Abstract
Impaired renal function is associated both with the development of cardiovascular disease and its prognosis. A new syndrome called ′Shrunken Pore Syndrome′ has been suggested, as the estimated glomerular filtration rate for cystatin C (eGFRcystatin C) is affected earlier due to differences in molecular size compared to eGFRcreatinine. The aim was to investigate if a lower eGFRcystatin C/eGFRcreatinine ratio in a prospective setting increases the risk of later developing a first-ever myocardial infarction (MI) independently of other cardiovascular risk factors. We used a nested case-referent study design within the Northern Sweden Health and Disease Study, and 545 subjects (29.0% women) were identified who prospectively developed a first-ever MI, and their 1054 matched referents. For women, but not for men, one standard deviation (SD) increase of ln z-scores of eGFRcystatin C/eGFRcreatinine ratio was associated with a lower risk of a future MI: odds ratio [95% confidence interval] 0.58 [0.34–0.99], adjusted for apolipoprotein B/A1 ratio, CRP, homocysteine, systolic blood pressure, body mass index, and diabetes. Furthermore, a high eGFRcreatinine associated independently with an increased risk of future MI in men only: OR 1.25 [1.05–1.48]. Thus, for women, a lower eGFRcystatin C/eGFRcreatinine ratio is associated with a higher risk of having a future first-ever MI, and it may be a valuable, easily implemented biomarker for risk of cardiovascular disease.
Introduction
Impaired renal function is a risk factor for cardiovascular disease (CVD) as well as for adverse outcomes for patients diagnosed with CVD [Citation1,Citation2]. The association between glomerular filtration rate (GFR) and cardiovascular disease has been studied, though mostly with case–control or retrospective designs [Citation1]. In a review of population-based studies, the prevalence of chronic kidney disease, defined as creatinine clearance or GFR less than 60 mL/min/1.73 m2, was estimated to be 7.2% in subjects older than 30 years [Citation3]. Most prospective studies on renal function and CVD risk are in cohorts in which a substantial fraction already have chronic kidney disease at inclusion, with the possibility of reverse causation. There are only a few prospective studies with subjects free from renal disease at baseline.
Creatinine and cystatin C are used as biomarkers of GFR, and both are freely filtered over the glomerular membrane in the healthy kidney. GFR–estimations based upon cystatin C are superior to GFR-estimations based upon creatinine to predict cardiovascular disease, end-stage renal disease, hospitalization, and death [Citation2,Citation4–6]. With increasing molecular masses of plasma biomarkers of GFR, the sieving coefficients of the molecules decrease [Citation7]. In the early stage of impaired renal function, a GFR reduction is not seen, and depicts how this can be conceptualized. This difference in sieving of biomarkers of different sizes depending on the type of kidney affection, quantitated by a ratio, eGFRcystatin C/eGFRcreatinine, is suggested as a more refined estimate of glomerular function, adding clinical and prognostic value [Citation7–9]. It has been termed ′Shrunken Pore Syndrome′ (SPS). Initially, the definition of SPS was a cystatin C-based estimated glomerular filtration rate (eGFRcystatin C), being less than 60% of creatinine-based estimated glomerular filtration rate (eGFRcreatinine) [Citation10].
Figure 1. Kidney with normal function and normal glomeruli, and examples of renal glomerular function impairment caused by fewer pores and shrunken pores.
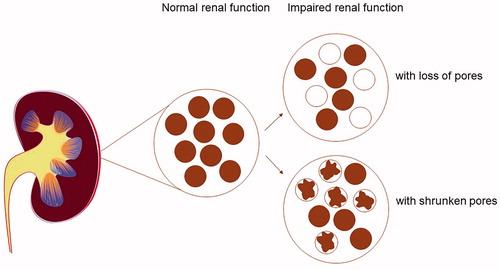
Recent studies have shown that the eGFRcystatin C/eGFRcreatinine-ratio associated with mortality in hip fracture patients [Citation11], cardiac surgery patients [Citation12], and with the development of aortic stenosis requiring valvular replacement [Citation13], notably mainly in women. As far as we know, there has been no prospective study concerning SPS and first-ever MI. This study aimed to test the hypothesis that an impaired renal function defined by the eGFRcystatin C/eGFRcreatinine ratio independently increases the risk of a first-ever MI in a prospective setting.
