different healthy donors.
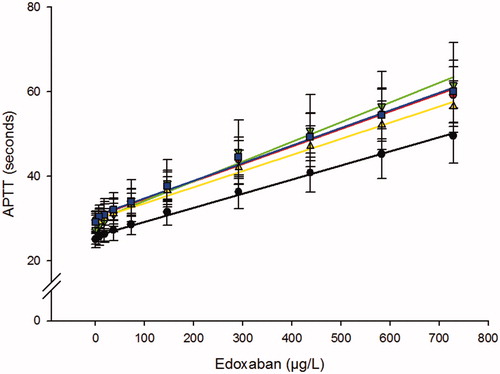
Table 1. Mean APTT (seconds) at different concentrations of edoxaban, increase in the APTT per 100 µg/L and concentrations required to double the APTT (CT2).
Effects on PT
The effect of edoxaban on seven different PT reagents is depicted in . All PT assays showed a linear relationship with a wide variation of the slopes between reagents. There was also a clear difference in sensitivities for edoxaban between Quick PT and Owren PT type reagents where the Owren PT type reagents were several times less sensitive compared to the Quick PT (). The mean edoxaban concentration for doubling the PT clotting time was found to be between 329 and 717 µg/L for the five Quick PT type reagents (). The corresponding CT2-values for the two Owren PT reagents were 1601 and 2505 µg/L, respectively. The most sensitive reagent was the Technoplastin, with a mean increase of 0.30 INR per 100 µg/L edoxaban. The least sensitive PT-type reagent was Innovin with a dose-response curve with a change of 0.09 INR per 100 µg/L edoxaban (). All samples analyzed with Quick PT type reagents, except two with Innovin, had INR above the upper reference range (>1.2) at an estimated therapeutic peak concentration of approx. 300 µg/L. For the less sensitive Owren PT type reagents, none of the samples from the 10 donors had an INR above the upper reference range (>1.2) at 300 µg/L edoxaban.
Figure 2. Effect of edoxaban on the PT assay expressed as INR. Analysis of five Quick PT assays based on plain thromboplastin reagents: STA-Neoplastine (■), RecombiPlastTin 2G (▼), Technoplastin HIS (▴), Thromborel S (■), Dade Innovin (♦), and two Owren type PT assays, based on combined rabbit thromboplastin reagents: SPA+ (●), Owren’s PT (●). Results are shown as INR (mean ± SD) of 10 different healthy donors.
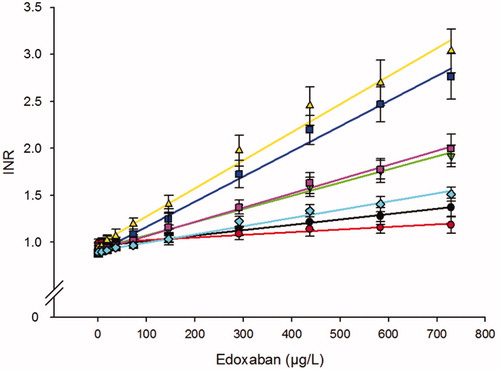
Table 2. Mean INR at different concentrations of edoxaban, mean INR increase per 100 µg/L and concentrations required to double the prothrombin time (CT2).
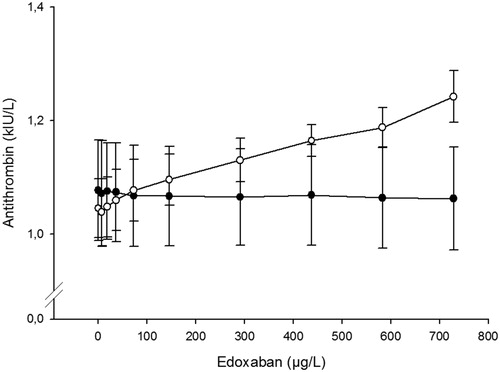
Effects on antithrombin
The antithrombin activity assay based on thrombin inhibition (Berichrom Antithrombin III) was not affected by edoxaban whereas the results with the other assay, based on factor Xa inhibition (Innovance Antithrombin), increased with the edoxaban concentration (). A linear regression analysis of the dose-response curve for the Xa-based assay indicates a weak effect of about 0.03 kIU/L per 100 µg/L (not shown).
Effects on APC resistance functional test
The APC resistance test (Pefakit APC-R Factor V Leiden) was not affected by edoxaban. Of the 10 donors, eight had a normal APC resistance ratio and two donors had a lower ratio indicating a heterozygous state of APC resistance, according to the cut-offs used by the laboratory. None of the classifications were changed depending on added edoxaban. There was a weak dose-response effect, with increasing clotting times and the edoxaban concentration, but the calculated APC resistance ratio was not affected (not shown).
