Abstract
Objective. Acid antisecretory agents are used for the prophylaxis of cancer chemotherapy (CT)-induced gastrointestinal (GI) mucositis. Although these drugs seem to be clinically beneficial, data on their effects on the GI mucosal defense during CT treatment are scant. The objective of this study was to compare the effects of omeprazole, lansoprazole, and lafutidine on mucin, a major mucus component, during 5-fluorouracil (5-FU) treatment, as a CT regimen. Material and methods. Rats, weighing approximately 230 g, were divided into five groups. The control group was administered 0.5% carboxymethylcellulose orally once daily for 5 days. The second, third, fourth, and fifth groups were treated with 5-FU (50 mg/kg), 5-FU plus omeprazole (10 mg/kg), 5-FU plus lansoprazole (10 mg/kg), and 5-FU plus lafutidine (30 mg/kg) in the same way, respectively. The rats were sacrificed on the sixth day, and their stomachs and small intestines were removed. Using anti-mucin monoclonal antibodies, we compared the immunoreactivity in different areas of the rats’ GI tracts as well as the mucin content. Results. Body-weight decreased in rats in the 5-FU group. Lafutidine, but neither omeprazole nor lansoprazole, inhibited the 5-FU-induced weight loss. Mucosal damage and reduced mucin content in stomach and small intestine were observed in rats receiving 5-FU alone. In the stomach, all antisecretory drugs caused the protective effects against 5-FU-induced mucosal injury and alleviation of the decreased mucin accumulation. In the jejunum and ileum, lafutidine, but neither omeprazole nor lansoprazole, ameliorated the 5-FU-induced mucosal damage and decreased mucin accumulation. Conclusion. Lafutidine could offer the possibility of more effective prevention of CT-induced mucositis through the activation of GI mucus cells.
Introduction
In 2004, the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer (MASCC) and the International Society for Oral Oncology (ISOO) published the clinical practice guidelines for the prevention and treatment of cancer chemotherapy (CT)-induced gastrointestinal (GI) mucositis Citation[1], Citation[2]. The updated guidelines recommend either ranitidine or omeprazole for the prophylaxis of epigastric pain after treatment with cyclophosphamide, methotrexate, and 5-fluorouracil (5-FU) or treatment with 5-FU with or without folinic acid Citation[3]. The potential utility of omeprazole in the prevention of CT-induced gastroduodenal injury has been clearly demonstrated by the randomized trials in Europe Citation[4], Citation[5]. In Japan, anti-ulcer drugs such as lansoprazole and lafutidine are given prophylactically to patients during CT treatment in the absence of randomized controlled trials. Although these drugs seem to be clinically beneficial in reducing gastric acid secretion Citation[6], Citation[7], data on their effects on the GI mucosal defense mechanisms during CT treatment are scant.
Mucin, a major component of mucus, is considered to be one of the principal factors in the physiological defense of the GI mucosa. In our previous studies, we have reported quantitative and qualitative changes in GI mucin in experimental animals treated with various drugs including 5-FU, and demonstrated its importance in the GI mucosal barrier Citation[8–11]. We have also established several monoclonal antibodies (mAbs) that react with mucin synthesized and secreted from specific mucus-producing cells of the rat GI mucosa Citation[12], Citation[13].
The first objective of the present study was to compare the efficacy of omeprazole, lansoprazole, and lafutidine against 5-FU-induced rat GI mucosal injury. Secondly, we sought to evaluate their effects on mucin accumulation in different areas of the GI tract.
Material and methods
Animals and drug treatment
Seven-week-old male Wistar rats purchased from CLEA-Japan (Tokyo, Japan) were used in this study. These animals were housed in our animal care facility for 1–2 weeks while body-weight stabilized. The animals were housed in individual cages with raised mesh bottoms and in a temperature- and humidity-controlled environment with a 12-h dark–light cycle (1800–0600 h dark cycle). At the beginning of the experimental period, the animals were weighed after fasting for 24 h. During the below-mentioned treatment, rats were given food and water ad libitum. After 24 h of food deprivation following final administration of drugs, the animals were again weighed, sacrificed, and their stomachs, proximal and distal small intestines (corresponding to the jejunum and ileum, respectively) were removed. The present study was conducted according to the guidelines of the Animal Laboratory Center of Kitasato University School of Medicine.
