Abstract
*Objective: Patients with potentially premalignant neoplastic pancreatic cysts without high-risk stigmata usually enter a surveillance program. Data on outcomes of such surveillance programs are scarce. We aimed to evaluate the resection rate and malignancy rate during follow-up.
Material and methods: From our prospective database (2006–2015) of patients with pancreatic cysts, we analyzed patients with pancreatic cysts without high-risk stigmata with at least six months follow-up.
Results: In total, 146 patients were followed for a median of 29 months (IQR 13.5–50 months). In 124 patients (84.9%), no changes in clinical or imaging characteristics occurred during follow-up. Thirteen patients (8.9%) developed an indication for surgery after a median follow-up of 25 months (IQR 12–42 months). Two patients did not undergo surgery because of comorbidity, 11 patients (7.5%) underwent resection. Indications for surgery were symptoms (n = 2), development of a pancreatic mass (n = 1), a new nodule (n = 2), thickened cyst wall (n = 1), pancreatic duct dilation (n = 3), and/or suspicion of mucinous cystic neoplasm (MCN) (n = 3). Postoperative histology showed one pancreatic malignancy not originating from the cyst, three mixed type-intraductal papillary mucinous neoplasm (IPMN), one side branch-IPMN, two MCN, one neuroendocrine tumor, one serous cystadenoma, one inflammatory cyst, and one lymphangioma. The highest grade of cyst dysplasia was borderline dysplasia.
Conclusions: Most neoplastic pancreatic cysts without high-risk stigmata at initial presentation show no substantial change during 1–4-year follow-up. Only 7.5% of patients underwent surgery and less than 1% of patients developed pancreatic malignancy. This indicates that additional markers are needed to tailor treatment of pancreatic cysts.
Introduction
An increasing number of pancreatic cysts is being identified because of the increased use of cross-sectional imaging.[Citation1] Although many cystic lesions are benign, a subgroup of pancreatic cysts is malignant or premalignant and requires resection or follow-up. It is therefore of critical importance to correctly classify each pancreatic cystic lesion at detection.
Treatment of post-pancreatitis collections and pure benign pancreatic cysts, such as serous cystic neoplasms (SCN), is only necessary when patients remain symptomatic.[Citation2,Citation3] Intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN) have malignant potential. Two international guidelines have been widely used in recent years: the International Association of Pancreatology (IAP) guideline [Citation4] and the European guideline of the European Study Group on Cystic Tumours of the Pancreas.[Citation3] These guidelines recommend surgery for all fit patients with main duct (MD)- and mixed type (MT)-IPMN with a pancreatic duct dilated up to 7 mm (European guidelines) or 10 mm (IAP guidelines), all MCNs and solid pseudopapillary neoplasms.[Citation3,Citation4] Management of patients with side branch (SB)-IPMN also differs between guidelines. Recommendations vary from surveillance to resection based on presence of malignant features and/or symptoms (). Patients who have premalignant pancreatic cysts and are fit enough to undergo surgery if needed, usually enter a surveillance program. According to the IAP [Citation4] and European [Citation3] guidelines, these patients should undergo lifelong surveillance with magnetic resonance imaging (MRI) or endoscopic ultrasound (EUS) every 6–24 months, depending on cyst size. These recommendations are in clear contrast to the recent guideline of the American Gastroenterological Association (AGA guidelines) [Citation5] suggesting wider surveillance intervals of 12–24 months, irrespective of the size of the cyst, and discontinuation of follow-up after five years if the cyst remains stable. In the AGA guideline, surgery is only recommended for pancreatic cysts with a solid component and a dilated pancreatic duct and/or positive cytology (). All current guidelines are based on existing studies with limited evidence. Because of the increasing number of pancreatic cysts under surveillance, it is important to evaluate the necessity for the intensive follow-up schedules as suggested by the IAP and European guidelines. The need for follow-up depends on the risk of malignant transformation during follow-up. The overall risk of malignancy associated with pancreatic cysts during follow-up varies widely between studies.[Citation3,Citation4,Citation6–16] Most patients present with a cyst that does not warrant a surgical resection at presentation. In these patients with initially non-suspicious pancreatic cysts the risk of malignant transformation during follow-up is not well described. Therefore, the aim of this study was to evaluate the resection rate and the risk of pancreatic malignancy in patients with pancreatic cystic lesions without high-risk stigmata who entered a surveillance program.
