Abstract
The objective of the study was to evaluate Capitainer’s quantitative dried blood spots (qDBS) card for Hemoglobin A1c (HbA1c) testing. qDBS cards can be used for at-home sampling for HbA1c determination in a Swedish laboratory setting. A total of 153 routine requested HbA1c samples were used in this evaluation of microfluidic cards (qDBS). The HbA1c was extracted from the disc and HbA1c was determined at cobas 6000 instruments with immunological technology. The results were compared with results from traditional venous HbA1c testing. The reproducibility of using this elution procedure was 4.0% measured as coefficient of variation at a HbA1c concentration of 51 mmol/mol. Analytical performance specifications for HbA1c < 52 mmol/mol using DBS card (c501) compared with assigned values from Capillarys 3 was (y) = 1.03 x Capillarys 3(x) − 0.87; R2 = 0.97. There is a good agreement between HbA1c determined by traditional HbA1c testing and determination from Capitainer’s qDBS cards. This shows that the technology could be used for out-of doctor’s office testing.
Introduction
Hemoglobin A1c (HbA1c) is one of the main diabetes markers and has a crucial role in the management of patients with type 1 and type 2 diabetes [Citation1,Citation2]. HbA1c is a marker of the average glucose levels over a two- to three-month period. [Citation3]. To measure HbA1c there are several different methods available such as ion exchange high-performance liquid chromatography (HPLC), boronate affinity chromatography, capillary zone electrophoresis (CZE), enzymatic assays and immunoassays [Citation4,Citation5]. Both HPLC and CZE methods show the presence of hemoglobin variants which is of importance when interpreting the results but is more time consuming and requires a higher competence of the staff evaluating the test results. The enzymatic and immunoassay-based methods give the operator an HbA1c concentration in mmol/mol and the result can be automatically validated.
All these methods above are using EDTA-blood, either venous or capillary from sample tubes. The most common way is to hemolyze erythrocytes in the instrument, but increasingly there are recommendations to either dry the blood or hemolyze it in a tube on the way to the laboratory [Citation6]. Another, and more convenient way, is to use quantitative dried blood spots (qDBS) cards for collecting blood samples. Using dried samples facilitates home testing and allows the samples to be transported through the regular mail service. The dried samples can then be used for HbA1c determination in a Swedish laboratory setting. However, so far the stability of using qDBS or the results achieved in comparison to traditional sampling has not been evaluated.
The aim of the present study was to evaluate quantitative DBS-cards (Capitainer qDBS) as a sampling solution for at-home sampling for HbA1c determination in a Swedish laboratory setting. In contrast to conventional DBS, the sample discs in the qDBS solution are pre-cut and the whole disc with an exact volume is used for analysis, thereby providing a standardized sample. Ten µL blood volume as a dried blood spot was used and determination of HbA1c was performed using clinical chemistry instruments with a spectrophotometer.
Materials and methods
Samples
Routine requested HbA1c samples at the Departments of Clinical Chemistry, Uppsala, were collected in K2-EDTA tubes (364664, BD Vacutainer Systems Plymouth, UK), using anonymized samples. A total of 153 samples from Uppsala were used in this evaluation of microfluidic cards as a pre-step before HbA1c was analyzed using immunological technology or capillary electrophoresis. This evaluation was approved by the ethical committee at Uppsala University (Dnr 01-367).
Dried blood spot microsampling card
Capitainer qDBS card is a new generation quantitative dried blood spot microsampling card based on qDBS technology, where capillary blood is metered within the device to deliver an exact volume of ten µL to a pre-cut DBS disc through a microfluidic channel. Each card collects samples in two separate paths (two discs). In this evaluation twenty-five µL EDTA-blood was added to each sample well. After approx. 10 s the metering process was completed, and a red dot appeared above the sample well which the control sign for successful sampling was. The card was then left at room temperature overnight to dry. The next day all samples were retrieved by opening the protective tabs on the back side of the cards and the dried sample discs were easily released and transferred to an empty 13 mm polypropylene tube. Time of elution from dried blood spots has been assessed for storage overnight in room temperature up to 1 month of dryness with samples with known concentrations of HbA1c.