5-FU was administered orally by gavage (50 mg/kg) once daily for 5 days. Anti-ulcer drugs used were omeprazole, lansoprazole (Sigma-Aldrich Corp., St. Louis, Mo., USA), and lafutidine (Taiho Pharm. Co. Ltd., Tokyo, Japan). All drugs were suspended in 0.5% carboxymethylcellulose (CMC) solution and prepared immediately before use. Each anti-ulcer drug (omeprazole 10 mg/kg; lansoprazole 10 mg/kg; lafutidine 30 mg/kg) was given orally 30 min before the respective 5-FU administration. Control animals received 0.5% CMC instead of 5-FU and anti-ulcer drugs.
Histological examination
Specimens of each tissue were immediately fixed for 3 h in freshly prepared Carnoy's solution following the method described elsewhere Citation[14]. After fixation, the materials were dehydrated through ethanol, cleared in xylene and embedded in paraffin. From these specimens, 3-µm paraffin sections were prepared for immunostaining with anti-mucin mAbs. Immunohistochemical staining was done using the avidin-biotin peroxidase method and an LSAB2 Kit (Dako, Carpinteria, Calif., USA). Briefly, endogenous peroxidase activity was blocked with 0.3% H2O2, and then the tissue was sequentially incubated with 10% (v/v) normal swine serum, the anti-mucin mAb (RGM21, RGM26, PGM34), biotinylated anti-mouse immunoglobulins, streptavidin horseradish peroxidase (HRP), and 0.02% 3,3-diaminobenzidine in 50 mM Tris-HCl, pH 7.6, containing 0.005% H2O2. The counterstaining was done with hematoxylin. The immunohistochemical reactivity of each of the mAbs was observed using an optical microscope. As previously described Citation[11], Citation[12], the immunohistochemical reactivities of RGM21 and RGM26 were located in the surface epithelial mucus cells of the rat corpus and antral mucosa, respectively. Regarding PGM34, it was recently shown that the epitope of this mAb was a specific sulfated oligosaccharide of the mucin molecule. This mAb stains all the goblet cells of rat small intestine Citation[13].
Biochemical examination
Specimens from each tissue were lyophilized and powdered for extraction of mucin by the previously described method Citation[8]. Each sample was suspended in 50 mM Tris-HCl, pH 7.2, containing 2% Triton X-100 (Triton-Tris buffer), homogenized and then incubated at 37°C for 1 h. After centrifugation at 8000 g for 30 min at 4°C, the supernatant was collected and an aliquot was applied to a Bio-Gel A-1.5 m column, and eluted with the Triton-Tris buffer. The void volume fraction (Fr-1) monitored by hexose measurement was collected as mucin. Hexose content in this fraction was measured by the phenol-sulfuric acid method using galactose as the standard. Mucin content (Fr-1 hexose value) was expressed as micrograms of hexose per tissue.
Statistical analysis
The difference in the mean values among the groups was analyzed by one-way ANOVA with Scheffe's test; a p-value of less than 0.05 was considered to indicate statistical significance.
Results
Body-weight change
The changes in the body-weight of the rats in each experimental group are recorded in . During a 6-day period, a body-weight gain was seen in rats of the control group, whereas a weight loss was found in the animals given 5-FU orally at a dose of 50 mg/kg once daily for 5 consecutive days. There were virtually no changes in the weights of rats administered 5-FU with either omeprazole or lansoprazole. There was a slight tendency toward an increase in the lafutidine plus 5-FU group, indicating that lafutidine inhibits 5-FU-induced body weight loss.
Table I. Weight changes in rats before and after the treatments.
