Abstract
Legumain is known to be regulated in atherosclerotic disease and may have both pro- and anti-atherogenic properties. The study aimed to explore legumain in individuals with familial hypercholesterolemia (FH), a population with increased cardiovascular risk. Plasma legumain was measured in 251 subjects with mostly genetically verified FH, of which 166 were adults (≥18 years) and 85 were children and young adults (<18 years) and compared to 96 normolipidemic healthy controls. Plasma legumain was significantly increased in the total FH population compared to controls (median 4.9 versus 3.3 pg/mL, respectively, p < 0.001), whereof adult subjects with FH using statins had higher levels compared to non-statin users (5.7 versus 3.9 pg/mL, respectively, p < 0.001). Children and young adults with FH (p = 0.67) did not have plasma legumain different from controls at the same age. Further, in FH subjects, legumain showed a positive association with apoB, and markers of inflammation and platelet activation (i.e. fibrinogen, NAP2 and RANTES). In the current study, we show that legumain is increased in adult subjects with FH using statins, whereas there was no difference in legumain among children and young adults with FH compared to controls. Legumain was further associated with cardiovascular risk markers in the FH population. However the role of legumain in regulation of cardiovascular risk in these individuals is still to be determined.
Introduction
Legumain, asparagine endopeptidase, is a cysteine protease of the C13 family. Although traditionally associated with its cysteine protease activity in lysosomes, where it contributes to antigen processing for MHC II presentation, it can also localize to the nucleus, cytosol and extracellularly, highlighting the adaptability and diversity of functions [Citation1].
Atherosclerosis, the main cause of cardiovascular disease (CVD), which is the number one cause of death in the world, is defined by a complex interaction between lipids and inflammation [Citation2]. Legumain is known to be regulated in atherosclerotic disease of both mice and men [Citation3], and so far the data point to a complex and dual role of legumain in these conditions. We have previously shown that legumain is upregulated in plasma and plaques from patients with carotid atherosclerosis, especially in patients with the most recent symptoms, and within the lesions, legumain localized to macrophages [Citation4]. More recently, high plasma legumain levels were observed in patients with complex coronary lesions [Citation5] and predicted all-cause mortality following myocardial infarction (MI) [Citation6]. Moreover, in an atherosclerotic mouse model, legumain infusion promoted vascular remodeling [Citation7]. Recently, however, we reported that high plasma levels of legumain was associated with improved outcome in patients with acute coronary events and to mediate anti-inflammatory effects within macrophages [Citation8]. In line with a potential protective effect of legumain in atherosclerotic disorders, we recently found high legumain levels to be associated with reduced risk of stroke in patients with acute coronary disease [Citation9].
Familial hypercholesterolemia (FH) is an inherited disorder resulting in increased low-density lipoprotein (LDL) cholesterol levels from birth, and an increased risk of premature coronary heart disease [Citation10–12]. Thus, the first-line therapy in these patients are long-term use of statins, with a recommended initiation at 8-10 years of age [Citation13], to lower their cholesterol burden. However, subjects with FH also display an inflammatory phenotype already from childhood [Citation14,Citation15], which does not seem to be fully normalized by lipid-lowering therapy [Citation16,Citation17]. To further elucidate the role of legumain in atherosclerosis, we investigated the effect of lifelong LDL cholesterol exposure and statin treatment on plasma legumain as well as its relation to markers of inflammation and platelet activation known to be related to cardiovascular risk in a well-characterized population of young and adult FH patients.
Materials and methods
Study population
In this cross-sectional study, we recruited subjects with mostly genetically verified FH ≥ 6 years of age from the outpatient Lipid Clinic, Oslo University Hospital, Norway [Citation18–22]. A Dutch Lipid Clinic Network score >8[11], or a clinical examination and the Simon Broome criteria [Citation23] were used to assess a clinical FH diagnosis in subjects without a positive DNA test. Healthy, normolipidemic controls were recruited among friends and colleagues at the university and the hospital. Exclusion criteria were homozygous FH, and pregnant and lactating women. Furthermore, as part of the regular follow-up of the children with FH, thirteen initiated statin therapy during the study [Citation20] and were included in an exploratory, pilot statin intervention.
