Abstract
Sepsis is the most frequent cause of death in the intensive care unit (ICU). A rapid and correct diagnosis and initiation of therapy is crucial for improving patient outcomes. The aim of this study was to compare the performance of calprotectin with the more widely used sepsis biomarker procalcitonin (PCT) in ICU patients. The performance of calprotectin and PCT as sepsis and prognostic markers for 30-d mortality was compared in a prospective, observational study in an eight-bed ICU. We investigated concentrations of the biomarkers in plasma collected at admission from all ICU patients admitted during a year (2012–2013, n = 271) together with simplified acute physiology 3 scores (SAPS3) and sequential organ failure assessment (SOFA) scores. The receiver operating characteristic (ROC) analysis showed a higher area under the curve (AUC) value for calprotectin (0.79) than for PCT (0.49) when used as a sepsis marker. The calprotectin concentrations at admission were higher in non-survivors than in survivors at day 30. In our study, calprotectin was superior to PCT for distinguishing between ICU patients with sepsis and non-sepsis patients. Calprotectin also had higher predictive ability regarding 30-d mortality.
Introduction
Infections cause the deaths of more than 10 million people per year [Citation1] and sepsis affects between 3 and 10 per 1000 people annually in industrialized countries [Citation2]. Sepsis is the most frequent cause of admission to intensive care units (ICUs) and the most common cause of death in ICUs [Citation3]. The sepsis incidence and prevalence have been increasing continuously over the last years [Citation4]. Early identification of sepsis and initiation of treatment is essential to minimize mortality and morbidity.
A problem is that sepsis often is associated with non-specific symptoms and is, therefore, difficult to diagnose [Citation5]. At the same time, any delay in treatment increases the mortality [Citation6].
One tool used to diagnose sepsis is sequential organ failure assessment (SOFA) score. It is considered a good predictor of outcome when used during the first few days in the ICU [Citation7], but it does not aid in identifying patients before they need to be admitted to the ICU.
Although SOFA helps to identify patients with increased risk of death, and it is a part of the current sepsis definitions [Citation8], it is by no means specific for infection or sepsis.
Biomarkers have an important role in detection of sepsis and estimation of the severity of the condition. Biomarkers are to distinguish between bacterial, viral and fungal infections as the different types of infections require different types of treatments. A widely used biomarker is C-reactive protein (CRP) that has been used for many years, but CRP is mainly an inflammation marker and the specificity in sepsis has been challenged [Citation9]. Procalcitonin (PCT) is together with CRP the most widely used sepsis biomarkers. PCT has been proposed as a more specific [Citation10] and better prognostic [Citation11] marker than CRP, but this has also been questioned [Citation12]. There is thus a continuous need for better sepsis biomarkers.
Neutrophil granulocytes react to bacterial infections and are one of the first-responders to bacterial infections. When the neutrophil is activated it releases calprotectin, one of the most abundant proteins in the neutrophil cytosol. Calprotectin consists of two subunits, S100A8 and S100A9. S100A8 has a molecular weight of 10.8 kDa, while S100A9 has a molecular weight of 13.2 kDa. This biomarker increases within hours in response to bacteria or endotoxin [Citation13]. Determination of serum calprotectin has been proposed as a valuable marker of acute appendicitis [Citation14], rheumatoid arthritis [Citation15,Citation16] and sepsis [Citation17–20]. Early release of calprotectin and rapid test turn-around-times suggest that calprotectin can become a useful biomarker with widespread clinical use.
The aim of this study was to compare the performance of calprotectin and PCT as markers for sepsis and 30-d mortality in a large cohort of ICU patients.
Materials and methods
Study population
This is a prospective observational study in an eight-bed county hospital mixed ICU at Östersund hospital, a 300-bed hospital in Sweden. The study was approved by the regional ethical review board in Linköping and has been performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Verbal consent was provided by the patient or next of kin if the patient was not able to provide the consent. Consent was documented on the patient’s inclusion sheet. Verbal as opposed to written consent was chosen as many of the ICU patients were not able to write due to severity of illness. This procedure was approved by the ethics review board. All patients admitted to the ICU, Östersund’s hospital between 1 February 2012 and 31 January 2013 were screened for inclusion in the study. Inclusion criteria were admission to the ICU and presence of or need for an arterial catheter to be inserted. Patients transferred from other ICUs and age less than 18 years were exclusion criteria. Final diagnosis was set retrospectively by chart review.