Changes in immunoreactivity and mucin content of the gastric mucosa
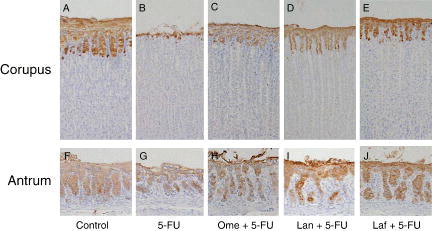
shows the morphological changes in corpus and antral mucosae after the treatments. In the control rat, gastric surface epithelial mucus cells were strongly stained with anti-mucin mAbs (RGM21 and RGM26, A and F, respectively). Treatment with 5-FU caused gastric mucosal damage restricted to the superficial epithelium Citation[11]. This was characterized by significant decreases in the RGM21- and RGM26-immunoreactivities in the corpus and antrum, respectively, when compared with the individual control (B, G). In contrast, significant observable damage could rarely be found in the gastric mucosae of the animals with the combined application of 5-FU and omeprazole, lansoprazole, or lafutidine (C–E, H–J).
Figure 1. Immunostaining of the gastric corpus (A–E) and antral (F–J) mucosae with anti-mucin monoclonal antibodies. Gastric tissues were obtained from control rats (A, F), rats treated with 5-fluorouracil (5-FU) alone (B, G), rats treated with omeprazole (Ome+ 5-FU (C, H), rats treated with lansoprazole (Lan) + 5-FU (D, I), and rats treated with lafutidine (Laf) + 5-FU (E, J). Notice that surface epithelial mucus cells in the corpus show positive staining with RGM21, and those in the antrum show positive staining with RGM26. Original magnification×25.
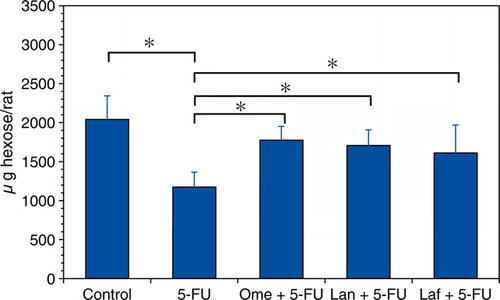
shows the effect of omeprazole, lansoprazole, or lafutidine treatment on the corpus mucin content in the 5-FU-induced gastric mucosal damage expressed as the Fr-1 hexose value. In the corpus of the rats treated with 5-FU, the mucin content was significantly decreased to 57.6% of the control. The 5-FU-induced mucin reduction was inhibited by the combination treatment of omeprazole, lansoprazole, or lafutidine.
Figure 2. Influence of acid antisecretory agents on the gastric corpus mucin accumulation in the 5-FU-induced gastric mucosal damage. Fr-1 hexose values corresponding to mucin content are expressed as micrograms of hexose per rat and represent means±SD. Abbreviations: 5-FU = 5-fluorouracil; Ome = omeprazole; Lan = lansoprazole; Laf = lafutidine. n=6–9 (each group); *p<0.05.
Changes in immunoreactivity and mucin content of the small-intestinal mucosa
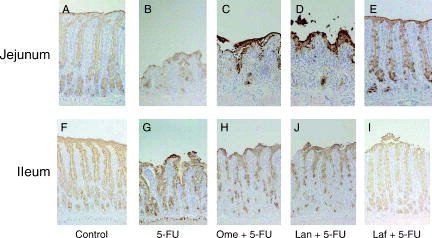
shows the morphological changes in the small-intestinal mucosa after treatments. In the control rats, immunohistochemical reactivity for PGM34 could be detected in the goblet cells, as well as the surface mucus gel layer, in the jejunum and ileum (A, F). As shown in B and G, 5-FU treatment caused a marked decrease in villus height and a remarkable reduction in the number of PGM34-positive goblet cells. In the animals treated with a combination of 5-FU and lafutidine, significant observable damage could rarely be found in the sections of the jejunal or ileal mucosa (E, J), whereas neither omeprazole (C, H) nor lansoprazole (D, I) was shown to prevent the 5-FU-induced intestinal mucosal damage.