Ethics approval and consent to participate
The Regional Committees for Medical and Health Research Ethics approved the protocols, ref. no. REK 2013/592, REK 2015/1577 and REK 2015/2392. The study confirms the principles outlined in the Declaration of Helsinki for use of human samples or subjects. Signed informed consent was obtained from all participants, or from one of their parents for the children below the age of 16 years.
Plasma analysis
Plasma legumain concentrations were determined by enzyme immunoassay (EIA) with antibodies from R&D Systems (Duoset DY4769), Stillwater, MN. Serum levels of LDL-associated phospholipase A2 (Lp-PLA2) and autotaxin were determined by EIA from R&D systems (DPLG70 and DENP20, respectively). Plasma levels of tumor necrosis factor (TNF) was determined by a custom human cytokine kit, V-plex (cat # K151A0H-1), from Mesoscale Discovery, Rockville, MD. Plasma levels of platelet-factor (PF)4 (Cat# DY795), neutrophil-activating protein (NAP2/CXCL7) (Cat#DY393), regulated on activation, normal T cell expressed and secreted (RANTES/CCL5) (Cat#DY278) and soluble CD40 ligand (CD40L) (Cat#DY617) were measured by EIAs obtained from R&D Systems. The intra- and inter-assay coefficients of variations were <10% for all EIAs. Routine biochemical analysis was performed in clinical routine laboratory at Oslo University Hospital.
Statistical analysis
We calculated descriptive summary statistics for display in tables. We used n (%) for categorical variables; for continuous variables, we used mean (SD) for normally distributed variables, and median (IQR) for right-skewed variables. For hypothesis testing, we used Pearson’s Chi-squared test for categorical variables; for continuous variables, we used the Welch Two Sample t-test for normally distributed variables, and the Wilcoxon rank sum test for right-skewed variables. We loge transformed legumain before analyses since it was right-skewed. In the figures, the axis labels for legumain are back-transformed for readability. In and , we display p-values from Welch Two Sample t-tests. In Figure S2, we display p-values from a paired t-test. For the 13 subjects with repeated measures of legumain, we excluded measurements for post-statin treatment in all analyses, except for data presented in Figure S2. We used multiple linear regression to explore the association between legumain and various clinical and biochemical markers among the entire population (adults and children, FH and controls, n = 347), including biomarkers of inflammation and platelet activation, adjusting for sex, age, BMI and statin use. Prior to modeling, to enable visualization in a forest plot, we scaled each clinical and inflammatory/platelet activation marker to a standard normal distribution by subtracting the mean and dividing by its standard deviation, using the scale function in R. All data analyses were performed in R version 4.2.0 [Citation24], using RStudio (Boston, MA, USA, www.rstudio.com) and the tidyverse framework [Citation25].
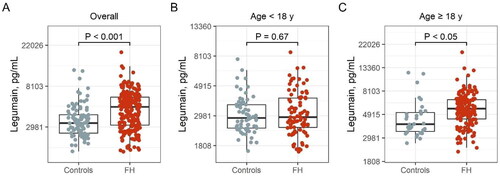
Figure 1. Plasma levels of legumain in the (A) total population of FH subjects and controls (controls, n = 96; FH, n = 251), (B) in children and young adults (controls, n = 64; FH, n = 85) and (C) in the adult populations (controls, n = 32; FH, n = 166). FH, familial hypercholesterolemia. P values are from Welch Two Sample t-tests.
Results
Characteristics
The study population consisted of a total of 251 FH subjects, divided in two different cohorts; children and young adults <18 years of age (n = 85) (Table1), and adults >18 years of age (n = 166) (). More than 97% of the FH subjects had a genetically-verified diagnosis. Normolipidemic healthy individuals were included as control subjects (64 children and young adults, 32 adults). The adult FH subjects were characterized by a higher age, than controls (median age 42 versus 29, p < 0.001); however, for children and young adults, FH subjects were younger than the control subjects (median age 12 versus 14, p = 0.001). In the children and young adults with FH, total cholesterol, LDL cholesterol, apolipoprotein B (apoB) and C reactive protein (CRP) were increased compared to control subjects (, 7.0 versus 4.0 mmol/L, 5.1 versus 2.2 mmol/L, 1.3 versus 0.6 g/L and 1.0 versus 0.6 mg/L in FH subjects and controls respectively, p < 0.001 for all). None of the children and young adults (both FH and controls) used statins (). In adults with FH (>18 years of age, n = 166), body mass index (BMI) and apoB were increased (25.9 versus 21.7 kg/m2 and 1.1 versus 0.8 g/L in FH subjects and controls, respectively), and there were more statin users among FH subjects, with 80% of FH subjects using statins, versus none in the control group (, p < 0.001 for all).
