Abstract
Monoclonal gammopathies involving immunoglobulin E (IgE) is a very rare phenomenon, with less than 70 cases being previously described in the literature. The IgE monoclonal gammopathies include malignant plasma cell disorders such as IgE multiple myeloma (MM), as well as the associated premalignant condition IgE monoclonal gammopathy of undetermined significance (MGUS). We report a case of a 41-year-old woman presenting with an IgE kappa monoclonal protein following routine laboratory testing. Serum protein electrophoresis (SPEP) initially showed a monoclonal protein in the beta-2 fraction, at an estimated concentration of <4 g/L. Subsequent serum immunofixation electrophoresis (SIFE) including antisera to Ig heavy chains delta and epsilon confirmed the presence of an IgE kappa monoclonal protein. Analysis of serum free light chains (FLCs) showed increased levels of kappa FLC, resulting in an abnormally elevated kappa/lambda FLC ratio. No Bence–Jones proteinuria was present. Bone marrow aspiration showed 6% plasma cells, and no sign of myeloma-associated end-organ damage was evident. Consequently, the patient was diagnosed with IgE kappa MGUS. In the present report, the clinical characteristics of the patient are compared to previous descriptions of IgE monoclonal gammopathy. The report further emphasizes the importance of considering the presence of monoclonal IgD or IgE when SIFE shows a clear band positive for a light chain but is negative for Ig heavy chains gamma, alpha and mu.
Introduction
Monoclonal gammopathies are a heterogeneous group of disorders characterized by the clonal proliferation of plasma cells, resulting in the production of a monoclonal immunoglobulin (Ig) [Citation1]. However, plasma cell dyscrasias resulting in the production of monoclonal IgE is a very rare phenomenon. A review of the cases of IgE monoclonal gammopathy described in the literature between 1967 and 2017 was recently published [Citation2], identifying only 63 cases since the first report in 1967 [Citation3]. Multiple myeloma (MM) is the most common malignant clonal plasma cell disorder, accounting for approximately 10% of all hematological malignancies [Citation4]. The most common MM subtypes, characterized by the resulting monoclonal Ig, are IgG MM and IgA MM, previously estimated to constitute 52% and 21% of MM cases, respectively. Light chain MM has been estimated to constitute 16%, while IgD MM and IgM MM constitute 2% and 0.5%, respectively [Citation5]. Indeed, IgE MM is the rarest subtype, estimated to constitute only about 0.1% of the MMs [Citation6]. MM is thought to be preceded by monoclonal gammopathy of undetermined significance (MGUS), a premalignant state characterized by the presence of a serum monoclonal Ig protein <30 g/L, <10% clonal bone marrow plasma cells and the absence of myeloma-associated end-organ damage or amyloidosis attributed to the underlying clonal plasma cell disorder [Citation7]. The prevalence of MGUS increases by age and has been estimated to about 0.3% and 3% in individuals <50 and ≥50 years old, respectively [Citation8,Citation9]. As could be expected, the distribution of the Ig isotypes in MGUS resembles that of MM [Citation9]. Hence, the prevalence of IgE MGUS is likely very low. To the best of our knowledge, only seven cases of IgE MGUS have been previously reported in the literature, five cases strictly meeting the current criteria according to the International Myeloma Working Group (IMWG) [Citation2,Citation7,Citation10–14].
Consequently, the knowledge regarding the IgE monoclonal gammopathies is scarce and largely based on single cases. In the present report, we describe a case of a 41-year-old female presenting with an MGUS consisting of a monoclonal Ig of the isotype IgE kappa.
Case presentation
The patient was a 41-year-old woman, previously diagnosed with irritable bowel syndrome (IBS), but otherwise fully healthy and without a family history of hematological disorders. In April 2020, she was diagnosed and empirically treated for a lobar pneumonia. No causative infectious agent was identified following microbiological testing. Due to a period of fatigue following the pneumonia, she visited her physician in May 2020 for an additional checkup, including routine laboratory testing. A serum protein electrophoresis (SPEP) (high resolution [HR] buffer, Capillarys 2, Sebia, Lisses, France) was performed, showing an increased beta-2 fraction in the electropherogram (). A subsequent serum immunofixation electrophoresis (SIFE) (Hydrasys 2, Sebia) using antisera to Ig heavy chains gamma, alpha, mu and Ig light chains kappa and lambda, showed a band positive for kappa chains (). A complementary SIFE including antisera to Ig heavy chains delta and epsilon confirmed the presence of an IgE kappa monoclonal protein (). The level of the IgE kappa monoclonal protein identified by SPEP was roughly estimated to 3.6 g/L by relating the area under the curve (AUC) of the monoclonal peak to the AUC of the albumin peak, the levels of albumin being known in g/L. Further routine laboratory testing showed normal results ().
Figure 1. Serum protein electrophoresis. Two electropherograms from the patient, before (March 2016, grey) and at the time of diagnosis (May 2020, black). The peak in the beta-2 fraction observed in May 2020 corresponds to an IgE kappa monoclonal protein co-migrating with the C3 fraction (arrow).