Figure 3. Immunostaining of the rat jejunal (A–E) and ileal (F–J) mucosae with anti-mucin monoclonal antibody PGM34. Small-bowel tissues were obtained from control rats (A, F), rats treated with 5-fluorouracil (5-FU) alone (B, G), rats treated with omeprazole (Ome) + 5-FU (C, H), rats treated with lansoprazole (Lan) + 5-FU (D, I), and rats treated with lafutidine (Laf) + 5-FU (E, J). Notice that goblet cells in the jejunum and ileum show positive staining with PGM34. Original magnification×25.
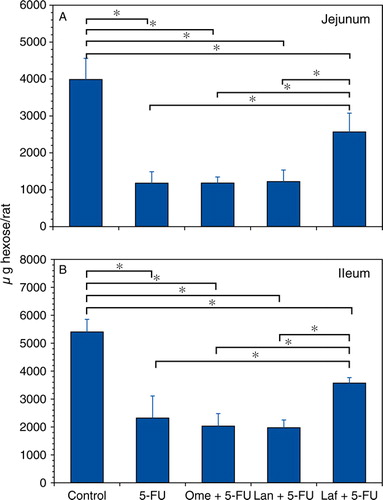
shows the comparison of the effects of the anti-ulcer drugs on the small-intestinal mucin contents in the 5-FU-induced mucosal damage. A decrease in the mucin content of the jejunum and ileum was observed after treatment with 5-FU (29.6% and 42.9% of the control mucin content, respectively). Lafutidine pretreatment significantly inhibited the 5-FU-induced mucin reduction in the jejunum and ileum mucin (75.8% and 66.1% of the control mucin, respectively), whereas no significant change could be detected in the mucin content in the small intestine by the 5-FU treatment with either omeprazole or lansoprazole.
Figure 4. Influence of acid antisecretory agents on the jejunal (A) and ileal (B) mucin accumulation in the 5-FU-induced small-bowel mucosal damage. Fr-1 hexose values corresponding to mucin content are expressed as micrograms of hexose per rat and represent means±SD. Abbreviations: 5-FU = 5-fluorouracil; Ome = omeprazole; Lan = lansoprazole; Laf = lafutidine. n=6–9 (each group); *p<0.05.
Discussion
Using the original anti-mucin mAbs RGM21 and RGM26, we demonstrated the protective effects of three anti-ulcer drugs, omeprazole, lansoprazole, and lafutidine, against 5-FU-induced gastric mucosal injury of the rat. From the randomized controlled studies, Sartori et al. Citation[4], Citation[5] documented that the strong and prolonged suppression of gastric acid secretion by omeprazole was effective in preventing and reducing CT-induced gastroduodenal mucosal injury, suggesting an important prophylactic role of the inhibition of acid secretion. Both lansoprazole and lafutidine possess a potent and long-lasting gastric antisecretory effect in humans Citation[6], Citation[7]. In the rat models, each drug at the dose used in this study has been shown sufficiently to decrease both the basal and the stimulated acid secretion Citation[15–18]. Our results strongly support the clinical studies showing that the acid-inhibitory drugs such as proton-pump inhibitors (PPIs) and H2-blockers are effective in reducing the frequency of gastric mucosal injury and upper GI symptoms caused by CT treatment Citation[4], Citation[5], Citation[19].
In the stomach, mucin is a key element in protecting the gastric epithelium against various irritants Citation[8], Citation[10], Citation[20]. Changes in gastric mucin content have been shown to occur in association with the oral administration of certain chemical agents including aspirin and 5-FU Citation[8], Citation[10], Citation[11]. In this study, a significant decrease in the mucin content of corpus mucosa was noted after oral administration of 5-FU at a dose of 50 mg/kg once daily for 5 consecutive days. Our most notable finding was that the mucin content did not decrease in animals given each of the anti-ulcer drugs used in this study. Accumulation of mucin in the gastric mucosa is closely related to mucosal protective capability Citation[8–10]. We have already reported that lafutidine, independent of its histamine H2-receptor antagonistic property, exerts a stimulant activity in the mucin accumulation and the protective effect against necrotizing-agent-induced gastric mucosal damage in the rat Citation[9]. Moreover, our recent study showed that lafutidine, given at clinical dosages, not only inhibits acid secretion but also strengthens the mucus barrier of the human gastric mucosa Citation[21]. The preventive effects of three anti-ulcer drugs against the 5-FU-induced gastric mucosal injury might also be associated with the non-acid inhibitory mechanism including mucosal defensive factors.