For eluting HbA1c from the DBS discs different methods were evaluated to find a procedure which works in a routine laboratory situation. For this purpose, seven different qDBS cards were evaluated, so that the dried blood was hemolyzed in a supernatant solution, and visible rated from 1–10 (see ). Rating of one was red disc (no elution had been done) and 10 was white disc (total elution had been done). Rating was lowest by using incubation for 30 min with 1000 µL hemolysate buffer without shaking (2 - almost total red disc) and highest by pre-preparation of discs with 100 µL PBS-buffer before 900 µL hemolysate buffer was added and shaken by using vortex for 5 s or a shake table at 300 rpm for 30 min (10 - total white disc). The total time for the elution procedure was 30 min, where two incubation times were included. To evaluate the reproducibility and repeatability of qDBS cards one EDTA blood sample with a known concentration of fifty mmol/mol was analyzed on cobas 6000 using 18 pre-cut filters, which was eluted according to the tested elution procedure.
Table 1. Testing various kinds of HbA1c elution procedures for DBS card according to visible elution rating.
Instruments
The HbA1c from the qDBS card was analyzed immunologically on cobas 6000 (c501; Roche Diagnostics) using Tina-Quant reagent (A1C-3 #05336163190). This reagent can be used for blood sample and samples hemolyzed offline of the system. The hemolysate step using Hemolysing Reagent for Tina-Quant HbA1c (#11488457122) in the ratio 1:101 (10 µL sample + 1000 µL hemolyzing reagent) according to method insert from Roche diagnostics. All routine samples had a known concentration of HbA1c based on routine analyzing with CZE, which was performed at Capillarys 3 Tera (Cap3) and the CAPI 3 HbA1c kit (#2515) from Sebia (Lisses, France). The Capillarys 3 is the instrument used for routine testing of patient samples at Departments of Clinical Chemistry. The instrument is participating in the external quality assurance program run by Equalis (Uppsala, Sweden).
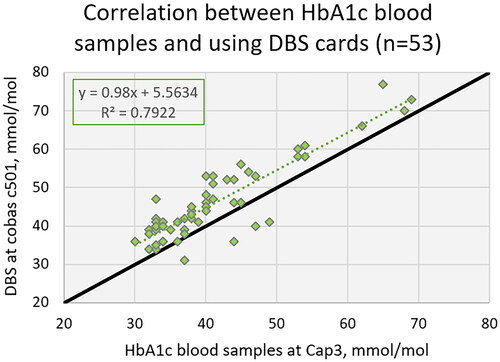
Correlation between HbA1c blood samples and using qDBS cards
A total of fifty-three blood samples were analyzed on the Capillarys 3 Tera (reference method in this study) without qDBS cards to get a known concentration of HbA1c for each sample. These samples were applied to qDBS cards to form a dried blood spot on the pre-cut filter. The dried blood spot was then eluted according to the tested procedure and analyzed on cobas 6000. The accuracy goal for HbA1c in Sweden, as agreed by Swedish external quality assurance organization (Equalis) and Swedish Society for Clinical Chemistry (SFKK); is that 95% of HbA1c results should be within 0.041xHbA1c ± 1.5 (mmol/mol) from the assigned value of EQA samples. In this evaluation no EQA samples were used, but both Capillarys 3 Tera and cobas 6000 fulfill this goal in the program [Citation7,Citation8]. Instead of using external control material assigned values of HbA1c, Capillarys 3 Tera assigned values have been referenced in this study.
Method comparison with 2nd generation of Capitainer qDBS cards
A total of 100 blood samples were analyzed at Capillarys 3 Tera and cobas 6000 without qDBS (e.g. whole blood samples in duplicate). All 100 blood samples were also added on 2nd generation of qDBS cards from Capitainer. During the time this study was done, Capitainer investigated an interference of a blue color which was used in the production of qDBS cards, which interfere with wavelengths above 600 nm (data not shown). Cobas 6000 using bicromatic analysis according to insert [Citation9] with primary wavelength of 376 and secondary wavelength of 660 nm. The secondary wavelength of 660 nm will interfere with the blue color from the production of filter. In the 2nd generation of qDBS cards from Capitainer the production is changed, and the blue color is removed from the filter. All 2nd generation filter were eluted according to the best procedure and analyzed with cobas 6000 in duplicate.
Statistical calculations
Statistical analysis (linear ordinary regression incl. correlation coefficient) was performed and calculated by Excel 365 (Microsoft Corp, Seattle, WA, USA).
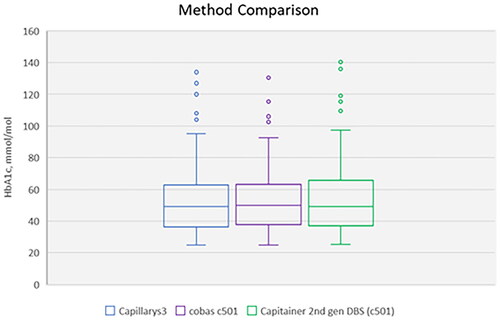
Bland Altman plots were used for comparison of HbA1c values by using qDBS cards for cobas 6000 and whole blood samples for Capillarys 3 Tera. Boxplots were used for method comparison of Capillarys 3, cobas 6000 and DBS analyzed with cobas 6000 in ; concentrations above 100 mmol/mol are presented in dots since >1.5 IQR value, which used for whiskers. As guidelines the accuracy goal for HbA1c in Sweden was used in and .
