Abstract
Objective: Patients with irritable bowel syndrome (IBS) in Asia show distinctive differences from those in the western world. The gastrointestinal endocrine cells appear to play an important role in the pathophysiology of IBS. The present study aimed at studying the density of chromogranin A (CgA) cells in the large intestine of Thai and Norwegian IBS patients.
Methods: Thirty Thai IBS patients and 20 control subjects, and 47 Norwegian IBS patients and 20 control subjects were included. A standard colonoscopy was performed in both the patients and controls, and biopsy samples were taken from the colon and the rectum. The biopsy samples were stained with hematoxylin–eosin and immunostained for CgA. The density of CgA cells was determined by computerized image analysis.
Results: In the colon and rectum, the CgA cell densities were far higher in both IBS and healthy Thai subjects than in Norwegians. The colonic CgA cell density was lower in Norwegian IBS patients than in controls, but did not differ between Thai IBS patients and controls. In the rectum, the CgA cell densities in both Thai and Norwegian patients did not differ from those of controls.
Conclusions: The higher densities of CgA cells in Thai subjects than Norwegians may be explained by a higher exposure to infections at childhood and the development of a broad immune tolerance, by differences in the intestinal microbiota, and/or differing diet habits. The normal CgA cell density in Thai IBS patients in contrast to that of Norwegians may be due to differences in pathophysiology.
Introduction
Irritable bowel syndrome (IBS) is a common chronic disorder of unknown etiology. However, several factors seem to play a role in its pathophysiology, including genetics, diet, intestinal bacterial flora, low-grade inflammation, and abnormalities of the gastrointestinal endocrine cells [Citation1]. Patients with IBS in Asia show distinctive differences from those in the Western world (USA and Europe), including the prevalence, gender, clinical presentation, and probably the pathophysiology.
The prevalence of IBS in Asia is reportedly 5–9% [Citation2–13], while in Western countries it is 10–20% [Citation14–25]. Furthermore, no female predominance was found in Asian IBS patients [Citation2], in contrast to in Western IBS patients [Citation22–25]. The clinical presentation of IBS differs between Asian and Western patients. Abdominal bloating is a more common complaint than pain in Asians, and this abdominal pain is localized to the upper abdomen rather than in the lower abdomen like it is in Western patients. Moreover, alteration in bowel habits is much less prominent in Asian IBS patients than in Western patients [Citation2–13].
Chromogranin A (CgA) is a common marker for endocrine cells [Citation26,Citation27], and hence the CgA cell density in the gastrointestinal tract reflects the total density of endocrine cells [Citation28]. The CgA cell density is reduced in the small and large intestines of Western IBS patients with the exception of the rectum [Citation29–32]. The gastrointestinal endocrine cells seem to play a pivotal role in the pathophysiology of IBS [Citation33–36]. The present study aimed to measure the density of CgA cells in the large intestine of Asian IBS patients and to compare it with that in European (Caucasian) IBS patients.
Material and methods
Patients and controls
Thirty patients who fulfilled the Rome III criteria for the diagnosis of IBS [Citation37,Citation38] were recruited at King Chulalongkorn Memorial Hospital, Bangkok, Thailand. These patients comprised 23 females and seven males with a mean age of 56 years (range, 45–70 years). The subtypes of IBS in these patients were distributed as follows: 11 with diarrhea as the predominant symptom (IBS-D), six with mixed diarrhea and constipation (IBS-M), and 13 with constipation as the predominant symptom (IBS-C). The control group comprised 20 subjects (14 females and six males; mean age 50 years, range 36–69 years) who had undergone a colonoscopy for colorectal cancer screening.
Forty-seven patients with IBS according to the Rome III criteria were recruited at Stord Hospital, Stord, Norway. These patients comprised 40 females and seven males with a mean age of 49.2 years (range 29–70 years): 18 with IBS-D, 14 with IBS-M, and 15 with IBS-C. The control group comprised 20 subjects (15 females and 5 males; mean age 50.3 years, range 38–67 years) who underwent colonoscopy because of gastrointestinal bleeding, where the source of bleeding was identified as hemorrhoids (n = 9) or angiodysplasia (n = 1), and health worries resulting from a relative being diagnosed with colon carcinoma (n = 10).
The onset of IBS symptoms was not associated with a gastrointestinal infection in the Thai and Norwegian patients. Patients taking antibiotics, immunosuppressants, or non-steroid anti-inflammatory agents within one month prior to study enrollment were excluded.
