Abstract
Objective: Iron isomaltoside (Monofer®) is a high-dose intravenous iron preparation with good tolerability and efficacy in inflammatory bowel disease (IBD) patients with iron deficiency anaemia (IDA). This trial evaluates the safety and efficacy, including effect on intact fibroblast growth factor 23 (iFGF23) of a high single dose and cumulative doses of iron isomaltoside in IBD patients with IDA.
Materials and methods: The trial was a prospective, open-label, multi-centre trial conducted in IBD patients with IDA. Based upon haemoglobin (Hb) levels at baseline and weight, the patients received 1500, 2000, 2500 or 3000 mg of iron isomaltoside infused in single doses up to 2000 mg. The outcome measurements included adverse drug reactions (ADRs) and changes in haematology and biochemistry parameters.
Results: Twenty-one IBD patients with IDA were enrolled, receiving 1500 (seven patients), 2000 (eight patients), 2500 mg (four patients) or 3000 (two patients) mg of iron. No serious ADRs were observed. Four patients experienced nine mild to moderate ADRs (hypersensitivity, pyrexia, vomiting, constipation, abdominal pain, dyspepsia (two events) and eye allergy (two events)). In total, 15 (75%) patients had an increase in Hb of ≥2.0 g/dL during the trial, with normalisation of ferritin. No changes in iFGF23 or clinically significant hypophosphataemia were found.
Conclusion: Rapid infusions of high-dose iron isomaltoside, administered as single doses up to 2000 mg and cumulative doses up to 3000 mg, were without safety concerns and were efficacious in increasing Hb levels in IBD patients. Iron isomaltoside did not induce profound phosphate wasting via increased iFGF23 levels.
Introduction
Iron deficiency anaemia (IDA) is a frequent complication of inflammatory bowel disease (IBD), with a prevalence of 36–76% [Citation1] and it is often not treated correctly despite it possibly impacting quality of life (QoL).[Citation2–6]
According to several international guidelines, intravenous (IV) iron reparation is preferred in the correction of moderate to severe active IBD and IDA because it is more effective, it is better tolerated and it improves QoL more rapidly than oral iron supplements.[Citation7,Citation8]
Anaemic patients rarely have deficits less than 1000 mg of iron, and high doses of IV iron, up to 3600 mg (administered as multiple smaller doses), have been administered in IBD patients.[Citation8] However, if the full iron replacement dose could be administered in fewer visits, then it would offer optimal convenience to patients and would also improve the overall pharmacoeconomics for both the patient and society.
Iron isomaltoside (Monofer®, Pharmacosmos A/S, Holbaek, Denmark) is high-dose IV iron for rapid infusion, and it can be administered in doses exceeding 1000 mg (maximum 20 mg/kg body weight), providing the opportunity for iron correction in one visit. Iron isomaltoside has previously been shown to be safe and well tolerated in patients with IBD,[Citation9,Citation10] chronic kidney disease,[Citation11–13] and chronic heart failure,[Citation14] and in patients undergoing elective or subacute coronary artery bypass graft procedures, valve replacement, or a combination thereof.[Citation15]
Whereas all parenteral irons can induce a small, transient decrease in serum phosphate (s-phosphate), probably related to increased erythropoiesis, some IV irons have been associated with phosphate wasting and potential clinically profound hypophosphataemia via intact fibroblast growth factor 23 (iFGF23)-induced urinary excretion of phosphate.[Citation16]
The present trial was a prospective, non-comparative, open-label, multi-centre, safety trial evaluating the safety of high single doses and cumulative doses of iron isomaltoside in IBD patients with IDA, including effect on iFGF23 and s-phosphate.
Methods
Trial design
A prospective, non-comparative, open-label, multi-centre, safety trial was conducted from July 2012 to November 2014 at six sites: three sites in Sweden, two sites in Denmark and one site in the Netherlands. For the patients in group A, the trial duration was approximately 10 weeks and they attended five visits (screening, baseline, week 1, week 4 and week 8). For the patients in treatment group B, the trial duration was approximately 18 weeks, and each patient attended eight visits (screening, baseline, week 1, week 4, week 8, week 9, week 12 and week 16).