Results
Elution procedure of micro fluid cards
Different HbA1c elution conditions for the DBS card were evaluated according to visible elution ratings (). Compared with dry discs as starting material, the best procedure was to pre-wet the disc with 100 µL Phosphate-buffered saline (PBS, 1x) solution during 10 min, then add 900 µL Hemolyzing Reagent for Tina-Quant HbA1c (#11488457122) and vortex at speed 10 for 5 s. To manage a larger number of samples, the vortex can be changed to shake table using 300 rpm for 30 min to get the same elution results. After 30 min (40 min with shake table) the supernatant was ready for analysis on the cobas 6000 instrument (Roche Diagnostics, Mannheim, Germany).
Storage and Reproducibility testing of micro fluid cards
After one month of dryness in qDBS filter of eight samples, the mean HbA1c concentration was 40.0 mmol/mol [37–47 mmol/mol], while the initial HbA1c concentration had a mean value of 37.4 mmol/mol [32–44 mmol/mol]. Reproducibility testing of eighteen filters with 50 mmol/mol HbA1c showed a mean HbA1c of 51 mmol/mol and CV of 4.0% according to the tested elution procedure. The bias between original measurement and repeated measurement one month later was 1 mmol/mol which corresponds to approximately 2% (p < 0.01) at 50 mmol/mol.
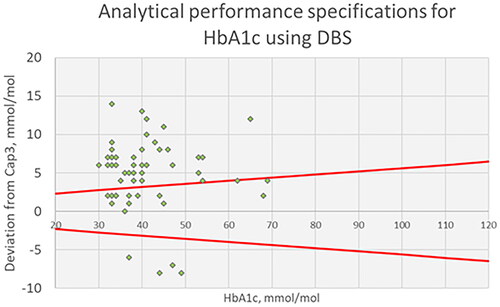
Correlation between HbA1c assigned value and using qDBS cards
The comparison between the assigned values of the 53 blood samples and using qDBS cards are presented in . The equation for the correlation between assigned values from Capillarys 3 and qDBS (c501) cards was (y) = 0.98 x Capillarys 3(x) + 5.56; R2 = 0.79. Analytical performance specifications using qDBS cards compared to assigned values showed a positive bias of 5.5 mmol/mol, which are above the national accuracy goal for HbA1c in Sweden for Hospital laboratory equipment (see ).
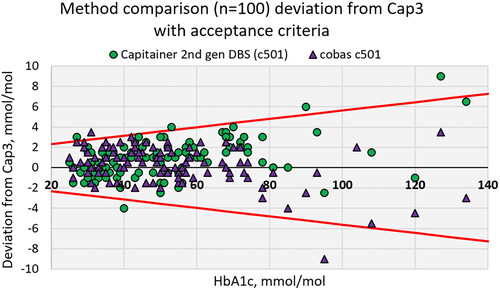
Method comparison with 2nd generation of Capitainer qDBS-cards
The method comparison of 100 routine blood samples using Capillarys 3, cobas 6000 and qDBS cards (c501) are presented in . The median for Capillarys 3 was 49 mmol/mol (qn(0.25) = 36.25; qn(0.75) = 62.75), cobas 6000 was 50 mmol/mol (qn(0.25) = 38; qn(0.75) = 63) and DBS (c501) was 49 mmol/mol (qn(0.25) = 37; qn(0.75) = 66). The total coefficient of variance (CV) in percent between the duplicates was 1.85 for cobas c501 and 2.13 with qDBS (c501). The equation for the correlation between assigned values from Capillarys 3 and cobas 6000 was (y) = 0.96 x Capillarys 3(x) + 2.14; R2 = 0.99 and for qDBS (c501) cards it was (y) = 1.05 x Capillarys 3(x) + 1.68; R2 = 0.99. The Swedish Medicines Agency’s treatment recommendation for glucose control in type 2 diabetes specifies a target value of 42–52 mmol/mol [Citation10]. In the range <52 mmol/mol the equation for the correlation for cobas 6000 was (y) = 0.99 x Capillarys 3(x) + 1.13; R2 = 0.97 and for DBS (c501) was (y) = 1.03 x Capillarys 3(x) − 0.87; R2 = 0.97. Analytical performance specifications using qDBS cards compared to laboratory methods showed an agreement up to 75 mmol/mol, with all samples inside the national accuracy goal for HbA1c in Sweden for Hospital laboratory equipment in target value of 42–52 mmol/mol (see ). Above 75 mmol/mol qDBS cards have an increasing bias of 5%.