Colonoscopy, histopathology, and immunohistochemistry
Both the patients and the controls underwent a standard colonoscopy: four biopsy samples were taken from the sigmoid colon at about 30 cm from the anus, and another four biopsy samples were taken from the rectum at about 15 cm from the anus. The biopsy samples were fixed overnight in 4% buffered paraformaldehyde, embedded in paraffin, and cut into 5-μm sections. The sections were stained with hematoxylin–eosin, and immunostained using the ultraView Universal DAB Detection Kit (version 1.02.0018, Venata Medical Systems, Basel, Switzerland) and the BenchMark Ultra IHC/ISH staining module (Venata Medical Systems, Basel, Switzerland). The sections were incubated with the primary antibody for 35 min at 37 °C. The primary antibody used was monoclonal mouse antibody raised against the N-terminal of purified CgA (code no. M869, Dako, Glostrup, Denmark). This antibody was used at a dilution of 1:1000.
Morphometry
Morphometric measurements were performed using Olympus cellSens imaging software (version 1.7) on a computer linked to an Olympus microscope (type BX 43) with an Olympus camera (DP 26). The morphometric method used has been validated previously [Citation39]. The number of CgA immunoreactive cells and the area of the epithelial cells were measured. The numbers of CgA cells in each field were counted manually by pointing and clicking the computer mouse. The area of epithelial cells was determined by manually drawing an enclosed region using the computer mouse. A ×40 objective was used, for which each frame (field) on the monitor represented a tissue area of 0.035 mm2. The density of CgA cells was expressed as the number of endocrine cells per square millimeter of epithelium. Measurements were performed in 10 randomly chosen microscopic fields. The measurements were made by the same person (M.E-S.), who was blind to the identities of the slides.
Statistical analysis
The Mann–Whitney nonparametric test was used to assess differences in the age distribution and the CgA cell density between Thai and Norwegian subjects. The gender difference between patients and controls was tested by Fisher’s exact test. Differences between controls, all IBS patients (IBS-total), and IBS-D, IBS-M, and IBS-C patients were assessed using the Kruskal–Wallis nonparametric test with Dunn’s posttest. The data are presented as mean ± SEM values (), and differences with p < .05 were considered to be statistically significant.
Table 1. Density of CgA cells in controls and IBS patients.
The study was performed in accordance with the declaration of Helsinki and was approved by the Regional Committee for Medical and Health Research Ethics West, Bergen, Norway, and the local Committee of Medical and Health Research Ethics at King Chulalongkorn Memorial Hospital, Bangkok, Thailand. All subjects gave informed oral and written consent to participate.
Results
Gender and age characteristics of patients and controls
The age distribution did not differ significantly between Thai IBS patients and controls (p = .2), between Norwegian IBS patients and controls (p = .7) between Thai and Norwegian controls (p = .3), or between Thai and Norwegian IBS patients (p = .5). The gender distribution did not differ between Thai IBS patients and controls (p = .7), between Norwegian IBS patients and controls (p = .3), between Thai and Norwegian controls (p = 1.0), or between Thai and Norwegian IBS patients (p = .4).
Colonoscopy, histopathology, and immunohistochemistry
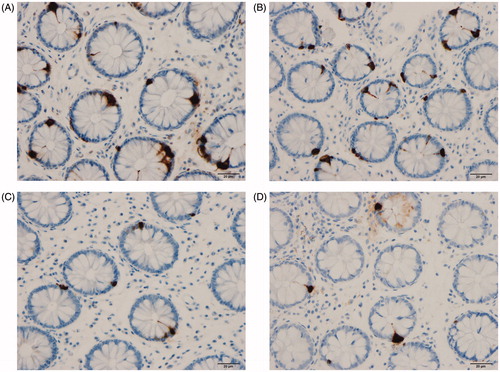
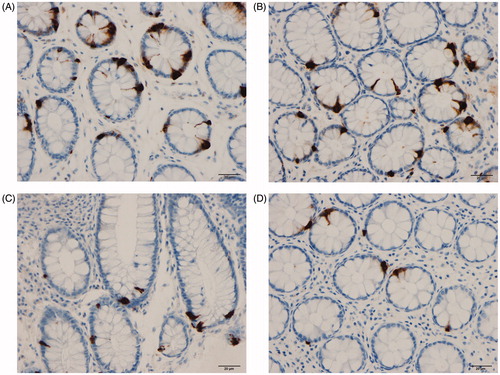
The colon and rectum of both the patients and the control subjects were macroscopically normal. The findings of histopathological examinations of the colon and rectum were normal in both the patients and the controls. CgA cells were found in the colon and rectum of patients and controls, mostly in the crypts. These cells were flask-shaped, sometimes with a basal cytoplasmic process.