The primary objective of the trial was to assess the type and incidence of adverse drug reactions (ADRs). The secondary objectives were changes in concentrations of haemoglobin (Hb), s-iron, s-ferritin, TSAT, reticulocytes, s-phosphate, iFGF23 and change in total QoL score (short-form health survey 12 Version 2 [SF-12 V2] and multidimensional fatigue inventory 20 [MFI-20] questionnaires).
Participants
Patients who were ≥18 years of age, diagnosed with IBD in remission or with disease activity and IDA (an Hb level of <12 g/dL for women and Hb <13 g/dL for men) were eligible to participate.
Patients were assessed as iron deficient if they had a C-reactive protein (CRP) greater than the upper limit of normal (ULN) and a ferritin level <100 μg/L, whereas patients with CRP ≤ ULN had to have a ferritin level <30 μg/L.[Citation7] The full inclusion and exclusion criteria are shown in Table S1.
Interventions
Based upon Hb level at baseline and weight, the patients were divided into two treatment groups: A and B. The treatment schedule is shown in .
Table 1. Intravenous dosing regimen of iron isomaltoside in IBD patients with iron deficiency anaemia.
The single dose of 1500 mg was administered at baseline in the 1500 mg group, whereas the cumulative doses of 2000 mg were administered in one or two doses (three patients received 2000 mg in one dose and five patients in two doses), and the cumulative doses of 2500 mg and 3000 mg were administered in two doses.
All of the administrations of iron isomaltoside (Monofer®, Pharmacosmos A/S, Holbaek, Denmark) were diluted in 100 mL of normal saline (0.9% sodium chloride) and were administered by infusion over approximately 15 min.
During the trial, any concomitant medications or treatments deemed necessary to provide adequate supportive care were allowed. Blood transfusions, erythropoiesis-stimulating agent treatment and any iron supplementation other than the investigational drug were prohibited.
Laboratory measurements of iFGF23 and phosphate
Intact FGF23 was measured using enzyme-linked immunoassay (Enzyme-linked Immunosorbent Assay Kit for FGF23, Cloud Clone Corp, Houston, TX) with an intra-assay specificity of <10% and inter-assay specificity of <12%. s-Phosphate was evaluated as a part of the standard safety laboratory assessment.
Sample size
There was no sample size calculation.
Statistical methods
In this safety trial, only one data set was constituted, i.e., the safety analysis set (N = 21). The safety analysis set comprised all patients who received at least one dose of the trial drug.
The primary safety data were tabulated. Descriptive statistics for continuous variables were presented with numbers of non-missing observations, means, standard deviations (SDs), medians and ranges when appropriate. Categorical data were presented by numbers of patients and percentages of observations in the various categories, where percentages were based on the patients for whom relevant measurements were obtained.
The changes in laboratory parameters over time were analysed and statistically evaluated with a repeated measures mixed model, with treatment and day as factors and baseline values as covariates. SAS® software, Version 9.1.3 (SAS Institute Inc., Cary, NC), was used for the analyses.
Ethical considerations
The trial protocol was approved by local ethics committees (Scientific Committees of Central Jutland no. 1-10-72-99-12) and competent authorities (EudraCT no. 2011-003121-94), and the trial was conducted in accordance with International Conference on Harmonization guidelines for good clinical practice and the Declaration of Helsinki of 1975, as revised in 1983.
The trial was registered on ClinicalTrials.gov (NCT01599702). The patients were informed by the investigator of the risks and benefits of the trial. Informed consent was obtained in writing prior to any trial-related activities.
Results
Patients
A total of 39 patients were screened in the period from 24 July 2012 to 4 September 2014, during which 21 patients were enrolled in group A (15 patients) and group B (six patients). The last patient’s final visit was on 27 November 2014. Of the 21 patients enrolled, 20 (95%) patients completed the trial, and one (5%) patient discontinued due to protocol non-compliance.