SOFA scoring was recorded daily. The patients were divided into four main groups. Sepsis group; Trauma group, presumed to have inflammation without infection on admission; Other medical conditions (OMC) group, presumed to have medical conditions without inflammation on admission; and the remaining patients were designated to the Miscellaneous group. The OMC group consisted of conditions normally treated by internal medicine doctors while the miscellaneous group consisted of patients treated by other specialties, mainly surgeons.
Blood sampling and biomarker analyses
Blood samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes on admission to the ICU and on the two following days. The samples were drawn from the arterial catheter. After centrifugation, the plasma was stored at −80 °C until analyzed.
PCT was analyzed by a commercial sandwich ELISA (DY8350-05, R&D Systems, Minneapolis, MN). Calprotectin was measured with particle enhanced turbidimetric assay (PETIA) methodology using calprotectin reagents from Gentian AS (Moss, Norway) and a Mindray BS380 chemistry analyzer (Mindray, Shenzhen, China).
SOFA scoring
Simplified acute physiology 3 scores (SAPS3) were recorded on admission [Citation21]. Sequential organ failure assessment (SOFA) scores were also recorded on admission and then on the two following days [Citation22]. Sepsis was defined according to Sepsis-3 as suspected infection and a SOFA >2 on admission [Citation8].
Statistical analysis
Data are presented as median (IQR) or as number of observations (percent of total number of observations). Mann–Whitney U test or Kruskal Wallis with Dunn’s multiple comparison test were used to compare two or more groups respectively. Logistic regression was performed with the diagnosis of Sepsis vs. Trauma and Sepsis vs. OMCs, and 30-d mortality as dependent variables with PCT or calprotectin as sole predictors. Area under the curve (AUC) of the receiver operating characteristic (ROC) was calculated. Best cut-off was defined at the maximal distance from ROC curve and the diagonal. In the same model after adjusting for age, sex, illness severity (SAPS3) and extent of organ failure (SOFA), we also assessed if the biomarker in the models was an independent predictor of sepsis or death and the magnitude of such an effect expressed as odds ratio. STATISTICA software version 13.2 (StatSoft, Tulsa, OK) and GraphPad Prism version 7.0 for Windows (GraphPad Software Inc., La Jolla, CA) were used for calculations and figures. p < .05 was considered significant where relevant.
Results
Calprotectin and procalcitonin as markers for sepsis
A total of 271 patients were included in the study. Basic demographic data for the patient group is presented in . Of the total number of patients, there were 77 patients with sepsis, 33 with trauma, 78 with OMCs and 83 with miscellaneous conditions as discharge diagnoses. Mortality at 30 d was 20% for sepsis patients, 9% for trauma patients, 21% for patients with OMC and 23% for patients with miscellaneous conditions. The most common diagnoses in the OMC group were in decreasing order ketoacidosis, intoxications, hyponatremia and stroke. In the miscellaneous group, the most common diagnoses were gastrointestinal bleeding, intestinal perforations, cardiac arrest and postoperative pneumonia.
Table 1. Patient clinical and demographics data.
The levels of plasma calprotectin were higher in sepsis vs. trauma patients (p < .001; ), sepsis vs. OMC (p < .01), sepsis vs. miscellaneous conditions (p < .01) and were higher in patients who did not survive to 30 d (p < .01, ). Plasma PCT did not differ significantly between the groups or for the outcome ( and ).
Figure 1. The levels of calprotectin and procalcitonin in plasma at admission in in the different patientgroups. *p < .05, **p < .01 and ***p < .001.