Table 1. Current guidelines for surgery of intraductal papillary mucinous neoplasm (IPMN).
Methods
Patients
Since November 2006, all patients presenting at our tertiary care center with a potential neoplastic pancreatic cyst are registered in a prospective database. We retrospectively analyzed all patients up to April 2015, who had no pancreatic cancer or indication for surgery at presentation and with at least six months of follow-up. The following features were considered as an indication for surgery: (suspicion of) a MT/MD-IPMN, MCN, solid pseudopapillary neoplasm or a SB-IPMN ≥3 cm (before 2012),[Citation17] or presence of jaundice, a nodule or mass, abrupt change in caliber of the pancreatic duct with distal pancreatic atrophy or a symptomatic cyst.[Citation4] Patients were discussed at a weekly multidisciplinary hepato-pancreato-biliary meeting and generally treated according to the IAP guidelines.[Citation4,Citation17] As stated in the IAP guidelines, timing of follow-up depended on the size of the cyst and most often imaging was repeated annually. Between 2006 and 2012 patients underwent follow-up with EUS after their initial MRI, after 2012 patients were followed with MRI and EUS was only performed when there was suspicion of PD involvement or to aspirate cyst fluid to differentiate between mucinous and non-mucinous cyst. Our local Medical Ethical Committee agreed with the execution of this retrospective analysis of prospectively collected observational data and informed consent was waived.
Outcome parameters
Data on patient demographics, symptoms, imaging, cyst fluid analysis and/or operation characteristics were extracted from the database. Follow-up duration was recorded as time in months between initial cyst diagnosis (the first cross-sectional imaging on which the cyst was detected) and last available imaging data. Reasons to stop surveillance were confirmation or high suspicion of non-premalignant cyst, patient request, patient inoperability, patient death, and referral to a different hospital or loss to follow-up. Cyst size was recorded as the maximum diameter on imaging (CT, MRI, or EUS). If multiple cysts were present, the size of the largest cyst was used for the analyses. Cyst growth was defined as increase in size of ≥10 mm between the first and latest imaging.[Citation12] The pancreatic duct was considered dilated when the diameter was ≥5 mm.[Citation4] Possible signs of malignant progression were development of a nodule, dilated PD, thickened cyst wall, obstructive jaundice, cyst growth, and a solid mass in the pancreatic parenchyma or weight loss without a clear other cause. Patients were referred for surgery after discussion at a multidisciplinary pancreatic meeting, following the indications as stated in the IAP guidelines ().[Citation4] Histopathological outcomes on definitive diagnosis and level of dysplasia were collected for all patients who underwent resection. Level of dysplasia was recorded as the highest grade of dysplasia, subdivided into non-dysplastic, low-grade, borderline or high-grade dysplasia, or invasive carcinoma.[Citation18]
Statistical analysis
Descriptive statistics were computed for all study variables. Data were analyzed with IBM SPSS Statistics version 20.0 (IBM Corp., Armonk, NY). Categorical data were reported as frequency or percentage. Continuous data were reported as mean ± standard deviation (SD) or as median and interquartile range (IQR), depending on the distribution. Cyst characteristics (cyst size, presence of an increased PD diameter, presence of a nodule) at baseline and at the last follow-up were compared with Wilcoxon signed rank test and McNemar test, depending on the type of data. The significance level was set at p < 0.05.
Results
Patient characteristics
From the 339 patients in our database we excluded all patients with malignancy at presentation (n = 51) or with an indication for surgery after initial presentation (n = 78), and patients with less than six months follow-up (n = 64). We included 146 patients (mean age 61 years (SD 11.0), 58% female) with pancreatic cysts, who were followed for a median of 29 months (IQR 13.5–50 months) (). Cyst(s) were identified on CT scan (57%), ultrasound (25%), MRI scan (14%), or unknown imaging modality (4%). Imaging was performed because of (suspicion of) a non-pancreatic disease (66%), abdominal pain (12%), weight loss (1%), abdominal pain, and weight loss (1%), pancreatitis (8%), preventive investigation without referral from a medical doctor (3%), a palpable mass in the abdomen (1%), familiar pancreatic cancer screening program (1%) or unknown indication (5%). Most patients (82%) underwent EUS at least once, with fine needle aspiration (FNA) in 62% of patients.