Methods
Study design and cohort
The study population is described in detail previously [Citation14]. It is a nested case-referent study design within the Northern Sweden Health and Disease Study (NSHDS), collected using a population-based methodology, consisting of the Västerbotten Intervention Project (VIP) [Citation15], the Northern Sweden WHO Monitoring of Trends and Cardiovascular Disease (MONICA), and the local Mammography Screening Project (MSP) [Citation16]. Cases of first-ever fatal and non-fatal MI and suspected fatal MI, according to ICD-10, that occurred prior to 1 January 2000 were included (n = 7337). Identification of cases was made, using WHO and MONICA criteria [Citation17]. Case ascertainment was performed with hospital records, general practitioners' reports, death certificates, and autopsy reports were screened. MI cases were excluded if they have had previous MI, stroke, or cancer diagnosis within five years before and one year after MI. A total of 545 subjects were included; 431, 44, and 70 from VIP, MONICA, and MSP, respectively. Two referents per case were selected within the NSHDS cohort, and matched for age, sex, time and type of survey, and geographical area. Exclusion criteria for referents were MI, stroke, cancer, or death before diagnosing the index case. Information on BMI, diabetes, hypertension, and smoking was collected. Diabetes was defined as either self-reported diabetes or fasting plasma glucose ≥7.0 mmol/L or post-load plasma glucose ≥11.0 mmol/L (12.2 mmol/L in VIP as capillary plasma was used). For classification as non-diabetic, measurement of fasting or post-load glucose was required. Hypertension was defined as either systolic blood pressure (BP) ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or taking antihypertensive medication. Current smokers were defined as self-reported daily smoking, excluding non-, previous-, and non-daily smokers: however, this variable had more missing data than the others. A study flowchart is shown in .
Figure 2. Flowchart showing the inclusion of study participants. All incident acute myocardial infarction (MI) from individuals in Norrbotten and Västerbotten counties of northern Sweden have been recorded since 1985 under the WHO's MONICA (Multinational Monitoring of Trends and Determinants in Cardiovascular Disease) project [Citation15]. Symptoms, biomarkers, and electrocardiogram (ECG) recordings were used for the diagnosis of MI. ECGs were evaluated according to the Minnesota code [Citation17]. The individuals with MI were cross-checked if they had participated in population-based risk factor surveys either as part of the Västerbotten Intervention Program (VIP), the MONICA project, or the Mammary Screening Program (MSP) within the Northern Sweden Health and Disease Study (NSHDS). At the MONICA Secretariat in Luleå and Umeå, research nurses validated MI cases that had participated. Two referent individuals with no previous MI were matched to every case based on sex, age (± 2 years), time of health examination and blood sampling, as well as geographical location and sub study.
![Figure 2. Flowchart showing the inclusion of study participants. All incident acute myocardial infarction (MI) from individuals in Norrbotten and Västerbotten counties of northern Sweden have been recorded since 1985 under the WHO's MONICA (Multinational Monitoring of Trends and Determinants in Cardiovascular Disease) project [Citation15]. Symptoms, biomarkers, and electrocardiogram (ECG) recordings were used for the diagnosis of MI. ECGs were evaluated according to the Minnesota code [Citation17]. The individuals with MI were cross-checked if they had participated in population-based risk factor surveys either as part of the Västerbotten Intervention Program (VIP), the MONICA project, or the Mammary Screening Program (MSP) within the Northern Sweden Health and Disease Study (NSHDS). At the MONICA Secretariat in Luleå and Umeå, research nurses validated MI cases that had participated. Two referent individuals with no previous MI were matched to every case based on sex, age (± 2 years), time of health examination and blood sampling, as well as geographical location and sub study.](/cms/asset/fb998683-931b-43cb-8af3-5aff5ecfad14/iclb_a_1941235_f0002_b.jpg)
Venous blood was collected in the morning in heparin-tubes, immediately centrifuged, and plasma was aliquoted, frozen, and stored at −80 °C before analysis.