Effects on dRVVT
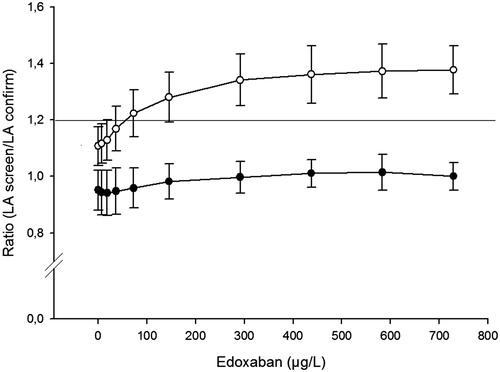
Different effects on two integrated dRVVT tests were observed. One of the tests (Staclot DRVV Screen/Confirm) was almost not affected at all whereas with increasing edoxaban concentration, the other test (Technoclot LA Screen/Confirm) displayed increasing ratios in a dose-dependent relation (). With the assumption that a screen/confirm clotting time ratio >1.20 indicates a possible positive result, all 10 samples analyzed with the Technoclot assay had a ratio above this cut-off. With the Staclot assay, none of the samples would be classified as positive. An evaluation of the underlying screen and confirm clotting times shows that the two clotting times of the Staclot assay are proportionally affected whereas for the Technoclot assay the screen clotting time is more affected by edoxaban than the confirm clotting time (not shown).
Figure 4. Effects of edoxaban on the lupus anticoagulant test. Two integrated dRVVT assays were evaluated: Technoclone LA (O) STA dRVVT (●). The assays consist of a screenclotting test (low phospholipid) and a confirm clotting test (phospholipid rich). The results are shown as the ratio (mean ± SD) of the screen/confirm test of 10 different donors and the horizontal line indicates the ratio of 1.2 that is the cut-off for suspicion of a positive result.
Measurement of edoxaban utilizing anti-Xa assays
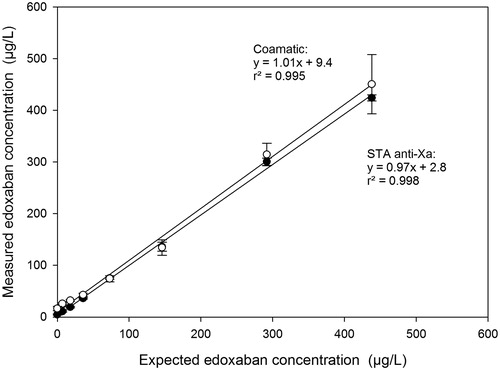
Edoxaban plasma concentration was determined with two different anti-Xa assays calibrated with edoxaban. One assay was the first commercially available and CE-labelled assay from Stago and the other was locally developed and based on the Coamatic anti-Xa reagent from Chromogenix. The linear correlations for the two assays between the expected concentration and the measured concentration are shown in . The calculated recoveries, based on the expected values for all samples with a concentration within the given measuring range of 20–400 µg/L, were between 93% and 106% for the Stago assay and 92–119% for the Coamatic assay.
Figure 5. Determination of the edoxaban plasma concentration measured by anti-Xa assays. Two assays, STA-Liquid anti-Xa using STA-Edoxaban calibrator on the STA-R MAX analyser (●), and Coamatic Heparin, calibrated with a locally produced edoxaban calibrator set and performed on the ACLTop (O). The results are shown as the anti-Xa activity (mean ± SD) of 10 different healthy donors. The solid lines are regression lines for the two assays and the equations and the r2-values of the lines are indicated in the plot.
Discussion
Edoxaban is an oral, direct factor Xa inhibitor with a dose-dependent inhibition of thrombin generation and thrombus formation [Citation23]. This implies that edoxaban also has the potential to interfere with coagulations assays dependent on factor Xa activity, which is valid for most routine coagulation tests. Edoxaban is characterized by linear and predictable pharmacokinetics and the plasma concentration is also linearly correlated with coagulation assay results, i.e. PT, APTT and anti-Xa activity [Citation7]. The linear correlation with APTT was also demonstrated in this study and this is in contrast to the three other DOAC-tests performed with the same study design, where second-order polynomial had to be used to describe the dose-response curves [Citation18–20].
