Abstract
Haemostatic treatment in women experiencing postpartum haemorrhage is increasingly based on point-of-care devices such as ROTEM® thromboelastometry. Recently, a fully automated successor of the ROTEM® Delta device, the ROTEM® Sigma was introduced. To determine whether these devices provide similar results, we compared ROTEM® parameters using the ROTEM® Delta and Sigma devices in women experiencing postpartum haemorrhage. Prospective observational cohort study of 23 women experiencing postpartum haemorrhage. ROTEM® INTEM, EXTEM, FIBTEM and APTEM measurements handled by the ROTEM® Delta and Sigma devices were compared. ROTEM® FIBTEM values were also related to Clauss fibrinogen values. A correlation of Spearman’s r (rs) varying between 0.76 and 0.95 was displayed between clot firmness measured in millimeters at 5 (A5), 10 (A10) and 20 (A20) minutes after start of clot formation measured by EXTEM, INTEM and APTEM assays executed on both devices; A5, A10 and A20 of FIBTEM correlated less well (rS between 0.71 and 0.74), especially after five and ten minutes. Correlation between both devices regarding clotting time (CT) was poor. The observed correlation between levels of Clauss fibrinogen and FIBTEM A5 was rs = 0.70, (95% confidence interval (CI): 0.38 to 0.87) for Delta and rs = 0.85, (CI 0.65 to 0.94) for Sigma. A5, A10 and A20 measured in EXTEM, INTEM and APTEM obtained from ROTEM® Delta and Sigma devices were similar. EXTEM, FIBTEM and APTEM CT values from both devices showed no correlation. Substantial variation was found between FIBTEM assays of the devices. Consequently, results of FIBTEM assays should always be interpreted in the context of device-specific reference values. Correlation with Clauss fibrinogen was better in the ROTEM® Sigma device.
Introduction
Postpartum haemorrhage continues to be one of the leading causes of maternal morbidity and mortality worldwide [Citation1]. By close monitoring of haemostasis, abnormalities in coagulation parameters may be detected soon after onset of bleeding. This could contribute to more individually targeted haemostatic therapy for women experiencing postpartum haemorrhage, potentially leading to better maternal outcomes [Citation2]. Some have suggested that a low fibrinogen concentration might be the earliest predictor of progression towards severe postpartum haemorrhage [Citation3–6]. Due to long turn-around times of traditional coagulation parameters like Clauss fibrinogen, their clinical applicability in presence of rapid bleeding is limited. For early detection of changes in coagulation parameters, point-of-care devices using a visco-elastometric method for haemostasis testing like ROTEM® thromboelastometry can be used. Several studies conducted in women during postpartum haemorrhage have confirmed that the ROTEM® FIBTEM A5 assay, available 7–10 min after sampling, provides an indication of the concentration of fibrinogen during postpartum haemorrhage [Citation2,Citation5,Citation7].
Until recently, the ROTEM® Delta device was the most common device to conduct thromboelastometry. To retrieve quick information on the coagulation status of a woman, users combine blood samples with reagents in several pipetting steps. Although this procedure is relatively user-friendly and the device is marketed as a point-of-care device, the procedure can be quite complicated for non-laboratory trained personnel. Now, the Werfen® company has introduced a fully automated successor of the ROTEM® Delta device, the Sigma. The fact that with this device there is no need for a pipetting procedure, makes it more applicable as a point-of-care device to be used at the bedside. We performed local validation of the new device. To the best of our knowledge no data have been published on comparability of measurements performed on the ROTEM® Delta and Sigma devices.
The aim of this study was to compare ROTEM® EXTEM, INTEM, FIBTEM and APTEM measurements conducted by the ROTEM® Delta and Sigma devices in women experiencing postpartum haemorrhage. Moreover, ROTEM® FIBTEM values obtained from both devices were compared to corresponding Clauss fibrinogen concentrations.