Biochemical analyses were performed at Umeå University Hospital, Department of Clinical Chemistry, Swedac accreditation no 1397. Creatinine, Cystatin C, Apo A1, and Apo B were analyzed with a Hitachi 911 multi analyzer (Roche/Boehringer, Mannheim, Germany). Creatinine was analyzed with kits from Roche, Crea plus, enzymatic method (Creatinine kit Cat. No. 11775669216), and creatinine results were IDMS-corrected. Cystatin C (Cat. No LX 00210, calibrator X097401), Apo A1, and Apo B (Cat. No Code No Q 0496 and Q 0497, respectively with the same calibrator X 0947) reagents were from DAKO (Copenhagen, Denmark). CRP was analyzed with a high sensitive automated method, IMMULITE 1 (Diagnostic Products Corporation, Los Angeles, CA, USA). Homocysteine was analyzed in batch with fluorescence polarization immunoassay on an IMx® unit (Abbott Laboratories, IL, USA). The laboratory technicians were blinded to case and referent status to reduce the risk of systemic analytical bias. The case-referent triplets were analyzed at the same time in batch to minimize methodological bias. All analyses had a low analytical imprecision [Citation14].
The estimated relative glomerular filtration rate (eGFR) was calculated according to Caucasian, Asian, Pediatric, Adult cohorts (CAPA) for eGFRcystatin C and Lund-Malmö-Revised formula (LMrev) for eGFRcreatinine [Citation18,Citation19].
The study adhered to the Declaration of Helsinki and was approved by the regional ethical board, Umeå, Sweden (Dnr 03-320). All participants gave their informed written consent before inclusion in the NSHDS.
Statistical analysis
We used the T-test for independent samples for continuous variables and the Chi-square test for independence for categorical variables to compare baseline characteristics in cases and referents. The T-test and the Chi-square test were also used to compare risk factors for non-smokers vs. smokers. p-values <.05 were considered significant.
The eGFR variables were categorized into quartiles based on the referents and separate for men and women. To evaluate the relationship between the risk of having a first-ever MI and quartiles of eGFRcreatinine, eGFRcystatin C, and the eGFRcystatin C/eGFRcreatinine ratio, respectively, we calculated odds ratios (OR) with 95% confidence intervals by conditional logistic regression analysis (rather than Cox regression) and the conditional maximum likelihood routine designed for matched analysis. We calculated p for trend using the quartiles as continuous variables.
Few subjects in this cohort of individuals free of CVD at entry fulfilled the criterion of clinically overt Shrunken pore syndrome, defined by a ratio of <0.6. Thus, we used the eGFRcystatin C/eGFRcreatinine ratio as a continuous variable. We performed univariable and multivariable analyses, adjusting for confounding factors. Due to skewness, we calculated ln z-scores, and this was done for men and women separately. In these models, we calculated the risk estimations for the eGFR variables for a change of one standard deviation (SD) in ln z-scores. In Model 1, we included plasma variables: the Apolipoprotein B/A1 ratio, ln CRP, and tHcy quartiles. This model included most subjects. In model 2, systolic blood pressure (SBP), Body Mass Index (BMI), and diabetes (yes/no) were added to the first model. In model 3, we added smoking to model 2. Missing data were treated as missing. Since some cardiovascular risk factors were not registered in MSP, we performed a sensitivity analysis excluding those participants.
We used IBM SPSS Statistics 26 (IBM Corporation, New York, NY, USA) for statistical analysis.
Results
Characteristics of cases and referents at the time of their health survey, including creatinine, and cystatin C concentrations with corresponding eGFR's and the eGFRcystatin C/eGFRcreatinine ratio, are presented in . The mean time from the health survey to first-ever MI was 4.3 years for men and 3.4 years for women. Women with future MI had higher plasma cystatin C and thus lower eGFRcystatin C, and lower eGFRcystatin C/eGFRcreatinine ratio than referents. In men, the glomerular biomarkers did not differ between cases and referents. BMI, hypertension, smoking, and diabetes differed significantly between male cases and referents, and hypertension and smoking differed significantly between cases and referents for women.
Table 1. Baseline characteristics of the study population.