Discussion
Many patients with diabetes monitor their blood glucose in the form of home testing either by continuous glucose monitoring or by analysis of capillary blood samples. Patients with diabetes are often elderly and may have problems getting to the primary care center. They also may require someone accompanying them which makes the trip to the primary care center costly. Previous studies have shown that diabetes patients were satisfied with dried blood sampling. The patients stated that the sample collection was easy to perform and that they preferred home sampling over traditional venous sampling at a medical center [Citation11,Citation12]. The volumes obtained by capillary samplings should be sufficient also for HbA1c testing. It is, however, not possible to send vacutainer tubes via regular mail. The use of dried blood samples could be sent by regular mail at room temperature, and it is expected that the transport time from all parts of Sweden would be one to three days at the most. Thus, the analytes must be stable at room temperature for at least this time and, preferably, longer.
The aim of the present study was to evaluate the new quantitative DBS card (Capitainer qDBS card) as a pre-step for HbA1c determination in a Swedish hospital laboratory setting with the potential to be used for home sampling. The use of capillary blood on filter paper to determine HbA1c has previously been reported [Citation13], but to have an exact volume of blood in each disc using microfluidics technology is new and enables an easy sampling at home. Improved sample quality and volumetric control in DBS, have previously been shown to improve analytical quality in other types of tests. Relative to liquid blood, the mean bias for conventional DBS cards was −32.6% [Citation14].
In this study, we showed that dried blood on the pre-cut filter in qDBS cards could be stored at room temperature for a month without any decrease in HbA1c values. When performing stability testing an acceptable difference of 10% is often used. The bias requirements should be lower for HbA1c and it is more appropriate to compare with the quality requirements for the national HbA1c. For a method to be used for diagnostics of diabetes type 2 in Sweden the instrument used for HbA1c analysis should have a margin of error which is less than ± 3.5 mmol/mol at the diagnostic limit of 48 mmol/mol. Thus, although the change of HbA1c results after one month was significant, the change is clearly lower than the national acceptance criteria for method differences. The stability data is in agreement with a previous study that showed that DBS samples could be stored for 44 days at room temperature before being analyzed using the Roche Tina-quant® II immunoturbidometric assay [Citation15]. A previous study showed that concentration of HbA1c (mmol/mol) in EDTA blood sample tubes decreased in room temperature in a brief period of days [Citation7]. This evaluation also showed a variation of 4% in repeatability, but this value includes two different steps, which can be the reason for the variation, elution procedure and instrument analyzing. According to the method sheet for HbA1c generation three on cobas 6000 [Citation9], the coefficient of variation can be between 1–2%, which indicates that 2–3% in variation will be related to the sample storage and preparation procedure. In this study, we showed that the hemolysate buffer was not efficient in directly eluting HbA1c from the filter paper. Thus, the addition of the PBS solution, before adding the hemolyzing reagent and vortexing, was needed to elute HbA1c from the filter papers efficiently. By analyzing the qDBS extracted samples on cobas 6000 and comparing the HbA1c results with the assigned value from Capillarys 3 Tera, using the same EDTA blood samples, the evaluation showed a positive bias using qDBS card, higher than the accuracy goal for HbA1c in Sweden allows for.
In a previous study, it has been shown that the correlation between HbA1c gen3 on cobas 6000 and Capillarys 3 Tera has a small positive bias also on liquid blood samples [Citation8]. However, even after adjustment for this the calculated positive bias using qDBS is still too high for the accuracy goal (data not shown). However, the Equalis’ recommendations about HbA1c [Citation16] have a notice about methods for point of care and allow wider boundaries for accuracy goals than for laboratory instruments. As qDBS is somehow a point-of-care method, it should be compared with this group of methods and are thus in parity with these methods in terms of accuracy goals. The positive bias could also be reduced by introducing a mathematical factor in laboratory information systems, which adjusts the result uniformly. A notice of this is that in this evaluation, no EQA samples have been used, just EDTA samples assigned with Capillarys 3, but Capillarys 3 have fulfilled these criteria which have been shown in Rollborn et.al. [Citation7]. The method comparison shows that 2nd generation of qDBS card and analyzing at cobas 6000, is similar to analyzing EDTA-blood samples. The difference of blue color in the 1st generation and 2nd generation qDBS cards from Capitainer had according to this evaluation generate a decreasing bias at the determined concentrations, which made the 2nd generations of qDBS cards comparable to laboratory system, such as Capillarys3 and cobas 6000.