Figure 1. Patient selection. MT/MD-IPMN: mixed type/main duct intraductal papillary mucinous neoplasm; MCN: mucinous cystic neoplasm; SB-IPMN: side branch-intraductal papillary mucinous neoplasm; solid pseudopap. Neoplasm: solid pseudopapillary neoplasm.
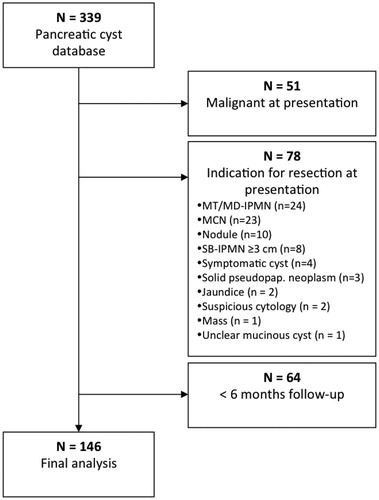
Baseline patient characteristics are summarized in . Most patients had a single cyst (60%) and the main location was the pancreatic head (37%). Overall, 19 patients (13%) with a suspected neoplastic pancreatic cyst had a history of acute (n = 14) or chronic (n = 5) pancreatitis. One patient (0.7%) with liver cirrhosis presented with jaundice that was not related to the pancreatic cyst. Ten patients (6.8%) had a, less than 10 mm, dilated PD at initial presentation. In two of these patients a benign cyst was suspected, in eight patients MT-IPMN was suspected. According to the IAP guidelines, surgery must be strongly considered in these patients, but close surveillance is allowed.[Citation4]
Table 2. Patient characteristics.
Changes during follow-up
The pancreatic cysts in most patients (n = 125, 85.6%) showed no changes in clinical or imaging characteristics during surveillance (median follow-up 29 months, IQR 13.0–49.5 months). Overall, there was no difference in median cyst size at baseline (2.4 cm (IQR 1.7–3.0 cm)) and last follow-up (2.4 cm (IQR 1.6–3.3 cm), p = 0.11). Also, the number of patients with an increased PD (6.8% vs. 7.5%, p = 0.63) or presence of a nodule (0% vs. 2.7%, p = 0.13) did not differ. One or more changes occurred during follow-up in 21 patients (14.4%).
Increase in cyst size was seen in 15 patients (10.3%). Median cyst growth was 1.4 cm (IQR 1.1–2.5 cm), after a median follow-up of 33 months (IQR 22.0–75.0). One patient had a presumed SB-IPMN that rapidly increased in size from 2.4 to 5.4 cm in one year. Four patients with cyst growth also developed a nodule, dilated PD, and/or new symptoms (pain). In the remaining 11 patients with cyst growth no other changes occurred.
Dilatation of the PD occurred in three patients (2.1%), of whom one had a dilated pancreatic duct at presentation that increased from 5 to 8 mm. Of the eight patients with suspected MT-IPMN at presentation, one also developed a new 8 mm nodule but without increased pancreatic duct dilatation and six (75%) showed no changes during surveillance. Overall, three patients (2.1%) developed a new nodule (8, 10, and 26 mm) and one patient (0.7%) developed a pancreatic mass.
Five patients (3.4%) developed new symptoms during follow-up: abdominal pain (n = 4) and/or weight loss (n = 2). In 3 patients these symptoms were thought to be related to the pancreatic cyst: weight loss combined with a thickened cyst wall, a growing SCN causing pain, and pain and weight loss in a patient who developed a pancreatic mass.
Surgical treatment and outcomes
According to the IAP guidelines, 133 patients (91.1%) did not develop an indication for surgery during a median follow-up of 30 months (IQR 13.5–50 months). At time of the analysis, 72/133 patients (54%) were still under surveillance. Follow-up was no longer considered indicated in 45 patients (34%, e.g., because of a benign cyst, comorbidity or death), 11 patients (10%) stopped surveillance at their own request and five patients (4%) were lost from follow-up.