As we have previously demonstrated Citation[11], oral administration of 5-FU caused the remarkable decreases in both the number of mucus cells and the mucin content in the rat small intestine, especially in the jejunum. Here we report on a preventive effect of lafutidine on 5-FU-induced alteration in the rat intestinal mucus. Although the protective property of intestinal mucin has received limited attention compared with gastric mucin Citation[22], our results suggest that lafutidine may be extremely useful in reducing CT-induced GI mucosal damage. Recent studies have documented the prophylactic effect of this drug on indomethacin-induced small-intestinal ulcers in rats Citation[23], Citation[24]. Moreover, our study showed that lafutidine had an effect on body-weight loss in the animals treated with 5-FU. Although accurate measurement of either food intake or fecal output was not done in our investigation, lafutidine appeared to prevent the 5-FU-induced hypophagia and ingestion. These findings should be confirmed in future large randomized controlled clinical trials of lafutidine during CT treatment.
Anti-neoplastic drugs may cause severe damage to normal cells in organs with a high cellular turnover. Because gastric and intestinal epithelia have a high growth fraction Citation[25], the potential risk of CT-induced injury is high. Therefore, it is possible that reduction of CT-induced GI injury may be related to a reduction in the growth-inhibitory ability of anti-cancer agents. However, to our knowledge, there are no previous reports to show that the anti-ulcer drugs used in this study will lead to a decreased anti-tumor efficacy in cancer CT treatment. Dilloway & Lant Citation[26] reported that a non-imidazole-based H2-blocker did not cause any significant effect on 5-FU pharmacokinetics in the rat and monkey, suggesting that lafutidine could not reduce the 5-FU blood levels. In the preliminary study using a Yoshida sarcoma-bearing rat model, lafutidine had no influence on the anti-tumor activity of TS-1 (30 mg/kg p.o.), a prodrug of 5-FU (data not shown). Our previous report showed that lafutidine directly stimulated the mucin production in the rat mucus cells Citation[27], Citation[28]. Thus, the preventive effect of lafutidine against 5-FU-induced intestinal damage may be attributed to the increased production of mucin by the goblet cells that remained alive after 5-FU treatment.
Omeprazole and lansoprazole have been shown to ameliorate intestinal mucosal damage induced by indomethacin or ischemia-reperfusion in rats, via the action being dependent on their anti-inflammatory and anti-oxidative responses Citation[29–31]. In this study omeprazole and lansoprazole failed to alleviate the changes in both the morphological defects and mucin contents in intestinal mucosae of rats treated with 5-FU. We previously found that omeprazole had no effect on mucin biosynthesis in the rat gastric mucosa Citation[27]. These findings suggest that the omeprazole and lansoprazole utilized in this study could not promote the goblet mucus cell function. Although further studies are needed to clarify the detailed mechanism for 5-FU-induced intestinal injury, the activation of the goblet cells, if appropriately manipulated, might lead to more effective prevention of 5-FU-induced GI mucositis.
To summarize, we present two important research findings. First, oral administration of omeprazole, lansoprazole, and lafutidine caused the protective effects against 5-FU-induced gastric mucosal injury through alleviation of the decreased mucin accumulation in the rat stomach. Second, lafutidine ameliorated the intestinal mucosal damage and the decreased body-weight gain induced by 5-FU treatment, raising the possibility of a more effective prevention of CT-induced GI mucositis.
Conflict of interests
No funding has been received for this work, which was conducted as part of the normal laboratory activities at the Department of Internal Medicine, Kitasato University School of Medicine, Japan. There are no conflicts of interest to declare.