A limitation of the study is that we compared venous blood sampling with dried blood spots from venous samples and not capillary samples. However, previous studies have shown a good agreement between HbA1c results obtained with venous and capillary sampling [Citation13,Citation17].
Conclusion
There is a good agreement between HbA1c determined by traditional HbA1c testing and determination from Capitainer’s qDBS cards. This shows that the technology could be used for out-of doctor’s office testing.
Ethical approval
The local ethical committee (01-367) approved the collection of samples. The ethical permit limited the patient information to age and gender.
Acknowledgements
The qDBS cards for the HbA1c evaluation were provided by Capitainer, Solna, Sweden.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371(21):1972–1982. doi:10.1056/NEJMoa1408214.
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the swedish national diabetes register (NDR). J Intern Med. 2010;268(5):471–482. doi:10.1111/j.1365-2796.2010.02265.x.
- Paprott R, Schaffrath Rosario A, Busch MA, et al. Association between haemoglobin A1c and all-cause mortality: results of the mortality follow-up of the german national health interview and examination survey 1998. Diabetes Care. 2015;38(2):249–256. doi:10.2337/dc14-1787.
- Weykamp C. HbA1c: a review of analytical and clinical aspects. Ann Lab Med. 2013;33(6):393–400. doi:10.3343/alm.2013.33.6.393.
- Weykamp C, John WG, Mosca A. A review of the challenge in measuring hemoglobin A1c. J Diabetes Sci Technol. 2009;3(3):439–445. doi:10.1177/193229680900300306.
- Colley J, Dambha-Miller H, Stuart B, et al. Home monitoring of haemoglobin A1c in diabetes: a systematic review and narrative synthesis on accuracy, reliability and patient acceptability. Diabet Med. 2023;40(4):e15033. doi:10.1111/dme.15033.
- Rollborn N, Åkerfeldt T, Nordin G, et al. Analysis of HbA1c on an automated multicapillary zone electrophoresis system. Scand J Clin Lab Invest. 2017;77(1):15–18. doi:10.1080/00365513.2016.1238507.
- Rollborn N, Nordin G, Mandic-Havelka A, et al. Good agreement Between Hba1c analyzed using capillary electrophoresis, HPLC, immunological and enzymatic methods. JDMC. 2019;1:1–7.
- Method sheet. Tina-quant hemoglobin A1c gen. 3. Roche Diagnostics 2018-02, v.7. 2018.
- Läkemedelsbehandling för glukoskontroll vid typ 2-diabetes – behandlingsrekommendation. Information från. Läkemedelsverket. 2017;28(4):29–48.
- Fokkema MR, Bakker AJ, de Boer F, et al. HbA1c measurements from dried blood spots: validation and patient satisfaction. Clin Chem Lab Med. 2009;47(10):1259–1264. doi:10.1515/CCLM.2009.274.
- Verougstraete N, Stove V, Stove CP. Remote HbA1c testing via microsampling: fit for purpose? Clin Chem Lab Med. 2023;62(1):3–17. in press doi:10.1515/cclm-2023-0228.
- Jeppsson JO, Jerntorp P, Almër LO, et al. Capillary blood on filter paper for determination of HbA1c by ion exchange chromatography. Diabetes Care. 1996;19(2):142–145. doi:10.2337/diacare.19.2.142.
- Carling RS, Emmett EC, Moat SJ. Evaluation of volumetric blood collection devices for the measurement of phenylalanine and tyrosine to monitor patients with phenylketonuria. Clin Chim Acta. 2022;535:157–166. doi:10.1016/j.cca.2023.117744.
- Jones TG, Warber KD, Roberts BD. Analysis of hemoglobin A1c from dried blood spot samples with the tina-quant® II immunoturbidimetric method. J Diabetes Sci Technol. 2010;4(2):244–249. doi:10.1177/193229681000400203.
- Nordin G. Kvalitetsmål för HbA1c-metoder som används för diagnostik av typ 2 diabetes, Equalis. https://www.equalis.se/media/m0ja141g/s006_kvalitetsm%C3%A5l-f%C3%B6r-hba1c_2-1.pdf
- Nathan DM, Krause-Steinrauf H, Braffett BH, et al. Comparison of Central laboratory HbA1c measurements obtained from a capillary collection versus a standard venous whole blood collection in the GRADE and EDIC studies. PLoS ONE. 2021;16(11):e0257154. doi:10.1371/journal.pone.0257154.