Materials and methods
Design and study population
We studied women who had been included in the Towards better Prognostic and Diagnostic strategies for Major Obstetric Haemorrhage (TeMpOH-2) study, a prospective cohort of pregnant women in The Netherlands between February 2015 and April 2018. Blood samples were drawn from women experiencing postpartum haemorrhage (blood loss ≥ 1000 mL). Up to three samples per woman were available, with the first sample drawn at a volume of blood loss between 800-1500 mL. Subsequent samples were drawn in case of an additional volume of blood loss of 1000-1500 mL. Besides ROTEM® assays INTEM, EXTEM, FIBTEM and APTEM, fibrinogen concentration was assessed by the Clauss method. During the inclusion period of the TeMpOH-2 study, the ROTEM® Sigma device was launched into the market. Subsequently, in one of the study sites measurements were performed simultaneously on both devices between July 2017 and April 2018 to study whether the devices yielded similar results. Data regarding maternal age, body mass index (BMI), ethnicity, gestational age at birth, mode of birth, primary cause of haemorrhage and total volume of blood loss were recorded from medical files available at the maternity ward and operating theatre. Approval for the study was obtained from the Ethical Committee of the Leiden University Medical Centre (P13.246). The study was registered at ClinicalTrials.gov (NCT02149472). Written informed consent was obtained from all women participating in the study. Women below 18 years of age or with a gestational age below 24 weeks at the time of birth were excluded. Women with coagulation disorders or who used anticoagulants were not excluded.
Handling of ROTEM® devices and measurements
Both the ROTEM® Delta and Sigma device were positioned in a utility room equipped to locate laboratory devices at the maternity ward. ROTEM® measurements were performed by well-trained study personnel including research nurses and clinical midwives. In case measurements could not be started immediately, citrated blood samples were stored and handled at 37 °C. Results were only taken into consideration when measurements had started within four hours post blood sampling as sample stability up to 4–6 hours has been confirmed in previous studies [Citation8,Citation9]. Blood withdrawal was performed by venepuncture using a 21 gauge blood collection needle or following insertion of a peripheral vein cannulation. Blood was collected in vacuum tubes (BD Vacutainer® Citrate tubes 3.2% and BD Vacutainer® spray-coated K2EDTA tubes) and the first 3 mL of blood was always discarded. Citrated tubes were collected before ethylenediaminetetraacetic acid (EDTA) tubes. Visual inspection was conducted to verify if the minimum acquired volume of blood was collected, otherwise the tube was discarded. The citrated tubes for ROTEM analyses were handled at the maternity ward, whereas tubes containing blood for other coagulation parameters were sent to the laboratory by pneumatic tube system transport. Blood samples for Clauss fibrinogen assays were handled immediately after arriving at the laboratory and centrifuged for 10 min at 2700 g at a temperature of 20 °C. External quality control for Clauss fibrinogen, Activated Partial Thromboplastin Time (APTT) and Prothrombin Time (PT) was secured based on the participation in the International External Quality Assessment Programme (EQAP). Internal quality control was performed weekly by using the quality control reagents ‘ROTROL N’ (normal control) and ‘ROTROL P’ (abnormal control) provided by the manufacturer. Also, daily, quarterly and yearly maintenance on the devices was carried out according to the manufacturer’s instructions. Single use reagents were used in the ROTEM® Delta device.
ROTEM® measurements
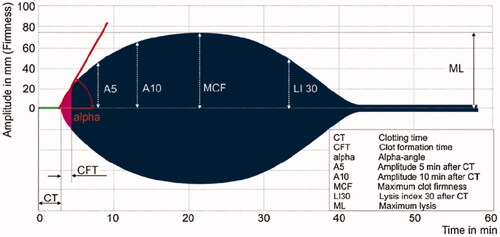
The ROTEM® Delta and Sigma analyses were performed according to the instructions provided by the manufacturer resulting in a visual display of the coagulation process (). Parameters considered include clotting time (CT) in seconds and amplitudes of clot firmness measured in millimeters at 5 (A5), 10 (A10) and 20 (A20) minutes after start of clot formation including maximum clot firmness (MCF). The following reagents were used: EXTEM to activate the extrinsic coagulation pathway, INTEM to analyse the intrinsic part of the coagulation cascade, FIBTEM to provide an indication of the fibrinogen concentration and APTEM to detect hyperfibrinolysis. All measurements on the ROTEM Delta® device were conducted with a single use reagent. On the ROTEM® Sigma device, the same measurements can be performed in a fully automated manner: a citrated tube with whole blood is inserted into a cartridge holding balls containing the four different reagents, which is then entered into the device initiating the measurements.
Statistical analysis
Characteristics of the women included are reported using descriptive statistics. Coagulation parameters are presented as median and interquartile ranges because of their non-Gaussian distribution. Non-Gaussian distribution was established by graphical visualisation and confirmed via Kolmogorov-Smirnov test. All ROTEM® parameters were verified by visually inspecting their corresponding temograms for normal lay-out and by checking all parameters for error codes. Unlikely values were excluded from the analyses. We first calculated the differences between values of ROTEM® parameters CT, A5, A10, A20 and MCF from the same samples on each of the two devices. Since the ROTEM® Sigma device was newly launched, the Delta was considered the reference. Statistical significance of the differences between the results from the two devices was tested with the Wilcoxon signed rank test. We also assessed Spearman’s rank correlation coefficients of ROTEM® Sigma and Delta results (rs) and for the correlation between ROTEM® FIBTEM values of both devices and fibrinogen concentration as obtained by the Clauss assay [Citation10].