The patient demographics and baseline characteristics are summarised in . The median age of the trial population was 43 years old (range: 19–74 years). More women (76%) than men (24%) participated and the majority of the patients were Caucasian (91%).
Table 2. Baseline demographics by infusion regime of intravenous iron isomaltoside.
Exposure to intravenous iron
All of the patients received the mean cumulative dose of iron isomaltoside as planned.
Group A consisted of 15 patients (seven single dose 1500 mg; three single dose 2000 mg; five cumulative dose 2000 mg [1500 + 500]).
Group B consisted of six patients (four cumulative doses of 2500 mg [two patients: 2000 + 500; two patients: 1500 + 1000]; two patients with cumulative doses of 3000 mg [one patient: 2000 + 1000; one patient: 1500 + 1500]).
Safety
All of the safety analyses were conducted on the safety analysis set (N = 21).
No patients experienced serious ADRs. A total of four patients experienced nine mild to moderate ADRs (hypersensitivity, pyrexia, vomiting, constipation, abdominal pain, dyspepsia (two events) and eye allergy (two events)).
The hypersensitivity event occurred in a 60-year-old woman dosed with 1500 mg of iron isomaltoside. Two days after dosing, the patient developed an allergic reaction (hypersensitivity), from which she recovered fully two days later. The event was mild and non-serious. No additional information was provided about the event. All cases were reported as recovered. There were no fatal events during the trial.
Efficacy
Haemoglobin
Hb increased from a median (range) of 10.7 (9.3–12.9) g/dL at baseline to 13.4 (11.3–14.5) g/dL at week 8 in Group A (p < 0.0001, n= 14) and from 8.8 (6.8–10.5) g/dL to 12.4 (6.6–15.5) g/dL at week 16 in Group B (p < 0.0001, n= 6) ( and Table S2).
Figure 1. Changes from baseline in haemoglobin, s-ferritin, transferrin saturation and reticulocyte concentration over time in patients treated with 1500–2000 mg iron isomaltoside (group A) and 2500–3000 mg iron isomaltoside (group B). Least square means (95% CIs) are derived from a repeated measures mixed model with treatment and week as factors and baseline value as covariate. *p < 0.05, **p < 0.01, ***p < 0.001 (test for difference from baseline value).
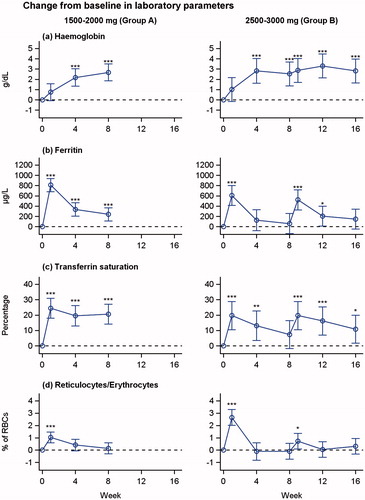
In total, 15 (75%) of 20 patients had an increase in Hb of ≥2.0 g/dL during the trial period.
s-Iron, s-ferritin, transferrin saturation and reticulocytes
There were increases in s-iron, s-ferritin and TSAT concentration from baseline ( and Table S2). There was a steady increase in concentration of reticulocytes from baseline to week 1, followed by a decrease throughout the trial.
Intact fibroblast growth factor 23 and phosphate levels
No overall changes in iFGF23 or clinically significant hypophosphataemia were reported (). The individual plots of s-phosphate and iFGF23 are shown in .
Figure 2. Change in iFGF23 and s-phosphate levels from baseline (blue line: change from baseline to week 8 in all patients after first dosing) and week 8 (red line: change from week 8 in patient dosed twice). Mean and 95% confidence interval of mean.