As this is the fourth DOAC we have investigated with the same study design, and reagents, it has been possible to compare sensitivities towards the APTT by comparing the calculated CT2-values for each DOAC. This indirect comparison resulted in the relative order of effect on the five different APTT reagents: dabigatran > rivaroxaban > edoxaban > apixaban, where dabigatran having the greatest effect and apixaban the least for all investigated reagents. In general, rivaroxaban and edoxaban were more similar in their in vitro effects on APTT as compared to apixaban, which had very little effect on the APTT reagents tested [Citation20]. For evaluating the effects of DOACs on coagulation assays, the expected drug concentrations during standard therapy is most relevant. However, the concentration in plasma will depend on dose, absorption, kidney function and timing of blood sampling, etc., so the concentrations will differ considerably between patients. Here, we have based our peak and trough concentrations from the samples with the edoxaban content that is closest to the published population data from safety and clinical studies with a daily dose of 60 mg edoxaban [Citation7,Citation9,Citation14]. We found that edoxaban at the chosen peak concentration gave a clotting time for APTT that was invariably above the mean baseline (with no added edoxaban) clotting time +2SD for the 10 donors, but with the variability observed, not all samples would have exceeded the upper reference limit for the various APTT reagents (usually about 35–45 seconds depending on the type of APTT system). A strength of our study is that the inter-individual variation can be identified since we have based our study on 10 individual samples instead of a pooled plasma. Thus, under regular edoxaban therapy it is not granted that a prolonged clotting time will be discovered if a screening for APTT is warranted.
We have expressed PT results as INR in this study in accordance with our previous studies on effects of DOACs on coagulation analysis [Citation18–20]. We are aware the INR-system is only intended for control of oral anticoagulation with warfarin. In this context, DOACs is a possible source of error in interpretations of results. The dose-responses of increasing edoxaban concentration on results with the different PT reagents were similar to what we previously found for rivaroxaban [Citation19]. The two Owren type PT reagents were the most insensitive for edoxaban and none of the samples exceeded INR of 1.2 at the estimated peak concentration. The calculated CT2-values were very high (). This is in contrast to all of the five Quick PT type reagents that yielded INR-values above the upper reference limit for all samples with peak concentrations of edoxaban. The large differences in sensitivities between Quick PT reagents are not easily explained, could be composition and concentration of lipids and/or tissue factor and/or source and species of thromboplastin. The difference in sensitivities between the Owren and Quick PT type of reagents is probably in part explained by the higher degree of sample dilution for the Owren PT type reagents (1:21) compared to the Quick PT type reagents (1:3). The calculated CT2-values in our study were substantially higher than the values reported by Douxfils et al. [Citation15]. Here, we calculated CT2-values that were 2–3 times higher for three specific PT reagents, Neoplastin CI plus, Recombiplastin and Innovin PT, compared to what was published by Douxfils. The reason for this is probably caused by differences in defining the concentrations needed to double the clotting times. Here, and in our previous studies [Citation18–20], we have used the plasma concentration whereas Douxfils et al. based their calculations on the final concentration in the reagent mixture [Citation15]. As the plasma dilution in the PT-assay is 1:3 it can be anticipated that there would be three times in difference between our calculations of the CT2-values. Thus, examination of the figure illustrating the PT dose-response curves published by Douxfils et al. indicates that the edoxaban concentration needed for a doubling of the PT baseline clotting time, according to our definition, would be close to the values we report here. Two other studies have also published PT response curves for edoxaban, without reporting any calculated CT2-values, and an examination of the graphs for those PT reagents used in our study it can be concluded that the edoxaban needed for doubling the basal PT clotting time is near what we report [Citation13,Citation16].
Chromogenic antithrombin assays based on factor Xa inhibition are likely to be affected by DOACs targeting Xa and here we show that edoxaban affects the Xa-based assay in a dose-dependent manner. However, the effect was not as pronounced as previously described for rivaroxaban [Citation19] and apixaban [Citation20]. At estimated peak concentration of approx. 300 µg/L none of the samples exceeded the upper reference limit of 1.20 kIU/L for antithrombin activity.
A clot-based APC resistance kit was also tested with increasing edoxaban concentration. Among our donors we had eight individuals with normal results and the remaining two individuals had a ratio that was classified as being APC resistant according to the cut-offs used by the laboratory. There was a slight but comparable increase in the two underlying clotting times with increasing edoxaban concentrations that resulted in unaffected APC resistance ratios and therefore did not influence the classification by the assay.