Results
Characteristics of the women included
During the inclusion period of this sub-study of the TeMpOH-2 study, samples of 23 women experiencing postpartum haemorrhage were analysed, simultaneously in both ROTEM® devices. Baseline characteristics of the study population are reported in . Women were on average 35 years of age (interquartile range (IQR) 31–38), gave birth at a median gestational age of 39 weeks (IQR 37.4–39.3) and in 43% (10/23) by caesarean section. Median total volume of blood loss was 1400 mL (IQR 1200–1800). One sample was available for 23 women and a second sample for three women. For EXTEM and INTEM assays, 26 valid measurements executed on both devices were available. For FIBTEM and APTEM assays, 23 and 25 valid combinations of measurements could be obtained. Of the total of 26 measurements, three FIBTEM and one APTEM measurement had to be excluded because of measurement errors, providing no valid result. Clauss fibrinogen assays were available for 21 women, with a median value of 4.3 (IQR 4.1–4.8).
Table 1. Characteristics of the women included.
EXTEM
Median difference between both devices for EXTEM CT was −6.5 seconds (IQR −9.25 to −1.75) with a poor rs = 0.18 (, and Supplementary Figure S1). Median differences between amplitudes at A5, A10, and A20 minutes and also MCF were minor and statistically non-significant: −1.5 mm (IQR −3.25 to 2.0), 0.5 mm (IQR −1.0 to 1.25), 0.0 mm (IQR −1.25 to 1.25) and −0.5 mm (−2.25 to 1.0), respectively; corresponding Spearman’s correlation coefficients were excellent ().
Figure 2. Scatterplots and Spearman’s correlation coefficients (rs) for EXTEM, INTEM, FIBTEM and APTEM parameters measured on ROTEM® Delta and Sigma devices.
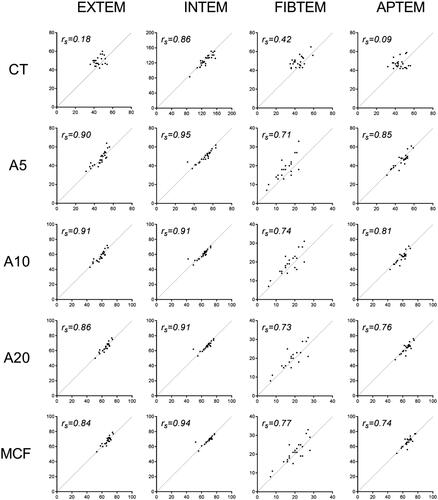
Table 2. Differences between ROTEM® Delta and Sigma for CT, A5, A10, A20 and MCF.a
Table 3. Correlation between ROTEM® Delta and Sigma for CT, A5, A10, A20 and MCF.
INTEM
INTEM values showed a median difference in CT between devices of +3.5 seconds (IQR −2to 7), rs = 0.86. Differences between amplitudes diminished over time: +2.0 mm (IQR 1–3) after 5 (A5) and 10 (A10) minutes, and +1 mm (IQR 0–2) at 20 (A20) minutes and MCF, with excellent Spearman’s correlation coefficients. Although the absolute differences between amplitudes were relatively small, these were statistically significant.
FIBTEM
The median FIBTEM CT value, performed on the ROTEM® Delta device, was 6 s (IQR −1 to −10) lower when compared to the values of the Sigma, rs = 0.42. This difference was also displayed in the amplitudes at 5, 10 and 20 minutes where some stabilisation occurred over time: A5: −4.0 mm (IQR − 5.0 to −2.0), A10: −3 mm (IQR −3. to −1) and A20: −2 mm (IQR −3to −1). The differences at A5 and A10, relevant for acute clinical decision making, were statistically significant. Spearman’s correlation coefficients for all FIBTEM measurements were only moderate compared to the results from the other assays, reflecting substantial variation between measurements.
APTEM
Variation between APTEM CT values on both devices was large, with a very poor Spearman’s correlation coefficient of −0.09. Median differences between amplitudes over time were small, but did show variation over time: A5: 0 mm (IQR −1 to 3), A10: 2mm (IQR 1–4), A20: 2mm (IQR 0.5–2.5) and MCF 0 mm (IQR −1to 2).