Table 1. FH subjects and healthy control <18 years of age.
Table 2. FH subjects and healthy control 18 years of age and older.
Plasma legumain is increased in adult subjects with FH using statins
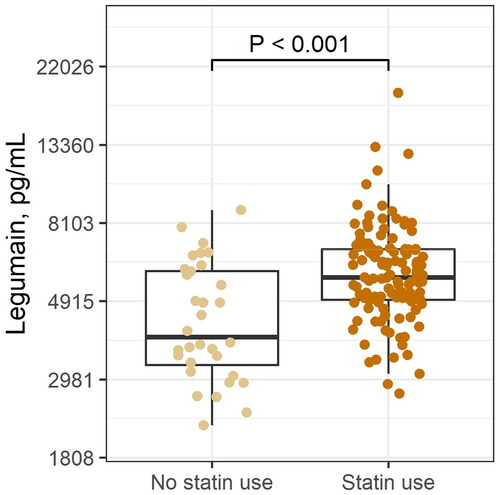
In the total FH population, plasma levels of legumain were significantly elevated compared to controls (median 4.9 versus 3.3 pg/mL, respectively, , p < 0.001) with no differences between male or female FH subjects (data not shown). Legumain showed a positive correlation with age (Spearman’s r = 0.36, p = 0.0003). When analyzing the cohorts separately, plasma legumain was increased in adult subjects with FH compared to healthy controls (5.6 and 4.0 pg/mL, respectively, p < 0.05, ), but not in FH children (2.9 and 2.9 pg/mL, respectively p = 0.67, ,). To examine the effect of statins on plasma legumain, we performed analyses in the adult FH population, in which we had statin users. Levels of legumain were higher among statin users as compared to non-statin users (5.7 versus 3.9 pg/mL, respectively, , p < 0.001), with no differences between sexes (males: 6.2 versus 3.8 pg/mL in males and 5.5 versus 4.1 pg/mL in females, respectively, Supplemental Figure 1). Statin use was further positively associated with legumain levels after adjusting for age, sex and BMI, in the total population (β = 0.29, p < 0.001) and even more so in the FH population (β = 0.34, p < 0.001). In an explorative pilot intervention study, we measured legumain levels before and after initiation of statin use in children and young adults with FH (n = 13, Supplemental Table 1). Although not significant, legumain levels seemed to increase after initiation of statin use in these individuals (Pre statin use, 2.2 pg/mL and after, 2.3 pg/mL, Supplemental Figure 2).
Legumain is associated with markers related to cardiovascular risk in adult FH subjects
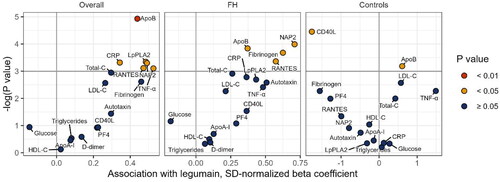
To further address the role of legumain in atherosclerosis, we performed a multiple regression analysis investigating the association between legumain and markers related to cardiovascular risk, including plasma lipids and markers of inflammation and platelet activation. In the total population, legumain showed a positive association with apoB, as well as markers of platelet activation and inflammation, i.e. CRP, LpPLA2, TNF, NAP2, and RANTES after adjusting for sex, age, BMI and statin use. In FH subjects alone, legumain showed a positive association with apoB, and fibrinogen, NAP2 and RANTES (β = 0.37, β = 0.63, β = 0.71 and β = 0.57, respectively, all p < 0.05, ).