The associations between quartiles of eGFR and the risk of future first-ever MI are presented in . In all, higher eGFRcystatin C and higher eGFRcystatin C/eGFRcreatinine ratio associated with reduced risk of a first-ever MI. After stratification for sex, these associations remained significant in women (, ). eGFRcreatinine showed a positive trend over quartiles in all subjects for an association with future MI. The association appeared to be J-shaped. After stratification, this was also significant for men, although not significant in women.
Figure 3. Univariable odds ratios with 95% confidence intervals for the risk of a first-ever MI in relation to glomerular function for men (▲) and women (■) separately. Risk through quartiles for (a) eGFRcreatinine, (b) eGFRcystatin C, and (c) the eGFRcreatinine/eGFRcystatin C ratio. p-Values ≤.05 are highlighted with *.
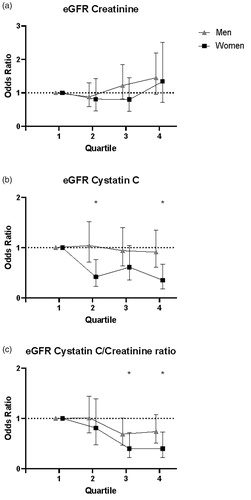
Table 2. Odds ratios (95% confidence intervals) for quartiles of glomerular function and risk of future MI.
Univariable and multivariable models for z-scores of ln eGFR's and ln eGFRcystatin C/eGFRcreatinine ratio and future MI risk are shown in . In the univariable analysis, an increase of one SD of ln eGFRcystatin C and of eGFRcystatin C/eGFRcreatinine ratio associated with a lower risk of future MI in women. This association remained for the eGFRcystatin C/eGFRcreatinine ratio after adjustments according to model 1 (apolipoprotein B/A1 ratio, ln CRP, and tHcy) and model 2 (model 1 plus systolic blood pressure, BMI, and diabetes). eGFRcystatin C remained associated with future MI after adjustments according to model 1 but not after further adjustments (model 2), although the OR remained numerically similar to previous models. In addition, eGFRcreatinine associated with increased risk of MI in men after adjustments in models 1 and 2.
Table 3. Univariable and multivariable odds ratios (95% confidence intervals) for z-scores of glomerular function and risk of future MI.
Further adjustment for smoking according to model 3 gave similar ORs as in previous models for eGFRcystatin C/eGFRcreatinine ratio, although not longer statistically significant. Therefore, we compared smokers and non-smokers (). Female smokers had lower eGFRcystatin C, eGFRcystatin C/eGFRcreatinine ratio, and BMI. Apolipoprotein B/A1 ratio, ln CRP, and tHcy were higher among smokers.
In a sensitivity analysis excluding the MSP survey (only women), the OR results remained unchanged in the remaining women (data not shown).
Discussion
In addition to conventional risk factors, biochemical biomarkers can be an essential addition to improving the prediction of myocardial infarction (MI) among patients with chronic kidney disease [Citation20] and mild impaired renal function. We have previously found a prospective association between a first-ever MI and apolipoproteins A1 and B [Citation14], CRP [Citation21], and total plasma homocysteine (tHcy) [Citation22] in our cohort. This study is, to our knowledge, the first prospective study of the association between eGFRcystatin C/eGFRcreatinine ratio and the risk of a first-ever MI.
This study's main finding was that a high eGFRcystatin C/eGFRcreatinine ratio was associated with approximately 40% lower risk of later developing an MI among women. The association remained significant after adjustment for apolipoprotein B/A1 ratio, tHcy and inflammation measured as high sensitive CRP. Sex differences has been described recently, with a tendency to higher mortality risk among women [Citation23]. We also show an association independent of tHcy despite the link to the one-carbon metabolism, where creatine synthesis accounts for 40% of methyl groups donated by S-adenosylmethionine [Citation24]. It is also known that tHcy is dependent on renal function as it is filtered in the glomeruli and then reabsorbed in the renal tubules [Citation25]. The association of MI with eGFR cystatin C/eGFR creatinine ratios among women was also independent of systolic blood pressure, BMI, and diabetes (, model 2).