Correlation between Clauss fibrinogen and ROTEM® FIBTEM parameters
To determine the correlation between fibrinogen concentration measured according to Clauss and ROTEM® FIBTEM parameters A5, A10, A20 and MCF, these values were compared for both devices. Spearman’s correlation coefficients for fibrinogen and FIBTEM A5 samples were r = 0.70, (95% CI: 0.377–0.8670.867) for the Delta device and r = 0.85, (95% CI: 0.651–0.935) for the Sigma, (Supplementary Figure (S2, S3)).
Discussion
A positive correlation was found for MCF, A5, A10 and A20 measured by ROTEM® assays EXTEM, INTEM and APTEM, executed on both the ROTEM® Delta and Sigma device in women experiencing postpartum haemorrhage. FIBTEM parameters however, showed rather wide variation, and only moderate correlation. Moreover, statistically significant differences were found between devices at A5 and A10, the most relevant parameters for acute clinical decision making. FIBTEM measurements from both devices showed moderate to good correlation with Clauss fibrinogen concentration, with a better result for the Sigma device.
Strength and limitations of this study
A strength of our study is that we were able to prospectively study ROTEM® measurements performed in women experiencing postpartum haemorrhage. Samples were handled on ROTEM® Delta and Sigma devices simultaneously and were not only compared to each other but also to the traditional Clauss fibrinogen assay. A limitation of our study is the small sample size, specifically a limited number of low fibrinogen concentrations. Since low fibrinogen concentrations represent women who are potentially in need of a haemostatic intervention, it is of high importance to also assess validity of samples in women with low fibrinogen concentrations.
Interpretation of results
The ability to perform point-of-care coagulation tests in acutely bleeding patients potentially provides a considerable clinical advantage. Yet, specifically in an acute situation, reliability of measurements is of utmost importance. For EXTEM, INTEM and APTEM assays, comparability was found to be excellent between both devices. This confirms the reliability of our measurements and study procedures. However, a statistically significant difference was found between FIBTEM measurements A5 and A10, the most relevant parameters for acute clinical decision making. For example, the use of an algorithm based on FIBTEM A5 on a Delta device will tend to provide a lower result for a given Clauss fibrinogen value. This will result in overtreatment and unnecessary interventions in case this difference remains unrecognized and the algorithm is not amended to account for this device specific difference. Consequently we can conclude that the two devices are not directly interchangeable and intervention points for algorithms must be tailored to the device used. When looking at the performance of both devices in our cohort of women experiencing postpartum haemorrhage, we did observe a better correlation with Clauss fibrinogen in the ROTEM® Sigma device (rs range 0.82–0.86) as compared to the Delta (rs range 0.66–0.7).
The question that remains unanswered is why this effect is only observed in FIBTEM measurements and not in EXTEM, INTEM and APTEM. Potentially, the absence of platelets in the FIBTEM assay, results in lower amplitudes when compared to the other assays increases the sensitivity of the FIBTEM measurement. This finding needs further evaluation because of its high importance to clinical practice with the use of practice flow-charts using exact FIBTEM cut-off points to initiate (haemostatic) treatments.
Very poor correlation between CT measured on both devices was observed for EXTEM, FIBTEM and APTEM assays. Correlation for INTEM CT however, was strong. A new mode of presenting the reagent (a ball constituted of reagent, located at the bottom of the cartridge) on top of a rather short dissolving time as currently programmed in the Sigma device seems to be a possible explanation for this problem. INTEM CT takes considerably longer compared to CT’s of the other assays, thereby providing more time for the process of liquefying. We conclude that CT values obtained by the Sigma device are unreliable. Future adaptation of the cartridge and elongation of the period of reagent dissolving are possible solutions for this issue.