Figure 3. Association between legumain and clinical and inflammatory/platelet activation markers in the total population of FH subjects and controls (right), only FH subjects (Middle) and controls (left). The volcano plots show regression coefficients and 95% CIs from multiple regression analyses adjusted for age, sex, BMI, and statin use (statin use not relevant for control group). Colors indicate p-value. Prior to modeling, to enable visualization in a volcano plot, we scaled each clinical and inflammatory/platelet activation marker to a standard normal distribution by subtracting the mean and dividing by its standard deviation. FH: familial hypercholesterolemia; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; apoB: apolipoprotein B; apoA-1: apolipoprotein A1; CRP: C reactive protein; Lp-PLA2: LDL-associated phospholipase A2; PF4: platelet factor 4; NAP2: neutrophil activating protein; TNF: tumor necrosis factor; SD: standard deviation.
Discussion
Legumain is known to be regulated in atherosclerotic disease [Citation4, Citation7–9], but its functional role is still not completely understood. Herein, we show that legumain levels is increased in adult FH subjects, but not in children and young adults with FH, compared to control subjects, driven by increased levels of legumain in adult FH subjects using statins. Furthermore, legumain was associated with several atherosclerotic markers such as apoB and other markers of inflammation and platelet activation related to increased cardiovascular risk.
Legumain was increased in adult FH subjects using statins, but not in adult non-statin users or children with FH. The positive association between legumain and age seen in the FH population, is in line with our previous findings in a large cohort of patients with acute coronary syndrome [Citation9]. However, in a population of osteoporotic women, an inverse relationship has been shown between serum legumain levels and age [Citation26]; illustrating that the association is not straight forward. Legumain is not previously studied in FH subjects, however levels of legumain is shown to be associated with vascular disease in other populations with increased cardiovascular risk, including type 2 diabetes, in which circulating legumain is associated with increased risk of peripheral artery disease [Citation27]. Herein, levels of legumain were positively correlated with apoB, as well as Lp-PLA2 and CRP in the total population, markers associated with increased risk of CVD [Citation28,Citation29]. In the FH population, the apoB association remained, and legumain was also associated with fibrinogen, which is suggested as a marker of thrombotic risk [Citation30]. However, further and larger studies are needed to examine if legumain could be a marker of future CVD in the FH population.
In FH subjects, higher plasma legumain was associated with statin use in age-adjusted analysis. In contrast, simvastatin has previously been shown to decrease maturation and activity of legumain in human myotubuli in vitro [Citation31], and treatment of monocytes with atorvastatin modestly reduce legumain mRNA expression [Citation32]. Further, and also in contrast to our present data, legumain levels was reduced after statin treatment in atherosclerotic rats [Citation33]. However, in vitro studies and animal models may not necessarily reflect the in vivo situation in humans and new studies are needed to address the statin – legumain axis.
Legumain has been shown to exhibit both pro- and anti-inflammatory effects, potentially related to the induction of a pro-resolving and anti-inflammatory phenotype in macrophages [Citation7,Citation8,Citation34]. The higher levels of legumain in statin users add to the complicated picture of legumain in CVD. In the total population, legumain levels were associated with CRP and TNF, and we have previously shown an association between CRP and legumain in a large population of ACS patients. However, herein, legumain was not associated with CRP in FH subjects. Legumain was, however, associated with markers of platelet activation, i.e. NAP2 and RANTES in the FH population. And we have previously shown that legumain is secreted from activated platelets, localized to platelets in the atherosclerotic plaque [Citation8] and associated with platelet count in a large population of ASC patients [Citation9]. Our findings in the present study support the notion that legumain is associated with activated platelets, also in individuals with FH, which may argue against a potential anti-inflammatory role of legumain in these subjects; but this needs further investigation.
This is the first study investigating plasma levels of legumain in an age-spanning, mostly genetically verified FH population. However, our study has some limitations. Some analyses herein had few individuals, and need to be verified in lager cohorts. Further, our study does not prove causality due to the cross-sectional design. Legumain should be measured in larger statin interventions in FH subjects to investigate the effect of statins on legumain further. Moreover in vitro and in vivo experiments should be performed to verify the association between legumain and the markers described herein. The association between legumain and several inflammatory markers does not necessarily mean that legumain mediate inflammatory effects in itself. It could represent a counteracting mechanisms against inflammation.
Our data show that legumain levels are increased in an adult FH population using statins, but not in FH children, and strongly associated with apoB, and platelet and inflammation markers. These data point to a coupled and complex role of legumain in atherosclerosis progression, cardiovascular risk and disorder.