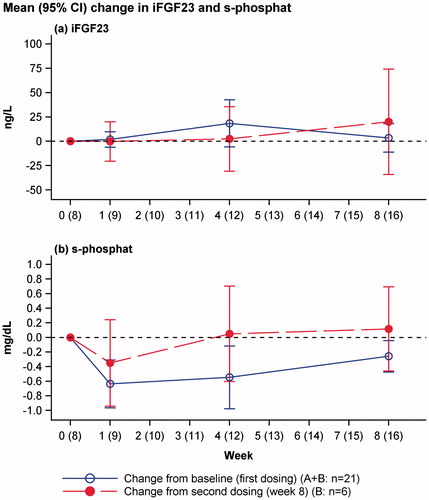
Figure 3. Individual plots of s-phosphate and iFGF23 levels. One patient was excluded due to having only baseline data.
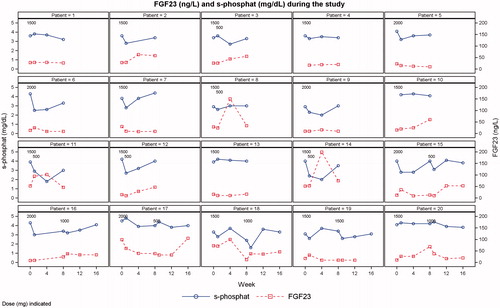
Two patients had transient events of moderate hypophosphataemia (s-phosphate level of 1-2 mg/dL) (patients 11 and 18 in ). Both patients had s-phosphate levels greater than 2 mg/dL at all other visits. No patients experienced severe hypophosphataemia (s-phosphate level <1 mg/dL).
Quality of life score
There were no significant changes in average QoL score (SF-12 version 2 and MFI-20) from baseline to week 8 in treatment groups A and B and from baseline to week 16 in treatment group B (Tables S3 and S4).
Discussion
In the present trial, we found no severe, serious or fatal ADRs. All ADRs were non-serious, mild or moderate and IBD patients with IDA received total cumulative doses of 1500–3000 mg of iron isomaltoside that were administered at 1–2 visits as single doses of 500–2000 mg; all recovered.
Anaemic IBD patients typically have been found to require iron supplementation at a mean dose 1500 mg of iron [Citation17] or even dose levels up to 3600 mg has been reported.[Citation18] Since most parenteral iron preparations have dose limitations of 1000 mg iron or less, the patients must undergo several administrations/visits to reach the full iron requirement.
Iron isomaltoside can be administered in doses up to 20 mg/kg body weight, enabling iron correction in one visit. By reducing the frequency of administrations, compliance and convenience for both patients and health care professionals are improved. In general, there are a number of cost savings associated with fewer IV iron infusions, including reduced time off of work for the patient, travel and transportation costs, equipment for administration, and time spent by the hospital staff.[Citation18,Citation19]
The use of a larger dose for administration and thereby fewer infusions might be an advantage because, according to the European Medicines Agency, there is a risk of an allergic reaction with every dose of IV iron that is administered, although the reactions are rare.[Citation20] By minimizing the number of administrations, the risk of a hypersensitivity reaction is kept to a minimum.
The primary goal of iron supplementation therapy for IDA is to increase Hb and s-ferritin to greater than the lower threshold reference range to ensure replenished iron stores and to increase QoL. The Hb improvement goal is to increase Hb >2 g/dL or to restore Hb to normal levels (women: Hb ≥12 g/dL; men: Hb ≥13 g/dL) in four weeks.[Citation8] In the present trial, there were increases in Hb and s-ferritin. In total, 15 (75%) of 20 patients had an increase in Hb of ≥2.0 g/dL during the trial period.
The reticulocyte count concentration increased from baseline to week 1 in both treatment groups, followed by a decrease. There was no change in average QoL scores, which might have been due to the small number of patients and the short duration of the trial.