Acknowledgements
We express our sincere appreciation to A. Ohkuma and S. Sugawara for technical assistance. Part of this work was supported by the Parents’ Association Grant of Kitasato University School of Medicine, grants from the Shin-Caterpillar Mitsubishi Co., Grants-in-aid for Scientific Research from the Japanese Ministry of Education, Science and Culture, grants from the Alumni Association of Medical Sciences, Kitasato University, and grants from the Integrative Research Program of the Graduate School of Medical Sciences, Kitasato University. We also thank Robert E. Brandt for editing the manuscript.
References
- Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 2004; 100: 2026–46
- Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, et al. Perspectives on cancer therapy-induced mucosal injury. Cancer 2004; 100: 1995–2025
- Keefe DM, Schubert MM, Elting ST, Sonis ST, Epsteine JB, Raber-Durlacher JE, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 2007; 109: 820–31
- Sartori S, Trevisani L, Nielsen I, Tassinari D., Abbasciano V. Misoprostol and omeprazole in the prevention of chemotherapy-induced acute gastroduodenal mucosal injury. A randomized, placebo-controlled pilot study. Cancer 1996; 78: 1477–82
- Sartori S, Trevisani L, Nielsen I, Tassinari D, Panzini I, Abbasciano V. Randomized trial of omeprazole or ranitidine versus placebo in the prevention of chemotherapy-induced gastroduodenal injury. J Clin Oncol 2000; 18: 463–7
- Brunner G, Hell M, Hengels KJ, Hennig U, Fuchs W. Influence of lansoprazole on intragastric 24-hour pH, meal-stimulated gastric acid secretion, and concentrations of gastrointestinal hormones and enzymes in serum and gastric juice in healthy volunteers. Digestion 1995; 56: 137–44
- Shimatani T, Inoue M, Kuroiwa T, Horikawa Y, Mieno H, Nakamura M. Effect of omeprazole 10 mg on intragastric pH in three different CYP2C19 genotypes, compared with omeprazole 20 mg and lafutidine 20 mg, a new H2-receptor antagonist. Aliment Pharmacol Ther 2003; 18: 1149–57
- Azuumi Y, Ohara S, Ishihara K, Okabe H, Hotta K. Correlation of quantitative changes of gastric mucosal glycoproteins with aspirin-induced gastric damage in rats. Gut 1980; 21: 533–6
- Ichikawa T, Ishihara K, Komuro Y, Kojima Y, Saigenji K, Hotta K. Effects of the new histamine H2-receptor antagonist, FRG-8813, on gastric mucin in rats with or without acidified ethanol-induced gastric damage. Life Sci 1994; 54: PL159–64
- Kojima Y, Ishihara K, Ohara S, Saigenji K, Hotta K. Effects of the M1 muscarinic receptor antagonist pirenzepine on gastric mucus glycoprotein in rats with or without ethanol-induced gastric damage. Scand J Gastroenterol 1992; 27: 764–8
- Saegusa Y, Ichikawa T, Iwai T, Goso Y, Okayasu I, Ikezawa T, , et al. Changes in the mucus barrier of the rat during 5-fluorouracil-induced gastrointestinal mucositis. Scand J Gastroenterol 2007. In press.