Authors’ contributions
IG, IN, JJC, BH, KBH contributed to the conception and design of the study; JJC, LKLØ, TU analyzed the data; IG, IN, JJC, BH, KBH contributed in writing and revising the manuscript. All authors interpreted and discussed the data; read and approved the final manuscript.
Supplemental Material
Download PDF (208.5 KB)Acknowledgements
The authors would like to acknowledge Ellen Lund Sagen, Navida Akther Sheikh and Annika Michelsen for technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s). Dr. Bogsrud has received research grants and/or personal fees from Amgen and Sanofi, none of which are related to the content of this manuscript. Dr. Retterstøl has received research grants and/or personal fees from Akcea, Amgen, Mills, Sanofi, and Sunnovion, none of which are related to the content of this manuscript. Dr. Holven has received research grants and/or personal fees from Mills, Amgen and Sanofi, none of which are related to the content of this manuscript.
Additional information
Funding
References
- Dall E, Brandstetter H. Structure and function of legumain in health and disease. Biochimie. 2016;122:126–150. doi: 10.1016/j.biochi.2015.09.022.
- Libby P. The changing landscape of atherosclerosis. Nature. 2021;592(7855):524–533. doi: 10.1038/s41586-021-03392-8.
- Clerin V, Shih HH, Deng N, et al. Expression of the cysteine protease legumain in vascular lesions and functional implications in atherogenesis. Atherosclerosis. 2008;201(1):53–66. doi: 10.1016/j.atherosclerosis.2008.01.016.
- Lunde NN, Holm S, Dahl TB, et al. Increased levels of legumain in plasma and plaques from patients with carotid atherosclerosis. Atherosclerosis. 2017;257:216–223. doi: 10.1016/j.atherosclerosis.2016.11.026.
- Umei TC, Kishimoto Y, Aoyama M, et al. High plasma levels of legumain in patients with complex coronary lesions. J Atheroscler Thromb. 2020;27(7):711–717. doi: 10.5551/jat.52027.
- Yang H, He Y, Zou P, et al. Legumain is a predictor of all-cause mortality and potential therapeutic target in acute myocardial infarction. Cell Death Dis. 2020;11(11):1014. doi: 10.1038/s41419-020-03211-4.
- Ozawa N, Sato Y, Mori Y, et al. Legumain promotes atherosclerotic vascular remodeling. Int J Mol Sci. 2019;20(9):2195. doi: 10.3390/ijms20092195.
- Lunde NN, Gregersen I, Ueland T, et al. Legumain is upregulated in acute cardiovascular events and associated with improved outcome - potentially related to anti-inflammatory effects on macrophages. Atherosclerosis. 2020;296:74–82. doi: 10.1016/j.atherosclerosis.2019.12.008.
- Gregersen I, Michelsen AE, Lunde NN, et al. Legumain in acute coronary syndromes: a substudy of the PLATO (platelet inhibition and patient outcomes). Trial. JAMA. 2020;9(17):e016360.
- Goldstein JL, Brown MS. Familial hypercholesterolemia: pathogenesis of a receptor disease. Johns Hopkins Med J. 1978;143(1):8–16.
- Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the european atherosclerosis society. Eur Heart J. 2013;34(45):3478–390a. doi: 10.1093/eurheartj/eht273.
- Mundal L, Veierød MB, Halvorsen T, et al. Cardiovascular disease in patients with genotyped familial hypercholesterolemia in Norway during 1994-2009, a registry study. Eur J Prev Cardiol. 2016;23(18):1962–1969. doi: 10.1177/2047487316666371.
- Wiegman A, Gidding SS, Watts GF, et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015;36(36):2425–2437. doi: 10.1093/eurheartj/ehv157.
- Narverud I, Christensen JJ, Bakke SS, et al. Profiling of immune-related gene expression in children with familial hypercholesterolaemia. J Intern Med. 2020;287(3):310–321. doi: 10.1111/joim.13001.
- Narverud I, Retterstøl K, Iversen PO, et al. Markers of atherosclerotic development in children with familial hypercholesterolemia: a literature review. Atherosclerosis. 2014;235(2):299–309. doi: 10.1016/j.atherosclerosis.2014.05.917.
- Holven KB, Narverud I, Lindvig HW, et al. Subjects with familial hypercholesterolemia are characterized by an inflammatory phenotype despite long-term intensive cholesterol lowering treatment. Atherosclerosis. 2014;233(2):561–567. doi: 10.1016/j.atherosclerosis.2014.01.022.