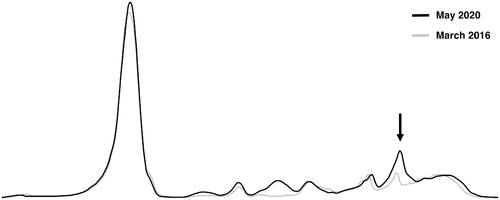
Figure 2. Serum immunofixation. (A) Serum immunofixation against immunoglobulin heavy chains gamma, alpha, mu and immunoglobulin light chains kappa and lambda, showing a band positive for kappa chains (arrow). (B) Serum immunofixation including antisera directed against immunoglobulin heavy chains delta and epsilon. The pattern corresponds to an IgE kappa monoclonal protein (arrows). ELP: serum protein electrophoresis; G: Ig heavy chain gamma; A: Ig heavy chain alpha; M: Ig heavy chain mu; D: Ig heavy chain delta; E: Ig heavy chain epsilon; K: Ig light chain kappa; L: Ig light chain lambda.
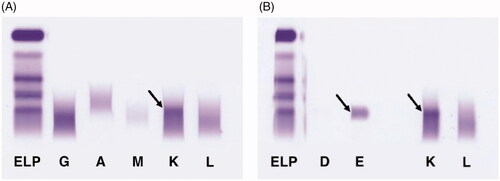
Table 1. Results of routine laboratory testing at admission.
As a consequence of the findings, the patient was referred to the Department of Hematology for further investigations. No clinical abnormalities associated with a malignant disorder were evident from the medical history or following physical examination. Whole-body low-dose computed tomography did not show any myeloma-associated skeletal lesions. Hence, no signs of myeloma-associated end-organ damage were evident [Citation4]. Analysis of serum free light chains (FLCs) (N Latex FLC kappa and N Latex FLC lambda assays; BN ProSpec System, both Siemens Healthineers, Erlangen, Germany) showed increased levels of kappa FLC of 224 mg/L (reference range: 6.7–22.4 mg/L). Lambda FLC was observed at normal levels of 16.3 mg/L (reference range: 8.3–27.0 mg/L), generating an abnormal kappa/lambda FLC ratio of 13.7 (reference range: 0.31–1.56) consistent with a monoclonal production of kappa FLC (). Serum FLCs were also analyzed using an alternative method (Freelite assay, Binding Site, Birmingham, UK; BN ProSpec System, Siemens Healthineers), showing similar results: kappa FLC of 374 mg/L (reference range: 3.0–19.0 mg/L), lambda FLC of 12.0 mg/L (reference range: 6.0–26.0 mg/L) and an abnormal kappa/lambda FLC ratio of 32.0 (reference range: 0.30–1.70) (). As expected, the Freelite assay showed somewhat higher values for kappa FLC and the kappa/lambda FLC ratio compared with the N Latex FLC assays [Citation15]. There was no presence of Bence–Jones proteinuria following urine analysis using protein electrophoresis and immunofixation. In addition, the total levels of serum IgE were analyzed (ImmunoCAP assay; Phadia 1000, both Thermo Fisher Scientific, Waltham, MA), showing markedly elevated levels of >5000 kU/L (reference range: <129 kU/L). Bone marrow aspiration showed 6% plasma cells morphologically. Flow cytometry analysis showed 3% kappa-monoclonal plasma cells with an abnormal immunophenotype (CD45dim, CD138+, CD38+, CD19−, CD56+, CD117−, CD27−, CD81dim) [Citation16]. Fluorescent in situ hybridization (FISH) showed an abnormal hybridization pattern with ins(11;14)(q13;q32q32), interpreted as a variant of the t(11;14)(q13;q32) translocation.
Table 2. Levels of the serum IgE kappa monoclonal protein and serum free light chains over time.
Consequently, the patient did not fulfill the criteria for MM, but was diagnosed with IgE kappa MGUS according to the revised criteria published by the IMWG [Citation7]. Regular follow-up was initiated to monitor an eventual increase in concentration of the monoclonal protein and a potential transition to MM. A persisting peak in the beta-2 region of the electropherogram corresponding to the IgE kappa monoclonal protein was evident during regular follow-up over eight months; the concentrations of the monoclonal protein were roughly estimated and varied between 2.4 and 2.8 g/L ().
Discussion
The patient was diagnosed with IgE kappa MGUS following the identification of an IgE kappa monoclonal protein, with an initially estimated concentration of 3.6 g/L in May 2020. Of note, the level of the monoclonal protein was then approximately 1 g/L higher compared to subsequent analyses. The fact that the patient was diagnosed with a pneumonia in April 2020 could possibly explain this observation. Accordingly, there was still evidence of an acute phase response with increased levels of CRP (13 mg/L; reference range: <3.0 mg/L) and orosomucoid (1.49 g/L; reference range: 0.52–1.17 g/L) in May 2020. In the electropherogram, the acute phase protein complement component 3 (C3) co-migrates with the peak of the monoclonal protein, affecting its approximated concentration. Therefore, it is reasonable to attribute the somewhat higher estimated level of the monoclonal protein observed in May 2020 to elevated levels of C3. At the subsequent follow-ups, the levels of measured acute phase proteins were normal, and the levels of the monoclonal protein consistently remained at a lower level. The presence of an IgE monoclonal protein involving the kappa light chain was consistent with the results from Hejl et al., reporting kappa as the most commonly involved light chain in patients with IgE monoclonal gammopathy (63% of the patients) [Citation2]. Interestingly, the translocation t(11;14)(q13;q32) identified in our patient has previously been suggested as a hallmark of IgE, IgM and non-secretory MM, due to a very high observed prevalence of the translocation in these MM subtypes (83%) [Citation17]. In unselected patients with MM, the prevalence of t(11;14)(q13;q32) has been estimated to 16–24% [Citation18]. However, the exact prevalence of the translocation in IgE MM is hard to establish due to the low prevalence of the disorder and the variable availability of historical cytogenetic information.