In 13 patients (8.9%) an indication for surgery developed after a median follow-up of 25 months (IQR 12–42 months) (). Changes were detected during routine follow-up in most patients (8/13, 62%) (). Two patients were not operated on because of comorbidity and/or patient preference. In a patient with SB-IPMN, EUS had detected a possible nodule of 26 mm, the other patient had a SB-IPMN that rapidly increased in size from 2.4 to 5.4 cm in one year. The remaining 11 (7.5%) patients underwent surgery: pylorus preserving pancreatoduodenectomy (n = 2), central pancreatectomy (n = 2), distal pancreatectomy (n = 3), explorative laparotomy (n = 2), or cyst enucleation (n = 2). Characteristics and pathological results are shown in .
Table 3. Characteristics of patients who developed an indication for surgery.
In three of the 11 patients who underwent surgery a cyst without malignant potential was diagnosed on histology. In seven patients a premalignant cyst was found, with no dysplasia (n = 1), low grade dysplasia (n = 3) or borderline dysplasia (n = 3). None of the cysts harbored high grade dysplasia or invasive malignancy. One patient developed an unresectable pancreatic malignancy distant from the presumed SB-IPMN after 50 months of follow-up. This patient was known with Peutz–Jeghers syndrome and developed interval symptoms in between the annual follow-ups, after which an extra CT scan was made. During exploratory laparotomy an unresectable pancreatic malignancy was confirmed.
Discussion
Our study shows that the resection rate during 2.5-year follow-up in 146 patients with pancreatic cysts without high-risk stigmata at initial presentation entering a surveillance program is relatively low: 7.5% of patients underwent surgery. The risk of developing pancreatic malignancy during surveillance was also low. None of the cysts developed high grade dysplasia or invasive malignancy. Pancreatic malignancy developed in one patient (0.7%), distant from the presumed SB-IPMN.
Our study demonstrates that most pancreatic cysts that enter a surveillance program have an indolent behavior. The most common change we saw was cyst growth (10% of patients), although there was no significant difference in cyst size between baseline and follow-up. Since cyst growth is not considered a worrisome feature, the 11 patients who only showed increase in size without other changes were not referred for surgery.[Citation4] Nevertheless, surveillance was planned at a shorter time interval in most of these patients. We only operated on 4/15 patients in whom cyst growth was seen, because of a changed presumptive diagnosis (n = 1) or because of development of a nodule, increased dilatation of the PD, and/or symptoms (n = 3).
Our findings on changes during follow-up correspond with recent studies, which report resection rates between 3.0 and 17.3% and pancreatic malignancy developing in 0–3.9% of patients (median follow-up 23–61 months).[Citation8–16] Vege et al. used most of these recent data for the AGA guideline, suggesting that a less intensive follow-up schedule might be adequate for surveillance of premalignant pancreatic cysts.[Citation5] The older European and IAP guidelines based their suggestions mainly on surgical series with high rates of invasive carcinoma: 11–30% for SB-IPMN, 33–60% for MD/MT-IPMN, and 7–12% for MCN.[Citation3,Citation4,Citation19,Citation20] This might explain why the recommendations of these guidelines are clearly conflicting. Our findings are in line with the recommendations of the recent AGA guideline regarding their suggestion for a less intensive surveillance strategy. Nevertheless, we have our concerns on the advice to discontinue follow-up if a cyst has been stable for five years, regardless of the patients’ age or cyst size. Evidence of follow-up results supporting this recommendation is lacking and a previous studies on IPMNs showed development of malignancy in 4% of patients after 84 months of follow-up.[Citation21]
Although the natural history of pancreatic cysts is not completely clear, it is known that in other types of tissues dysplasia does not necessarily develop into invasive malignancy.[Citation22,Citation23] Since pancreatic surgery has a 2–3% mortality rate and a 30–40% morbidity rate, it seems inappropriate to resect all potentially premalignant cysts.[Citation24,Citation25] Patients with high grade dysplasia could possibly benefit most from surgery, by preventing progression to invasive malignancy. In our cohort the highest grade of dysplasia of the resected cysts was borderline dysplasia; no cysts with invasive malignancy or high grade dysplasia were found. Since surveillance and surgery are probably most likely to be indicated in patients with high grade dysplasia or cancer, non-surgical differentiation between low grade/borderline dysplasia and high grade dysplasia/invasive malignancy is important. Several studies evaluated possible markers for malignant progression of IPMNs. Although carcinoembryonic antigen (CEA) can be helpful to differentiate between mucinous and non-mucinous cysts, CEA does not correspond with the level of dysplasia.[Citation26] Outcomes on KRAS and GNAS mutation studies vary between increased, decreased and similar number of mutations between the different levels of dysplasia.[Citation27–30] The discrepancy between these studies might be explained by a difference in number of patients per IPMN subtype (intestinal, gastric, pancreatobiliary, and oncocytic) per study, and the difference in prevalence of KRAS and GNAS mutations between these subtypes.[Citation27,Citation29] Mutations in p16 and p53 are often found in IPMN with invasive carcinoma. However, there is no clear difference in mutation rate of these genes between IPMNs with borderline and high grade dysplasia.[Citation27,Citation30,Citation31] Therefore, although it may help to detect invasive malignancy, p16 and p53 seem to be inadequate markers for detection of high grade dysplasia. Currently, the most reliable markers for differentiation between IPMNs with low grade/borderline dysplasia and those with high grade malignancy/invasive carcinoma seem to be human telomerase reverse transcriptase (hTERT) (16–36% vs. 85–89%) and Sonic hedgehog (Shh) (36–69% vs. 80–91%).[Citation31,Citation32] However, these numbers are based on small studies and should be validated in larger cohorts. Furthermore, the possibility to detect these mutations in pancreatic cyst fluid obtained via EUS should be evaluated to determine the preoperative diagnostic value.