- Bekkering S, Stiekema LCA, Bernelot Moens S, et al. Treatment with statins does not revert trained immunity in patients with familial hypercholesterolemia. Cell Metab. 2019;30(1):1–2. doi: 10.1016/j.cmet.2019.05.014.
- Øyri LKL, Hansson P, Bogsrud MP, et al. Delayed postprandial TAG peak after intake of SFA compared with PUFA in subjects with and without familial hypercholesterolaemia: a randomised controlled trial. Br J Nutr. 2018;119(10):1142–1150. doi: 10.1017/S0007114518000673.
- Narverud I, Bogsrud MP, Øyri LKL, et al. Lipoprotein(a) concentration is associated with plasma arachidonic acid in subjects with familial hypercholesterolaemia. Br J Nutr. 2019;122(7):790–799. doi: 10.1017/S0007114519001600.
- Narverud I, Halvorsen B, Nenseter MS, et al. Oxidized LDL level is related to gene expression of tumour necrosis factor super family members in children and young adults with familial hypercholesterolaemia. J Intern Med. 2013;273(1):69–78. doi: 10.1111/j.1365-2796.2012.02584.x.
- Torvik K, Narverud I, Ottestad I, et al. Dietary counseling is associated with an improved lipid profile in children with familial hypercholesterolemia. Atherosclerosis. 2016;252:21–27. doi: 10.1016/j.atherosclerosis.2016.07.913.
- Narverud I, Iversen PO, Aukrust P, et al. Maternal familial hypercholesterolaemia (FH) confers altered haemostatic profile in offspring with and without FH. Thromb Res. 2013;131(2):178–182. doi: 10.1016/j.thromres.2012.11.008.
- Austin MA, Hutter CM, Zimmern RL, et al. Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol. 2004;160(5):407–420. doi: 10.1093/aje/kwh236.
- R Core Team. R: a language and environment for statistical computing. R foundation for statistical computing., Vienna, Austria. 2022; Available from: URL https://www.R-project.org/.
- Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. JOSS. 2019. 2019;4(43):1686. doi: 10.21105/joss.01686.
- Jafari A, Qanie D, Andersen TL, et al. Legumain regulates differentiation fate of human bone marrow stromal cells and Is altered in postmenopausal osteoporosis. Stem Cell Reports. 2017;8(2):373–386. doi: 10.1016/j.stemcr.2017.01.003.
- Wei W, Chen S, Huang J, et al. Serum legumain Is associated with peripheral artery disease in patients with type 2 diabetes. J Diabetes Res. 2021;2021:5651469–5651467. doi: 10.1155/2021/5651469.
- Cojocaru M, Cojocaru IM, Silosi I. Lipoprotein-associated phospholipase A2 as a predictive biomarker of Sub-clinical inflammation in cardiovascular diseases. Maedica. 2010;5(1):51–55.
- Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4(3):337–345. doi: 10.1161/CIRCOUTCOMES.110.959247.
- Stec JJ, Silbershatz H, Tofler GH, et al. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the Framingham offspring population. Circulation. 2000;102(14):1634–1638. doi: 10.1161/01.cir.102.14.1634.
- Smith R, Solberg R, Jacobsen LL, et al. Simvastatin inhibits glucose metabolism and legumain activity in human myotubes. PLOS One. 2014;9(1):e85721-e85721. doi: 10.1371/journal.pone.0085721.
- Wang ZH, Liu XL, Zhong M, et al. Pleiotropic effects of atorvastatin on monocytes in atherosclerotic patients. J Clin Pharmacol. 2010;50(3):311–319. doi: 10.1177/0091270009340889.
- Fang Y, Duan C, Chen S, et al. Increased legumain/Smad3 expression in atherosclerotic plaque of rat thoracic aorta. Biomed Pharmacother. 2019;119:109353. doi: 10.1016/j.biopha.2019.109353.
- Jia D, Chen S, Bai P, et al. Cardiac resident macrophage-derived legumain improves cardiac repair by promoting clearance and degradation of apoptotic cardiomyocytes after myocardial infarction. Circulation. 2022;145(20):1542–1556. doi: 10.1161/CIRCULATIONAHA.121.057549.