There were discordant effects on the two tested assays for LA. The LA ratio for one assay, Staclot DRVV Screen/Confirm, was unaffected by edoxaban whereas the other assay, Technoclot LA Screen/Confirm, resulted in higher ratios with increasing edoxaban concentration (). The mechanism behind this discrepancy is not obvious. Both assays are based on Russell’s viper venom as activator and utilize two clotting reagents (screen and confirm) which may differ in the composition and concentrations of phospholipids [Citation15,Citation24]. However, detailed information is not provided by the manufacturers. Here, we observe that the screen and confirm tests of the Staclot assay are equally sensitive for edoxaban leaving the screen/confirm ratio intact over the entire edoxaban concentration range. The screen and confirm tests of the Technoclot assay are also prolonged in a dose-dependent way, but the screen test is more sensitive for edoxaban, which leads to a false positive LA ratio. The same observation was also seen with rivaroxaban using another integrated dRVVT test from Siemens [Citation20].
The findings of unaffected Staclot LA assay and Pefakit APC resistance assay are not in line with the results by Douxfils et al. that reported a dose-dependent increase of the LA ratio as well as the APC resistance ratio [Citation15]. The variable effect, between reagents and between studies using the same reagents, is difficult to explain. It may be attributed to lot-to-lot variations of the phospholipid concentrations used in these assays as we observe that the screen and confirm tests sometimes can be equally or differently affected by DOACs. The heterogeneity in detection of LA during DOAC treatment was recently reviewed [Citation24]. It seems that most reports of false positive LA come from dRVVT-based assays, where the screen test is more prolonged compared to the confirm test, whereas several other LA assays, not based on the dRVVT, display comparable prolongation times in both the screen and confirm tests.
The effects of direct oral anticoagulants on many coagulation assays are trouble-some and may cause erroneous interpretation if the presence of drug is unknown. We believe there is a need for a simple and cheap screening test, an attempt in this direction is published recently [Citation25].
Chromogenic anti-Xa assays are the most common methodology for measuring the concentrations of indirect and direct Xa inhibitors and the first commercially available assay was recently released from Stago in collaboration with Daiichi Sankyo. It is based on the STA-Liquid anti-Xa with an edoxaban-specific calibration and set-up. In a recent publication by He et al., it was shown that the assay demonstrated good correlation and accuracy relative to a reference method based on mass spectrometry [Citation26]. In our study, we had our spiked samples analyzed with the Stago assay and with a local anti-Xa assay based on the Coamatic anti-Xa reagent calibrated with edoxaban. There were linear relationships between the expected and observed anti-Xa activities with good correlation for both assays (). However, the Stago assay resulted in mean recoveries that were more close to 100% for all samples in the measuring range as compared to the local anti-Xa assay (not shown). Furthermore, the mean results of the samples analyzed with the Stago assay had less variation compared with the local assay and had better accuracy in the very low measuring range (not shown).
Conclusions
As this is the fourth DOAC we have investigated with the same study design and reagents, it was possible to compare sensitivities towards APTT and PT. This indirect comparison gave the relative order of effect on the five different APTT reagents: dabigatran > rivaroxaban > edoxaban > apixaban. The dose-responses of increasing edoxaban concentration on results with the different PT reagents were similar to what we previously found for rivaroxaban. Edoxaban interferes with antithrombin assays based on factor Xa but it did not influence the APC resistance assay used in this study. A contradictory result on the effect of integrated LA assays was found. The reason for this discrepancy is unknown but is explained by variable effect on the underlying screen and confirm clotting times. Commercially available chromogenic anti-Xa assays gave good correlations between measured and expected edoxaban concentrations.
Acknowledgments
The expert technical assistance of the following biomedical scientists is gratefully acknowledged: Peter Nemeczek (Halmstad); Ewa Lönn Karlsson (Linköping); Heidi Isacson (Malmö). Daiichi-Sankyo provided the drug edoxaban. Special thanks to the QC-team at Stago for verification of the edoxaban content in our working solutions. We are also thankful for all support from Mrs Anne Frösegård, Elisabeth Eriksson Boija and Dr Gunnar Nordin at the EQUALIS office in Uppsala.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Brekelmans M, Middeldorp S, Coppens M. Direct factor Xa inhibitor edoxaban: from bench to clinical practice. Expert Rev Hematol. 2015;8:707–702.
- Poulakos M, Walker JN, Baig U, et al. Edoxaban: a direct oral anticoagulant. Am J Health Syst Pharm. 2017;74:117–129.
- Reiffel JA, Weitz JI, Reilly PA, et al. NOAC monitoring, reversal agents, and post-approval safety and effectiveness evaluation: a cardiac safety research consortium think tank. Am Heart J. 2016;177:74–86.