Profound hypophosphataemia has previously been reported with other IV iron preparations,[Citation21] and clinical studies have suggested that prolonged and severely reduced s-phosphate in response to IV iron could be caused by an increase in iFGF23, leading to an increase in the excretion of phosphate in the urine.[Citation22,Citation23]
FGF23 is a hormone produced by the osteocytes, and it increases the rate of urinary excretion of phosphate and inhibits renal production of 1,25-dihydroxyvitamin D (1,25D).[Citation24] A proportion of FGF23 is normally cleaved before secretion, resulting in an intact, active hormone (iFGF23) and biologically inactive C-terminal (cFGF23) fragments.[Citation25] An animal study showed that iron deficiency induced FGF23 transcription in the osteocytes, but a counterbalancing increase in FGF23 cleavage ensured that the level of iFGF23 was maintained. Thus, the level of iFGF23 was unchanged, whereas cFGF23 was increased in iron-deficient animals.[Citation26]
Wolf and colleagues evaluated the associations of IDA with iFGF23 and cFGF23 levels in 55 women with heavy uterine bleeding treated with equivalent doses of 1000 mg of ferric carboxymaltose or iron dextran.[Citation16] Iron deficiency was associated with markedly increased levels of cFGF23, but the iFGF23 level was normal at baseline. After administration of iron, cFGF23 decreased by approximately 80% in both treatment groups, indicating that fgf transcription was normalised. However, in women treated with ferric carboxymaltose, iFGF23 increased with hypophosphataemia in 10 of 17 women, whereas women treated with iron dextran had normal levels of iFGF23 and no events of hypophosphataemia. The authors suggested that the carbohydrate moieties in certain iron preparations, such as ferric carboxymaltose, might temporarily increase iFGF23 levels by inhibiting the degradation of FGF23 in osteocytes, leading to hypophosphataemia.[Citation16] Similar findings were reported in a comparison of iron sucrose and ferric carboxymaltose, in which ferric carboxymaltose induced an increase in FGF23 and urinary phosphate wasting; this was not the case for iron sucrose.[Citation27] Hence, profound urinary phosphate wasting does not seem to be a class effect of IV irons per se, but it might be a mechanism related to specific compounds that alter iFGF23 release, which is not seen with iron sucrose [Citation27] or iron dextran.[Citation16]
In the present trial, two patients had transient events of hypophosphataemia (s-phosphate level <2 mg/dL) of 1.6 and 1.8 mg/dL, and no significant changes in iFGF23 were observed, even when iron isomaltoside was administered in cumulative doses of up to 3000 mg and single infusions of up to 2000 mg. Although this trial was based upon a small number of patients, it suggested that iron isomaltoside does not induce hypophosphataemia through an FGF23-mediated pathway despite the high iron doses administered that exceeded the dosing applied in the trial by Wolf and colleagues.
In conclusion, single high doses of iron isomaltoside, up to 2000 mg within 15 min, were well tolerated and considered safe in patients with IDA secondary to IBD, and these doses did not induce profound changes in phosphate or iFGF23 levels after infusion. The safety profile of the high dosing regimen was in agreement with current knowledge about iron isomaltoside safety in lower dosing regimens.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding information
This work was funded by Pharmacosmos A/S.
IBD-02_supplementary_tables_20160525_EMN.docx
Download MS Word (92.6 KB)Acknowledgements
The authors gratefully acknowledge all of the investigators and trial personnel for their contributions to the trial, as well as Jens-Kristian Slott Jensen, Slott Stat, who provided statistical support, and Eva-Maria Damsgaard Nielsen, who provided medical writing assistance.
Disclosure statement
Eva-Maria Damsgaard Nielsen is employed by Pharmacosmos A/S. Lars L. Thomsen is employed by Pharmacosmos A/S and the investigators/institutions received a fee per patient. Bent A. Jacobsen is a member of the advisory board of Tillotts Pharma. Jens Frederik Dahlerup, Janneke van der Woude, Lars-Åke Bark and Stefan Lindgren have no further conflicts of interest.
References
- Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7:599–610.