- Ishihara K, Kurihara M, Goso Y, Ota H, Katsuyama T, Hotta K. Establishment of monoclonal antibodies against carbohydrate moiety of gastric mucins distributed in the different sites and layers of rat gastric mucosa. Glycoconj J 1996; 13: 857–64
- Tsubokawa D, Goso Y, Sawaguchi A, Kurihara M, Ichikawa T, Sato N, et al. A monoclonal antibody, PGM34, against 6-sulfated blood-group H type 2 antigen, on the carbohydrate moiety of mucin. FEBS J 2007; 274: 1833–48
- Ota H, Katsuyama T. Alternating laminated array of two types of mucin in the human gastric surface mucous layer. Histochem J 1992; 24: 86–92
- Blandizzi C, Natale G, Gherardi G, Lazzeri G, Marveggio C, Colucci R, et al. Acid-independent gastroprotective effects of lansoprazole in experimental mucosal injury. Dig Dis Sci 1999; 44: 2039–50
- Onodera S, Shibata M, Tanaka M, Inaba N, Yamaura T, Ohnishi H. Gastroprotective activity of FRG-8813, a novel histamine H2-receptor antagonist, in rats. Jpn J Pharmacol 1995; 68: 161–73
- Shibata M, Yamaura T, Inaba N, Onodera S, Chiba Y, Ohnishi H. Gastric antisecretory effect of FRG-8813, a new histamine H2-receptor antagonist, in rats and dogs. Eur J Pharmacol 1993; 235: 245–53
- Takeuchi K, Konaka A, Nishijima M, Kato S, Yasuhiro T. Effects of pantoprazole, a novel H + /K + -ATPase inhibitor, on duodenal ulcerogenic and healing responses in rats: a comparative study with omeprazole and lansoprazole. J Gastroenterol Hepatol 1999; 14: 251–7
- Mori K, Tominaga K, Yokoyama K, Suga Y, Kishiro I, Tsurui M. Efficacy of famotidine in patients with acute gastric mucosal injury after continuous infusion of cisplatin plus vindesine. J Cancer Res Clin Oncol 1995; 121: 367–70
- Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, et al. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 2004; 305: 1003–6
- Ichikawa T, Ota H, Sugiyama A, Maruta F, Ikezawa T, Hotta K, , et al Effects of a novel histamine H2-receptor antagonist, lafutidine, on the mucus barrier of human gastric mucosa. J Gastroenterol Hepatol 2007. In press.
- Specian RD, Oliver MG. Functional biology of intestinal goblet cells. Am J Physiol 1991; 260: 183–93
- Kato S, Tanaka A, Kunikata T, Umeda M, Takeuchi K. Protective effect of lafutidine against indomethacin-induced intestinal ulceration in rats: relation to capsaicin-sensitive sensory neurons. Digestion 2000; 61: 39–46
- Tanaka A, Mizoguchi H, Hase S, Miyazawa T, Takeuchi K. Intestinal protection by lafutidine, a histamine H(2)-receptor antagonist, against indomethacin-induced damage in rats: role of endogenous nitric oxide. Med Sci Monit 2001; 7: 869–77
- Mitchell EP. Gastrointestinal toxicity of chemotherapeutic agents. Semin Oncol 1992; 19: 566–79
- Dilloway MR, Lant AF. Effect of H2-receptor antagonists on the pharmacokinetics of 5-fluorouracil in the rat and monkey. Biopharm Drug Dispos 1991; 12: 17–28
- Ichikawa T, Ishihara K, Saigenji K, Hotta K. Effects of acid-inhibitory antiulcer drugs on mucin biosynthesis in the rat stomach. Eur J Pharmacol 1994; 251: 107–11
- Ichikawa T, Ishihara K, Shibata M, Yamaura T, Saigenji K, Hotta K. Stimulation of mucin biosynthesis in rat gastric mucosa by FRG-8813 and its structural analogs. Eur J Pharmacol 1996; 297: 87–92
- Ichikawa H, Yoshida N, Takagi T, Tomatsuri N, Katada K, Isozaki Y, et al. Lansoprazole ameliorates intestinal mucosal damage induced by ischemia-reperfusion in rats. World J Gastroenterol 2004; 10: 2814–7
- Kuroda M, Yoshida N, Ichikawa H, Takagi T, Okuda T, Naito Y, et al. Lansoprazole, a proton pump inhibitor, reduces the severity of indomethacin-induced rat enteritis. Int J Mol Med 2006; 17: 89–93
- Pozzoli C, Mezozii A, Grandi D, Solenghi E, Ossiprandi MC, Zullian C, et al. Protective effects of proton pump inhibitors against indomethacin-induced lesions in the rat small intestine. Naunyn Schmiedebergs Arch Pharmacol 2007; 374: 283–91