- Gibson CM, Finks SW. Edoxaban: how does the newest agent fit into the DOAC landscape? Am J Med. 2017;130:900–906.
- Baglin T, Hillarp A, Tripodi A, et al. Measuring oral direct inhibitors of thrombin and factor Xa: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2013;11:756–760.
- Dale BJ, Chan NC, Eikelboom JW. Laboratory measurement of the direct oral anticoagulants. Br J Haematol. 2016;172:315–336.
- Ogata K, Mendell-Harary J, Tachibana M, et al. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol. 2010;50:743–753.
- Mendell J, Noveck RJ, Shi M. A randomized trial of the safety, pharmacokinetics and pharmacodynamics of edoxaban, an oral factor Xa inhibitor, following a switch from warfarin. Br J Clin Pharmacol. 2013;75:966–978.
- Ruff CT, Giugliano RP, Braunwald E, et al. Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomized, double-blind, ENGAGE AF-TIMI 48 trial. Lancet. 2015;385:2288–2295.
- Morishima Y, Kamisato C. Laboratory measurements of the oral direct factor Xa inhibitor edoxaban. Comparison of prothrombin time, activated partial thromboplastin time, and thrombin generation assay. Am J Clin Pathol. 2015;143:241–247.
- Gosselin R, Grant RP, Adcock DM. Comparison of the effect of the anti-Xa direct oral anticoagulants apixaban, edoxaban, and rivaroxaban on coagulation assays. Int J Lab Hematol. 2016;38:505–513.
- Adcock DM, Gosselin RC. The danger of relying on the APTT and PT in patients on DOAC therapy, a potential patient safety issue. Int J Lab Hematol. 2017;39:37–40.
- Samama MM, Mendell J, Guinet C, et al. In vitro study of the anticoagulant effects of edoxaban and its effect on thrombin generation in comparison to fondaparinux. Thromb Res. 2012;129:e77–e82.
- Zahir H, Matsushima N, Halim AB, et al. Edoxaban administration following enoxaparin: a pharmacodynamic, pharmacokinetic, and tolerability assessment in human subjects. Thromb Haemost. 2012;108:166–175.
- Douxfils J, Chatelain B, Chatelain C, et al. Edoxaban: impact on routine and specific coagulation assays. Thromb Haemost. 2016;115:368–381.
- Iba T, Emmi M, Hiki M, et al. Comparison of prothrombin time tests used in the monitoring of edoxaban and their evaluation as indicators of the reversal effect. Int J Hematol. 2016;103:665–672.
- Sezai A, Osaka S, Yaoita H, et al. Safety of the direct oral anticoagulant edoxaban for atrial fibrillation after cardiac surgery: pilot study. J Atr Fibrillat. 2016;9:1456.
- Lindahl TL, Baghaei F, Fagerberg Blixter I, et al. Effects of the oral, direct thrombin inhibitor dabigatran on five common coagulation assays. Thromb Haemost. 2011;105:371–378.
- Hillarp A, Baghaei F, Fagerberg Blixter I, et al. Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost. 2011;9:133–139.
- Hillarp A, Gustafsson KM, Faxälv L, et al. Effects of the oral, direct factor Xa inhibitor apixaban on routine coagulation assays and anti-FXa assays. J Thromb Haemost. 2014;12:1545–1553.
- Lindahl TL, Egberg N, Hillarp A, et al. INR calibration of Owren-type prothrombin time based on the relationship between PT% and INR utilizing normal plasma samples. Thromb Haemost. 2004;91:1223–1231.
- Mani H, Rohde G, Stratmann G, et al. Accurate determination of rivaroxaban levels requires different calibrator sets but not addition of antithrombin. Thromb Haemost. 2012;108:191–198.
- Furugohri T, Isobe K, Honda Y, et al. DU-176b, a potent and orally active factor Xa inhibitor: in vitro and in vivo pharmacological profiles. J Thromb Haemost. 2008;6:1542–1549.
- Hoxha A, Banzato A, Ruffatti A, et al. Detection of lupus anticoagulant in the era of direct oral anticoagulants. Autoimmun Rev. 2017;16:173–178.
- Lindahl TL, Arbring K, Wallstedt M, et al. A novel prothrombin time method to measure all non-vitamin K-dependent oral anticoagulants (NOACs). Ups J Med Sci. 2017;122:171–176.
- He L, Kochan J, Lin M, et al. Determination of edoxaban equivalent concentrations in human plasma by an automated anti-factor Xa chromogenic assay. Thromb Res. 2017;155:121–127.