When we included smoking in model 3, the OR of the eGFRcystatin C/eGFRcreatinine ratio was no longer statistically significant, but the OR was 0.66, i.e. of the same magnitude as in model 1. This may suggest a lack of statistical power. Another reason could be that smoking is also associated with many other risk factors. As shown in Supplement Table 1, smoking in women affected almost all variables in models 1 and 2.
Among subjects with heart failure, the Shrunken pore syndrome (SPS), defined as an eGFRcystatin C/eGFRcreatinine ratio <0.6, has been associated with poor right ventricular systolic function [Citation26], and with proteins involved in atherosclerosis [Citation27]. In another study, SPS associated independently with higher mortality among patients undergoing elective coronary bypass grafting [Citation28] and this was seen both in patients with low eGFR (<60 mL/min/1.73m2) as well as in the group with normal eGFR (≥60 mL/min/1.73m2). Our subjects had a relatively normal eGFR at inclusion, and the association found between future MI and SPS was based on using the eGFRcystatin C/eGFRcreatinine ratio as a continuous variable expressed as a z-score. This is according to the results of a recent large study, including 4007 patients, demonstrating that a decrease in the eGFRcystatin C/eGFRcreatinine ratio from 1.0 to 0.9 associated with increased postoperative mortality after elective cardiac surgery [Citation12]. Furthermore, we have recently shown that a high eGFRcystatin C/eGFRcreatinine ratio expressed as ln z-scores associated with a lower risk of future surgery for aortic stenosis in women [Citation13]. This lower risk was observed only among those with coronary artery disease at the time of surgery, indicating that changes in the eGFRcystatin C/eGFRcreatinine ratio may be associated with atherosclerosis in women. This association of early changes in glomerular function with MI and future surgery for aortic stenosis with concurrent coronary artery atherosclerosis was independent of apolipoproteins B/A1 ratio, diabetes, hypertension, BMI, and high sensitive CRP. The link with atherosclerosis is multifaceted, as shown by the association between the SPS and several proteins promoting atherosclerosis studied with a proteomic approach [Citation27,Citation29]. Calcification is a late stage in the complex process of atherosclerosis, and calcium metabolism may be disturbed in late-stage renal disease. There is a recent publication showing that Shrunken pore syndrome is associated with osteoporosis among 831 subjects (appr 90% women) with rheumatoid diseases [Citation30]. PTH was increased among the 4% with Shrunken pore syndrome in that study, but total calcium did not differ between groups.
In our study, a higher eGFRcystatin C associated with a lower risk of MI in women after adjusting for measured biomarkers. This association has previously been seen in two prospective studies, including men only, the first on CVD [Citation31] and the second on first MI [Citation32] in the latter study, no association was seen when adjusting for CRP. In a meta-analysis of cross-sectional studies, cystatin C associated with MI, though in that meta-analysis, possible differences due to sex were not addressed [Citation33]. This is the first study reporting prospective results for women, and in contrast to the other studies, we found no association in men. Our results remained significant in the model, including CRP and other plasma variables, but not after further adjustments.
Another finding in our study was the significant positive association between eGFRcreatinine and MI in men but not in women. eGFRcreatinine had a significant p for trend through quartiles for men and was associated with an increased risk of a first-ever MI in multivariable model 2. Although BMI was higher, we cannot exclude a lower muscle mass in cases. A higher eGFRcreatinine may also reflect glomerular hyperfiltration. This has been reported related to a high BMI [Citation34] and an inflammatory status reflected by CRP [Citation35]. In addition, all-cause mortality, cardiovascular disease [Citation36,Citation37], and subclinical CVD [Citation38,Citation39] are reported to be associated with glomerular hyperfiltration. In the Tromsø study [Citation38], higher GFR (by iohexol clearance) was associated with more subclinical coronary atherosclerosis and left ventricular hypertrophy.
There are many proposed reasons for sex differences in risk and pathophysiology for cardiovascular disease, including sex hormones, especially estrogen [Citation40]. Differences in muscle mass may be another reason for the sex differences. These differences are reflected by higher reference intervals for both creatinine and creatine kinase in plasma for men compared to women. In part, this is taken into account by including sex and age in the formula when calculating eGFRcreatinine [Citation19], and cases and referents were matched for age and sex. For eGFRcystatin C, there are no differences based on sex or muscle mass. In the Dallas Heart Study, many cardiometabolic biomarkers differed between men and women [Citation41]. Among those that differed were eGFRcreatinine and cystatin C after adjustments.