Since a definitive marker to detect malignant progression is not yet available, combinations of established and new clinical and molecular factors to help predict progression are currently evaluated. A recent study investigated if malignant behavior could be predicted based on the presence of a combination of clinical features (size >3 cm, growth rate >3 mm/year, duct dilation >10 mm, CEA >1000 ng/ml, atypical cells on cytology) and molecular criteria (availability of high-quality DNA, presence of KRAS, and/or GNAS mutations or loss of heterozygosity (LOH) of tumor suppressor genes).[Citation33] Patients were stratified into four diagnostic categories: benign, indolent, higher risk, and aggressive cysts. Compared to the surgical criteria as stated by the IAP guideline, this method had higher specificity (90.6% vs. 46.2%, p < 0.0001) and positive predictive value (57.9% vs. 20.8%, p < 0.0001). There was no significant difference in sensitivity (83.3% vs. 90.9%) and negative predictive value (97.2% vs. 97.0%).[Citation4,Citation33] These results are promising and should be validated in a prospective, and preferably randomized, study.
Our study has some limitations. First of all, this was a post hoc analysis at a single tertiary hospital of prospectively maintained database. Patients were treated according to the IAP guidelines but without using a fixed follow-up protocol. Our patient population consists of initially non-suspicious pancreatic cysts, outcomes of patients presenting with suspicious cysts were not evaluated in this study. Furthermore, our median follow-up was 29 months; therefore we still require more data on long-term follow-up beyond five years of surveillance. Only a relatively small number of patients underwent resection. The final histopathological diagnosis and the level of dysplasia are unknown for the 135 patients who did not undergo surgery. Nevertheless, this limitation is inevitable to answer our current research question, since many initially non-suspicious cysts do not require surgery and histopathology will therefore never be obtained. When these patients would be excluded, only the follow-up of high-risk cyst will be analyzed.
In conclusion, most pancreatic cysts that undergo surveillance do not show substantial changes during more than two-year follow-up. Only 7.5% of patients underwent resection and less than 1% of patients developed pancreatic malignancy. Of the patients who underwent surgery 20% was found to have a completely benign and non-premalignant lesion. Our data suggest that additional markers are urgently needed for an evidence based, tailored follow-up and treatment of (potentially) premalignant pancreatic cysts. Future guidelines should specifically attempt to include more data from non-surgical series since this might influence decisions making.
Disclosure statement
Jeanin van Hooft is consultant for Covidien and Boston Scientific and received grants from Cook Medical and Abbott, outside the submitted work. Paul Fockens is consultant for Covidien, Olympus and Fujifim. All other authors have nothing to declare. The authors alone are responsible for the content and writing of this article.
References
- Canto MI, Hruban RH. Managing pancreatic cysts: less is more? Gastroenterology. 2015;148:688–691.
- Farrell JJ, Fernández-del Castillo C. Pancreatic cystic neoplasms: management and unanswered questions. Gastroenterology. 2013;144:1303–1315.
- Del Chiaro M, Verbeke C, Salvia R, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45:703–711.
- Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197.