The presence of MGUS is associated with the risk of malignant transformation to MM [Citation19]. IgE MM has generally been associated with a poor prognosis compared to non-IgE MM [Citation20]. Furthermore, patients with IgE MM undergoing autologous transplantation have also been shown to have an inferior overall survival compared with patients suffering from common MMs (IgG, IgA and light chain MM) [Citation6]. However, the previous studies include patients treated before the introduction of modern drugs such as bortezomib and lenalidomide [Citation21]. Hence, an improved prognosis could possibly be expected with the use of current treatment regimens. Even though IgE MM seems to have a dismal prognosis relative to other MM subtypes, cases of IgE monoclonal gammopathy with benign and prolonged courses have been reported [Citation2,Citation13,Citation14]. However, due to the rarity of the IgE monoclonal gammopathies, it is hard to draw definitive conclusions regarding the prognosis of the patient described in the present report.
In conclusion, we report a rare case of IgE kappa monoclonal gammopathy, classified as IgE kappa MGUS. The report illustrates the importance of considering performing SIFE using complementary antisera to Ig heavy chains delta and epsilon when investigating a monoclonal protein positive for Ig kappa or lambda light chains that is found to be negative for Ig heavy chains gamma, alpha and mu.
Acknowledgments
Dr Anders Lindblom at the Department of Hematology, Skåne University Hospital, Malmö is acknowledged for his clinical advice during the writing of the manuscript.
Disclosure statement
The authors report no conflicts of interest.
References
- Kyle RA, Rajkumar SV. Epidemiology of the plasma-cell disorders. Best Pract Res Clin Haematol. 2007;20:637–664.
- Hejl C, Mestiri R, Carmoi T, et al. IgE monoclonal gammopathy: a case report and literature review. Clin Biochem. 2018;51:103–109.
- Johansson SG, Bennich H. Immunological studies of an atypical (myeloma) immunoglobulin. Immunology. 1967;13:381–394.
- Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95:548–567.
- Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33.
- Morris C, Drake M, Apperley J, et al. Efficacy and outcome of autologous transplantation in rare myelomas. Haematologica. 2010;95:2126–2133.
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548.
- Landgren O, Graubard BI, Kumar S, et al. Prevalence of myeloma precursor state monoclonal gammopathy of undetermined significance in 12372 individuals 10-49 years old: a population-based study from the National Health and Nutrition Examination Survey. Blood Cancer J. 2017;7:e618.
- Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362–1369.
- Malling HJ, Djurup R, Stahl Skov P, et al. Clinical and immunological aspects of a case of monoclonal hyper-IgE. Isolation of IgE-protein, estimation of basophil cell bound IgE and histamine release. Allergy. 1985;40:250–256.
- Brivio R, Cappellani A, Carpenedo M, et al. IgE monoclonal gammopathy: the clinical relevance to perform the immunofixation using IgE antisera. Int J Lab Hematol. 2020;42:e237–e239.
- Gamundi Grimalt E, Morandeira Rego F, Clapes Puig V, et al. IgE multiple myeloma: a new case report. Clin Chem Lab Med. 2017;55:e37–e40.
- Caldini A, Balboni F, Parronchi P, et al. A rare condition: IgE type monoclonal gammopathy of undetermined significance. Clin Chem Lab Med. 2014;52:e183–e185.
- Ludwig H, Vormittag W. Benign" monoclonal IgE gammopathy. Br Med J. 1980;281:539–540.
- Schieferdecker A, Horber S, Ums M, et al. Comparison of three different serum-free light-chain assays-implications on diagnostic and therapeutic monitoring of multiple myeloma. Blood Cancer J. 2020;10(2):2.
- Rawstron AC, Orfao A, Beksac M, et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008;93:431–438.
- Avet-Loiseau H, Garand R, Lode L, et al. Translocation t(11;14)(q13;q32) is the hallmark of IgM, IgE, and nonsecretory multiple myeloma variants. Blood. 2003;101:1570–1571.
- Lakshman A, Alhaj Moustafa M, Rajkumar SV, et al. Natural history of t(11;14) multiple myeloma. Leukemia. 2018;32(1):131–138.
- Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569.
- Macro M, Andre I, Comby E, et al. IgE multiple myeloma. Leuk Lymphoma. 1999;32:597–603.
- Rajkumar SV, Kumar S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020;10:94.