It has been shown that estimated GFR is associated with many cardiovascular risk factors [Citation42,Citation43] and mortality [Citation44] independent of measured GFR. This suggests that eGFR is partially determined by factors other than GFR, and these factors more often influence eGFRcystatin C compared to eGFRcreatinine [Citation42]. Despite these associations with surrogate markers for atherosclerotic diseases, we found a significant risk reduction for the hard endpoint first-ever MI with higher eGFRcystatin C/eGFRcreatinine ratio, even after adjustment for other risk factors. According to our results, the ratio also seems to be a better early risk marker for first-ever MI in clinical practice than eGFRcystatin C, judging from the association through quartiles (, ). Results above the median of the ratio associated with decreased risk of MI. Such a cut-off is not possible using either eGFRcystatin C or eGFRcreatinine separately.
A limitation in studies of SPS is its low prevalence among healthy subjects. By using the criteria eGFRcystatin C/eGFRcreatinine ratio of ≤0.60 [Citation10,Citation29], we found only 32 subjects (2.1%) fulfilling these criteria. More recent studies suggest a proportionally increasing CVD risk with a lower eGFRcystatin C/eGFRcreatinine ratio [Citation12,Citation13]. Thus, we used this ratio as a continuous variable in our study (). Physiological clearance measurements of GFR with iohexol or CrEDTA were not available, as they were not performed at the time of study inclusion. This may not have been a disadvantage, as eGFR is also partially determined by other cardiovascular risk factors [Citation42,Citation43].
The study's main strengths were the population-based study design, the population homogenous regarding ethnicity, representative of the population of Northern Sweden, and rigorous case ascertainment. The blood samples were taken years before MI, reducing the risk of reverse causation. All plasma samples were analyzed in one batch, reducing time-dependent methodological variability. We used an IDMS-corrected enzymatic creatinine method, with no interference from pseudo-creatinine chromogens.
In conclusion, we showed in a prospective setting that in women, a lower eGFRcystatin C/eGFRcreatinine ratio was independently associated with an increased risk of later developing a first-ever MI. This was not seen in men. The eGFRcystatin C/eGFRcreatinine ratio seems to have a more consistent risk reduction of having a future first-ever MI, through quartiles, than both eGFRcystatin C and eGFRcreatinine used separately. To determine if the eGFRcystatin C/eGFRcreatinine ratio improves the prediction of MI needs further investigation. Still, our results suggest that this ratio might be a valuable and easily implemented addition in the hospital laboratory toolbox of biomarkers for the risk of a future first MI in women.
Supplemental Material
Download PDF (67.2 KB)Acknowledgments
We would like to thank the participants in the Västerbotten Intervention Program, the Mammography screening project, and the Northern Sweden MONICA study. In particular, we acknowledge the case ascertainment made within the MONICA study. We also thank the Department of Biobank Research at Umeå University (https://www.umu.se/en/biobank-research-unit/), the Northern Sweden Health and Disease Cohort, and Västerbotten County Council for delivering data and blood samples and acknowledge the contribution of Biobank Sweden. We also thank laboratory staff at health care centers and VIP-administrators, as well as Åsa Ågren at the Department of Biobank Research, Umeå University. We also appreciate the assistance provided by Eva Samuelsson and the staff at Clinical Chemistry, Laboratory Medicine, Umeå University Hospital.
Disclosure statement
Dr. Söderberg reports personal fees and other from Actelion Ltd, outside the submitted work. No potential conflict of interest was reported by the author(s).
Data availability statement
Aggregate data are included in the manuscript and its Supporting Information files. Individual data is not publicly available, as it contains potentially identifying patient information. Data are available upon request from the Northern Sweden Health and Disease Study Biobank. To request data, interested researchers must complete a formal application (available at https://www.umu.se/en/biobankresearch-unit/research/access-to-samples-anddata/access-to-nsdd/) and submit it to The Biobank Research Unit at Umeå University (contact via [email protected]).