- Stein J, Dignass AU. Management of iron deficiency anemia in inflammatory bowel disease – a practical approach. Ann Gastroenterol. 2013;26:104–113.
- Gisbert JP, Bermejo F, Pajares R, et al. Oral and intravenous iron treatment in inflammatory bowel disease: hematological response and quality of life improvement. Inflamm Bowel Dis. 2009;15:1485–1491.
- Gomollon F, Gisbert JP. Anemia and inflammatory bowel diseases. World J Gastroenterol. 2009;15:4659–4665.
- Wells CW, Lewis S, Barton JR, et al. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis. 2006;12:123–130.
- Gasche C, Lomer MC, Cavill I, et al. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53:1190–1197.
- Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9:211–222.
- Gasche C, Berstad A, Befrits R, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545–1553.
- Reinisch W, Staun M, Tandon RK, et al. A randomized, open-label, non-inferiority study of intravenous iron isomaltoside 1,000 (Monofer) compared with oral iron for treatment of anemia in IBD (PROCEED). Am J Gastroenterol. 2013;108:1877–1888.
- Reinisch W, Altorjay I, Zsigmond F, et al. A 1-year trial of repeated high-dose intravenous iron isomaltoside 1000 to maintain stable hemoglobin levels in inflammatory bowel disease. Scand J Gastroenterol. 2015;50:1226–1233.
- Wikstrom B, Bhandari S, Barany P, et al. Iron isomaltoside 1000: a new intravenous iron for treating iron deficiency in chronic kidney disease. J Nephrol. 2011;24:589–596.
- Bhandari S, Kalra PA, Kothari J, et al. A randomized, open-label trial of iron isomaltoside 1000 (Monofer®) compared with iron sucrose (Venofer®) as maintenance therapy in haemodialysis patients. Nephrol Dial Transplant. 2015;30:1577–1589.
- Kalra PA, Bhandari S, Saxena S, et al. A randomized trial of iron isomaltoside 1000 versus oral iron in non-dialysis-dependent chronic kidney disease patients with anaemia. Nephrol Dial Transplant. 2015;31:646–655.
- Hildebrandt PR, Bruun NE, Nielsen OW, et al. Effects of administration of iron isomaltoside 1000 in patients with chronic heart failure. A pilot study. Transfus Altern Transfus Med. 2010;11:131–137.
- Johansson PI, Rasmussen AS, Thomsen LL. Intravenous iron isomaltoside 1000 (Monofer®) reduces postoperative anaemia in preoperatively non-anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: a randomized double-blind placebo-controlled clinical trial (the PROTECT trial). Vox Sang. 2015;109:257–266.
- Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28:1793–1803.
- Koch TA, Myers J, Goodnough LT. Intravenous iron therapy in patients with iron deficiency anemia: dosing considerations. Anemia. 2015. doi:10.1155/2015/763576.
- Gozzard D. When is high-dose intravenous iron repletion needed? Assessing new treatment options. Drug Des Devel Ther. 2011;5:51–60.
- Bager P, Dahlerup JF. The health care cost of intravenous iron treatment in IBD patients depends on the economic evaluation perspective. J Crohns Colitis. 2010;4:427–430.
- European Medicine Agency. New recommendations to manage risk of allergic reactions with intravenous iron-containing medicines, EMA/377372/2013; 2013.
- Blazevic A, Hunze J, Boots JM. Severe hypophosphataemia after intravenous iron administration. Neth J Med. 2014;72:49–53.
- Schouten BJ, Hunt PJ, Livesey JH, et al. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab. 2009;94:2332–2337.
- Shimizu Y, Tada Y, Yamauchi M, et al. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone. 2009;45:814–816.
- Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435.
- Shimada T, Muto T, Urakawa I, et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143:3179–3182.
- Farrow EG, Yu X, Summers LJ, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci USA. 2011;108:E1146–E1155.
- Bregman DB, Tokars ML. inventors. Patent application publication. Patent number US 2014/0162974 A1, application number US 13/821,072, 12; Jun 2014.