Comparison with other studies
To the best of our knowledge no previous studies have been conducted into the agreement between ROTEM® Delta and Sigma measurements in women experiencing postpartum haemorrhage. When looking at a non-pregnant population, we only found a trial registered by Tem Innovations GmbH (NCT02379104, Germany) into the method comparison of the ROTEM® Sigma with its predecessor model ROTEM® Delta to confirm equality of reference intervals; results are expected in December 2018. Some have studied agreement between ROTEM® FIBTEM parameters and Clauss fibrinogen concentrations. The peripartum use of thromboelastometry and ROTEM® Delta were studied among 153 women and compared with fibrinogen concentrations assessed by the Clauss method [Citation11]. Samples drawn within one hour after childbirth showed only moderate correlation between ROTEM® FIBTEM parameters and fibrinogen concentrations: Spearman’s rank correlation coefficients were 0.57 (A10), 0.56 (A20) and 0.56 (MCF). Another study into the bedside assessment of fibrinogen concentration in postpartum haemorrhage by thromboelastometry reported on results of 37 women experiencing postpartum haemorrhage [Citation12]. Correlation between Clauss fibrinogen concentration and ROTEM® FIBTEM parameters A 5, A15 and MCF was found to be strong (rs 0.84–0.86). The correlation between ROTEM® FIBTEM and Clauss fibrinogen displayed in our cohort lies between values of correlation described in these earlier studies. In both articles, the authors did not state whether single or multiple-use reagent was used.
Clinical implications
During acute situations like postpartum haemorrhage, clinicians are keen to get fast, yet reliable results on a patient’s fibrinogen status. Results from ROTEM® FIBTEM assays of the devices differed significantly, especially in the earlier measurements (A5 and A10) emphasizing the need to validate new devices before implementation and obtain device specific reference ranges, to inform appropriate device specific intervention points on an algorithm. Results of FIBTEM assays should be used with caution and should always be interpreted in the context of the specific device used and all available patient and bleeding characteristics, because of the potential variation between measurements. Parallel sampling of Clauss fibrinogen level is advised to be used as a comparison in the course of ongoing haemorrhage.
Conclusion
In our cohort of 23 women experiencing postpartum haemorrhage, we displayed a positive correlation between thromboelastometry assays EXTEM, INTEM and APTEM executed on the ROTEM® Delta and Sigma device: results of these assays from both devices are similar. CT values as obtained by the Sigma device are unreliable. A wide variation was shown between ROTEM® FIBTEM assays performed on both devices, especially in the earlier measurements (A5 and A10), important to acute clinical decision-making. Consequently, results of FIBTEM assays should be interpreted with caution and always in the context of device-specific reference values determining the intervention points on an algorithm. Correlation with Clauss fibrinogen was better in the ROTEM® Sigma device as compared to the Delta.
Acknowledgements
We would like to thank scientific laboratory technician D. Priem- Visser, research nurses C. Kolster-Bijdevaate, M.S. Bourgonje-Verhart, C.E. Bleeker-Taborh, E. Roos-van Milligen, R.J. M. Berkhout, E. Sucu, E. C. Willems of Brilman-Tuinhof de Moed and M. Stigter-Dekker and medical students M. van de Sande, R.H. Wouters, and L.S. Smits and the clinical midwifes of the Leiden University Medical Center for their contributions to the TeMpOH-2 study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–e333.
- Solomon C, Collis RE, Collins PW. Haemostatic monitoring during postpartum haemorrhage and implications for management. Br J Anaesth. 2012;109:851–863.
- Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007;5:266–273.
- Cortet M, Deneux-Tharaux C, Dupont C, et al. Association between fibrinogen level and severity of postpartum haemorrhage: secondary analysis of a prospective trial. Br J Anaesth. 2012;108:984–989.
- Collins PW, Lilley G, Bruynseels D, et al. Fibrin-based clot formation as an early and rapid biomarker for progression of postpartum hemorrhage: a prospective study. Blood. 2014;124:1727–1736.
- Collis RE, Collins PW. Haemostatic management of obstetric haemorrhage. Anaesthesia. 2015;70 (Suppl 1): 78–86, e27–e8.
- van Rheenen-Flach LE, Zweegman S, Boersma F, et al. A prospective longitudinal study on rotation thromboelastometry in women with uncomplicated pregnancies and postpartum. Aust N Z J Obstet Gynaecol. 2013;53:32–36.
- Lang T, Bauters A, Braun SL, et al. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis. 2005;16:301–310.
- Kuiper GJ, Kleinegris MC, van Oerle R, et al. Validation of a modified thromboelastometry approach to detect changes in fibrinolytic activity. Thromb J. 2016;14:1.
- Mackie IJ, Kitchen S, Machin SJ, et al. Guidelines on fibrinogen assays. Br J Haematol. 2003;121:396–404.
- de Lange NM, van Rheenen-Flach LE, Lance MD, et al. Peri-partum reference ranges for ROTEM(R) thromboelastometry. Br J Anaesth. 2014;112:852–859.
- Huissoud C, Carrabin N, Audibert F, et al. Bedside assessment of fibrinogen level in postpartum haemorrhage by thrombelastometry. BJOG. 2009;116:1097–1102.