Additional information
Funding
References
- Sarnak MJ, Levey AS, Schoolwerth AC, American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Hypertension. 2003;42(5):1050–1065.
- Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293(14):1737–1745.
- Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117.
- Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305(15):1545–1552.
- Shlipak MG, Matsushita K, Arnlov J, CKD Prognosis Consortium, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932–943.
- Nerpin E, Ingelsson E, Riserus U, et al. The combined contribution of albuminuria and glomerular filtration rate to the prediction of cardiovascular mortality in elderly men. Nephrol Dial Transplant. 2011;26(9):2820–2827.
- Grubb A. Shrunken pore syndrome – a common kidney disorder with high mortality. Diagnosis, prevalence, pathophysiology and treatment options. Clin Biochem. 2020;83:12–20.
- Zhou H, Yang M, He X, et al. eGFR, cystatin C and creatinine in shrunken pore syndrome. Clin Chim Acta. 2019;498:1–5.
- Grubb A. Glomerular filtration and shrunken pore syndrome in children and adults. Acta Paediatr. 2021; doi: https://doi.org/10.1111/apa.15846.
- Grubb A, Lindström V, Jonsson M, et al. Reduction in glomerular pore size is not restricted to pregnant women. Evidence for a new syndrome: 'Shrunken pore syndrome'. Scand J Clin Lab Invest. 2015;75(4):333–340.
- Jonsson MH, Åkesson A, Hommel A, et al. Markers of renal function at admission and mortality in hip fracture patients – a single center prospective observational study. Scand J Clin Lab Invest. 2021;81(3):201–207.
- Herou E, Dardashti A, Nozohoor S, et al. The mortality increase in cardiac surgery patients associated with shrunken pore syndrome correlates with the eGFRcystatin C/eGFRcreatinine-ratio. Scand J Clin Lab Invest. 2019;79(3):167–173.
- Ljungberg J, Johansson B, Bergdahl IA, et al. Mild impairment of renal function (shrunken pore syndrome) is associated with increased risk for future surgery for aortic stenosis. Scand J Clin Lab Invest. 2019;79(7):524–530.
- Söderström E, Eliasson M, Johnson O, et al. Plasma folate, but not homocysteine, is associated with Apolipoprotein A1 levels in a non-fortified population. Lipids Health Dis. 2013;12(74):1–11.
- Norberg M, Wall S, Boman K, et al. The Vasterbotten Intervention Programme: background, design and implications. Glob Health Action. 2010;3. doi: https://doi.org/10.3402/gha.v3i0.4643
- Hallmans G, Agren A, Johansson G, et al. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort – evaluation of risk factors and their interactions. Scand J Public Health Suppl. 2003;61:18–24.
- Stegmayr B, Lundberg V, Asplund K. The events registration and survey procedures in the Northern Sweden MONICA Project. Scand J Public Health Suppl. 2003;61:9–17.
- Grubb A, Horio M, Hansson LO, et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem. 2014;60(7):974–986.
- Nyman U, Grubb A, Larsson A, et al. The revised Lund-Malmo GFR estimating equation outperforms MDRD and CKD-EPI across GFR, age and BMI intervals in a large Swedish population. Clin Chem Lab Med. 2014;52:815–824.
- Park SH, Stenvinkel P, Lindholm B. Cardiovascular biomarkers in chronic kidney disease. J Ren Nutr. 2012;22(1):120–127.
- Wennberg P, Wensley F, Di Angelantonio E, et al. Haemostatic and inflammatory markers are independently associated with myocardial infarction in men and women. Thromb Res. 2012;129(1):68–73.
- Van Guelpen B, Hultdin J, Johansson I, et al. Plasma folate and total homocysteine levels are associated with the risk of myocardial infarction, independently of each other and of renal function. J Intern Med. 2009;266(2):182–195.
- Åkesson A, Lindström V, Nyman U, et al. Shrunken pore syndrome and mortality: a cohort study of patients with measured GFR and known comorbidities. Scand J Clin Lab Invest. 2020;80(5):412–422.