- Vege SS, Ziring B, Jain R, et al. American Gastroenterological Association Institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819–822.
- Gardner TB, Glass LM, Smith KD, et al. Pancreatic cyst prevalence and the risk of mucin-producing adenocarcinoma in US adults. Am J Gastroenterol. 2013;108:1546–1550.
- Scheiman JM, Hwang JH, Moayyedi P. American Gastroenterological Association Technical Review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824–848.e22.
- Munigala S, Gelrud A, Agarwal B. Risk of pancreatic cancer in patients with pancreatic cyst. Gastroint Endosc. 2015; Epub ahead of print; 1–6.
- Wu BU, Sampath K, Berberian CE, et al. Prediction of malignancy in cystic neoplasms of the pancreas: a Population-Based Cohort Study. Am J Gastroenterol. 2014;109:121–129.
- Lahav M, Maor Y, Avidan B, et al. Nonsurgical management of asymptomatic incidental pancreatic cysts. Clin Gastroenterol Hepatol. 2007;5:813–817.
- Tanno S, Nakano Y, Nishikawa T, et al. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: long-term follow-up results. Gut. 2008;57:339–343.
- Das A, Wells CD, Nguyen CC. Incidental cystic neoplasms of pancreas: what is the optimal interval of imaging surveillance? Am J Gastroenterol. 2008;103:1657–1662.
- Sawai Y, Yamao K, Bhatia V, et al. Development of pancreatic cancers during long-term follow-up of side-branch intraductal papillary mucinous neoplasms. Endoscopy. 2010;42:1077–1084.
- Malleo G, Marchegiani G, Borin A, et al. Observational study of the incidence of pancreatic and extrapancreatic malignancies during surveillance of patients with branch-duct intraductal papillary mucinous neoplasm. Ann Surg. 2015;261:984–990.
- Marchegiani G, Malleo G, D’Haese JG, et al. Association between pancreatic intraductal papillary mucinous neoplasms and extrapancreatic malignancies. Clin Gastroenterol Hepatol. 2015;13:1162–1169.
- Chung JW, Chung MJ, Park JY, et al. Clinicopathologic features and outcomes of pancreatic cysts during a 12-year period. Pancreas. 2013;42:230–238.
- Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32.
- Bosman F, Carneiro F, Hruban R, Theise N. WHO classification of tumors of digestive system. Geneva, Switzerland: WHO Press; 2010. p. 304e13.
- Reddy RP, Smyrk TC, Zapiach M, et al. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol. 2004;2:1026–1031.
- Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247:571–579.
- Khannoussi W, Vullierme MP, Rebours V, et al. The long term risk of malignancy in patients with branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatology. 2012;12:198–202.
- Östör A. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12:186–192.
- Wani S, Puli S, Shaheen N, et al. Esophageal adenocarcinoma in Barrett's esophagus after endoscopic ablative therapy: a meta-analysis and systematic review. Am J Gastroenterol. 2009;104:502–513.
- Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210.
- Newhook TE, LaPar DJ, Lindberg JM, et al. Morbidity and mortality of pancreaticoduodenectomy for benign and premalignant pancreatic neoplasms. J Gastrointest Surg. 2015;19:1072–1077.
- Ngamruengphong S, Bartel MJ, Raimondo M. Cyst carcinoembryonic antigen in differentiating pancreatic cysts: a meta-analysis. Dig Liver Dis. 2013;45:920–926.
- Amato E, Molin MD, Mafficini A, et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol. 2014;233:217–227.
- Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:1–19.
- Dal Molin M, Matthaei H, Wu J, et al. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol. 2013;20:3802–3808.
- Wada K. p16 and p53 gene alterations and accumulations in the malignant evolution of intraductal papillary-mucinous tumors of the pancreas. J Hepatobiliary Pancreat Surg. 2002;9:76–85.
- Paini M, Crippa S, Partelli S, et al. Molecular pathology of intraductal papillary mucinous neoplasms of the pancreas. World J Gastroenterol: WJG. 2014;20:10008–10023.
- Nissim S, Idos GE, Wu B. Genetic markers of malignant transformation in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Pancreas. 2012;41:1195–1205.
- Al-haddad M, Kowalski T, Siddiqui A, et al. Integrated molecular pathology accurately determines the malignant potential of pancreatic cysts. Endoscopy. 2015;47:136–142.