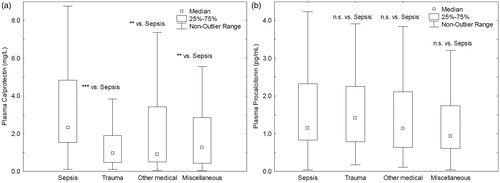
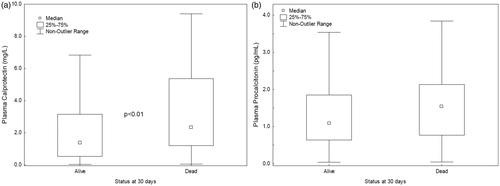
Figure 2. Calprotectin and procalcitonin in plasma at admission in survivor vs. non-survivor at 30 d. **p < .01.
AUC values for discriminating () sepsis vs. non-sepsis patients, sepsis vs. trauma patients, sepsis vs. OMC and survivor vs. non-survivor at 30 d were higher for calprotectin than for PCT.
Figure 3. Receiver operating characteristic curves for plasma calprotectin and procalcitonin. Discrimination between sepsis and non-sepsis, sepsis and other medical conditions, sepsis and trauma, as well as death at 30 d are displayed.
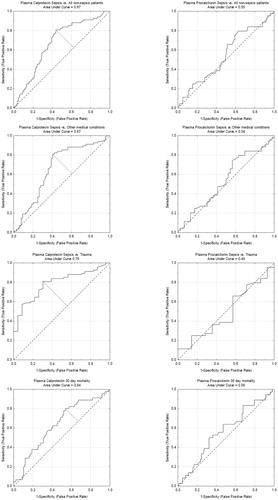
Using the best cut-off for calprotectin sensitivity values for sepsis vs. non-sepsis patients, sepsis vs. trauma patients, sepsis vs. OMC and survivor vs. non-survivor were equal or above 80% (), while specificity values were lower. Due to low AUC values for PCT cut-off values were not identified ().
Table 2. Diagnostic performance of calprotectin and PCT for discrimination between sepsis and other patients.
Calprotectin was an independent predictor for sepsis vs trauma after adjustment for age, sex, SAPS3 and SOFA scores ().
Table 3. Odds ratios for calprotectin and procalcitonin for sepsis or death as outcome after adjustment for age, sex, SAPS3 and SOFA scores.
Discussion
When bacteria or virus invade the body, the immune system is activated to counter the infection and reduce the damage. The first step in the process is the innate immunity, which responds rapidly in a non-specific fashion. The neutrophil is the first cell to reach the affected location [Citation23]. High neutrophil counts are often used as a sign of sepsis, but low neutrophil counts may also be found in sepsis patients. Both low and high neutrophil values are associated with a poor prognosis in patients with suspected sepsis. Neutrophils adhere to the vessel wall and penetrate the blood vessels to reach the site of infection. This extravasation leads to lower neutrophil numbers in the circulation [Citation24]. Neutrophil activation markers will be released from the neutrophil when activated. The measurement of neutrophil activation markers may overcome the problem of low values due to extravasation of the cells. The aim of this study was thus to evaluate the performance of calprotectin, a neutrophil activation marker, with PCT which is a widely used biomarker for sepsis. We found significantly higher calprotectin concentrations in sepsis patients while the PCT concentrations did not distinguish between sepsis patients and the other patient groups. The AUC for calprotectin was also higher and calprotectin remained an independent predictor for sepsis also after adjustment for age, sex, SAPS and maximum SOFA scores. Calprotectin concentrations were higher in non-survivors than in survivors while PCT showed no significant difference between the groups. The significance in calprotectin was lost after adjustment for age, sex SAPS and maximum SOFA scores.
The calprotectin assay used was a PETIA. PETIA is a technology that can be applied on routine chemistry analyzers available at most clinical chemistry laboratories allowing short test turnaround times. In comparison with ELISA it is designed for measuring single samples, and thus, shortens the analysis times significantly.
This is important as sepsis is an acute condition that requires rapid test results. In comparison, the available commercial PCT assays are either microtiter based or can only be performed on a limited number of instruments.
The patients in this study were severely ill and had systemic inflammatory response which was a challenge for the tested sepsis biomarkers. This is probably the reason for PCT having such poor performance in contrast to previous studies. Despite the disease severity in the study population calprotectin had a high sensitivity for the detection of sepsis.