- Brosnan JT, da Silva RP, Brosnan ME. The metabolic burden of creatine synthesis. Amino Acids. 2011;40(5):1325–1331.
- Elshorbagy AK, Oulhaj A, Konstantinova S, et al. Plasma creatinine as a determinant of plasma total homocysteine concentrations in the Hordaland Homocysteine Study: use of statistical modeling to determine reference limits. Clin Biochem. 2007;40(16-17):1209–1218.
- Christensson A, Grubb A, Molvin J, et al. The shrunken pore syndrome is associated with declined right ventricular systolic function in a heart failure population – the HARVEST study. Scand J Clin Lab Invest. 2016;76(7):568–574.
- Xhakollari L, Jujic A, Molvin J, et al. Proteins linked to atherosclerosis and cell proliferation are associated with the shrunken pore syndrome in heart failure patients: Shrunken pore syndrome and proteomic associations. Proteomics Clin Appl. 2021;2000089. doi: https://doi.org/10.1002/prca.202000089.
- Dardashti A, Nozohoor S, Grubb A, et al. Shrunken Pore Syndrome is associated with a sharp rise in mortality in patients undergoing elective coronary artery bypass grafting. Scand J Clin Lab Invest. 2016;76(1):74–81.
- Almen MS, Björk J, Nyman U, et al. Shrunken pore syndrome is associated with increased levels of atherosclerosis-promoting proteins. Kidney Int Rep. 2019;4(1):67–79.
- Yoshii I, Nishiyama S. The impact of shrunken pore syndrome in patient with rheumatic diseases on bone mineral metabolism. Scand J Clin Lab Invest. 2021;81(1):72–81.
- Astor BC, Levey AS, Stevens LA, et al. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol. 2009;20(10):2214–2222.
- Luc G, Bard JM, Lesueur C, PRIME Study Group, et al. Plasma cystatin-C and development of coronary heart disease: The PRIME Study. Atherosclerosis. 2006;185(2):375–380.
- Bi M, Huang Z, Li P, et al. The association between elevated cystatin C levels with myocardial infarction: a meta-analysis. Int J Clin Exp Med. 2015;8(11):20540–20547.
- Bachorzewska-Gajewska H, Malyszko J, Malyszko J, et al. Obesity as a risk factor of chronic kidney disease in patients undergoing primary angioplasty. Pol Arch Med Wewn. 2006;116:916–923.
- Stuveling EM, Hillege HL, Bakker SJ, et al. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int. 2003;63(2):654–661.
- Park M, Yoon E, Lim YH, et al. Renal hyperfiltration as a novel marker of all-cause mortality. J Am Soc Nephrol. 2015;26(6):1426–1433.
- Dupuis ME, Nadeau-Fredette AC, Madore F, et al. Association of glomerular hyperfiltration and cardiovascular risk in middle-aged healthy individuals. JAMA Netw Open. 2020;3(4):e202377.
- Eriksen BO, Lochen ML, Arntzen KA, et al. Subclinical cardiovascular disease is associated with a high glomerular filtration rate in the nondiabetic general population. Kidney Int. 2014;86(1):146–153.
- Rognant N, Laville M. To live with normal GFR: when higher is not better. Kidney Int. 2014;86(1):10–13.
- Westerman S, Wenger NK. Women and heart disease, the underrecognized burden: sex differences, biases, and unmet clinical and research challenges. Clin Sci. 2016;130(8):551–563.
- Lew J, Sanghavi M, Ayers CR, et al. Sex-based differences in cardiometabolic biomarkers. Circulation. 2017;135(6):544–555.
- Mathisen UD, Melsom T, Ingebretsen OC, et al. Estimated GFR associates with cardiovascular risk factors independently of measured GFR. JASN. 2011;22(5):927–937.
- Melsom T, Fuskevag OM, Mathisen UD, et al. Estimated GFR is biased by non-traditional cardiovascular risk factors. Am J Nephrol. 2015;41(1):7–15.
- Sundin PO, Sjostrom P, Jones I, et al. Measured glomerular filtration rate does not improve prediction of mortality by cystatin C and creatinine. Nephrol Dial Transplant. 2017;32(4):663–670.