A strength of this study was the consecutive inclusion of a comparatively large number of patients with a wide variety of diagnoses, representative for a medium‐sized mixed ICU.
Amongst the limitations of the study are that only 59 patients with severe sepsis were included and that the patients were all caucasians and recruited from a single ICU in Sweden. This limits the generalizability of our results to other ethnic groups.
Conclusions
In summary, calprotectin was superior to PCT for distinguishing between patients with sepsis and non-sepsis patients. Calprotectin had also higher predictive ability regarding 30-d mortality.
Acknowledgments
We thank Ms Carina Brännström for technical assistance.
Disclosure statement
The authors have no financial conflicts of interest.
References
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544.
- Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272.
- Perner A, Gordon AC, De Backer D, et al. Sepsis: frontiers in diagnosis, resuscitation and antibiotic therapy. Intensive Care Med. 2016;42(12):1958–1969.
- Martin GS. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Exp Rev Anti-Infect Ther. 2012;10(6):701–706.
- Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629–1638.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical Care Med. 2006;34(6):1589–1596.
- Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810.
- Yeh CF, Wu CC, Liu SH, et al. Comparison of the accuracy of neutrophil CD64, procalcitonin, and C-reactive protein for sepsis identification: a systematic review and meta-analysis. Ann Intens Care. 2019;9:5.
- Wacker C, Prkno A, Brunkhorst FM, et al. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(5):426–435.
- Arora S, Singh P, Singh PM, et al. Procalcitonin levels in survivors and nonsurvivors of sepsis: systematic review and meta-analysis. Shock. 2015;43(3):212–221.
- Tang BM, Eslick GD, Craig JC, et al. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis. 2007;7(3):210–217.
- Lipcsey M, Hanslin K, Stålberg J, et al. The time course of calprotectin liberation from human neutrophil granulocytes after Escherichia coli and endotoxin challenge. Innate Immun. 2019;25(6):369–373.
- Schellekens DH, Hulsewe KW, van Acker BA, et al. Evaluation of the diagnostic accuracy of plasma markers for early diagnosis in patients suspected for acute appendicitis. Acad Emerg Med. 2013;20(7):703–710.
- Bae SC, Lee YH. Calprotectin levels in rheumatoid arthritis and their correlation with disease activity: a meta-analysis. Postgrad Med. 2017;129(5):531–537.
- Mansour HE, Abdullrhman MA, Mobasher SA, et al. Serum calprotectin in rheumatoid arthritis: a promising diagnostic marker, how far is it related to activity and sonographic findings? J Med Ultrasound. 2017;25(1):40–46.
- Terrin G, Passariello A, Manguso F, et al. Serum calprotectin: an antimicrobial peptide as a new marker for the diagnosis of sepsis in very low birth weight newborns. Clin Dev Immunol. 2011;2011:1.
- Pepper RJ, Hamour S, Chavele KM, et al. Leukocyte and serum S100A8/S100A9 expression reflects disease activity in ANCA-associated vasculitis and glomerulonephritis. Kidney Int. 2013;83(6):1150–1158.
- Gao S, Yang Y, Fu Y, et al. Diagnostic and prognostic value of myeloid-related protein complex 8/14 for sepsis. Am J Emerg Med. 2015;33(9):1278–1282.
- Jonsson N, Nilsen T, Gille-Johnson P, et al. Calprotectin as an early biomarker of bacterial infections in critically ill patients: an exploratory cohort assessment. Crit Care Resusc. 2017;19(3):205–213.
- Moreno RP, Metnitz PG, Almeida E, et al. SAPS 3-From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345–1355.
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22(7):707–710.
- Voisin MB, Nourshargh S. Neutrophil transmigration: emergence of an adhesive cascade within venular walls. J Innate Immun. 2013;5(4):336–347.
- Lerman YV, Kim M. Neutrophil migration under normal and sepsis conditions. Cardiovasc Hematol Disord Drug Targets. 2015;15(1):19–28.