Morphometry
Comparison between Thai and Norwegian subjects
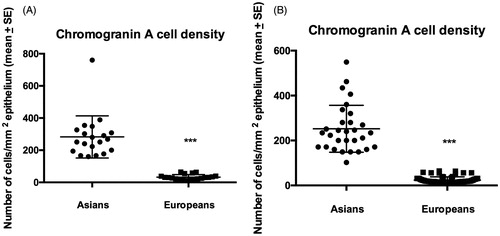
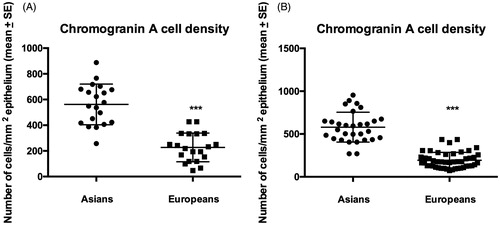
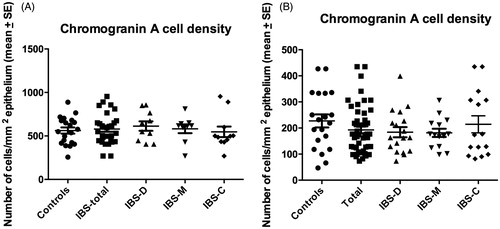
The CgA cell densities in the colon were significantly higher in Thai controls and IBS patients than in the corresponding Norwegians (p < .0001 for both) (). Similar to the colon, the CgA cell densities were significantly higher in Thai controls and IBS patients than in the corresponding Norwegians (p < .0001 for both) ().
CgA cell density in the colon
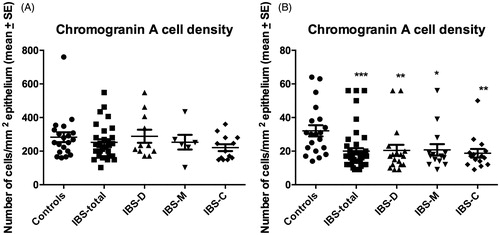
The densities of CgA cells in the colon of Thai and Norwegian controls and IBS patients are given in . The Kruskal–Wallis nonparametric test revealed no significant difference between Thai controls, IBS-total, IBS-D, IBS-M, and IBS-C (p = .5). Dunn’s posttest showed no significant differences between controls and IBS-total, IBS-D, IBS-M, and IBS-C (p = .3, p = .9, p = .7, and p = .07, respectively) ( and ). In the Norwegian subjects, there was a significant difference between controls, IBS-total, and the IBS subgroups (p = .007). The density of CgA cells was lower in IBS-total, IBS-D, IBS-M, and IBS-C than in controls (p < .0001, p = .002, p = .005, and p = .0007, respectively) ( and ).
CgA cell density in the rectum
The densities of CgA cells in the rectum of Thai and Norwegian controls and patients IBS are summarized in . The density of CgA did not differ between Thai controls, total-IBS, and the IBS subgroups (p = .9). The densities of CgA in IBS-total, IBS-D, IBS-M, and IBS-C did not differ from that in controls (p = .9, p = .6, p = .8, and p = .7, respectively) ( and ). Similarly the Kruskal–Wallis nonparametric test found no significant difference in the CgA cell density between Norwegian controls, IBS-total, and the IBS subgroups (p = .7). There were no significant differences in the density of CgA cells between controls and IBS-total, IBS-D, IBS-M, and IBS-C (p = .2, p = .2, p = .2, and p = .7, respectively).
Discussion
The present study found that the density of CgA cells was much higher in Thai subjects than in Norwegian subjects in both the colon and rectum. This could be explained by a high exposure of Thai subjects to infections at childhood and the development of a broad immune tolerance, by a difference in intestinal microbiota, and/or differences in diet habits.
CgA belongs to the family of granins [Citation40], and is localized to the stomach and the small and large intestines [Citation41,Citation42]. Although the density of CgA cells during exposure to infections is not known, other similar process such as intestinal inflammation affects the density of CgA. Thus, the number of CgA cells is reportedly increased in ulcerative colitis, Crohn’s disease, and lymphocytic colitis as well as in animal models of inflammatory bowel disease [Citation43–48]. CgA-derived peptides reduce the release of interleukin (IL)-16 and IL-5, hence reducing the number of lymphocytes at inflammatory sites and the proinflammatory action of lymphocytes and monocytes [Citation49,Citation50]. Thus, CgA is considered to act as an anti-inflammatory. Interactions between the neuroendocrine peptides/amines of the gastrointestinal tract and the immune system have been suggested to play an important role in the inflammatory/infectious process in the gastrointestinal tract [Citation51–56]. It is therefore likely that the increase in CgA density in Thai subjects is a response defense mechanism against inflammation/infection that developed because of the high exposure to infectious agents and the development of a broad immune tolerance.
A change in diet or intestinal microbiota has been found also to induce changes in intestinal endocrine cells [Citation57,Citation58]. Thus, difference in intestinal microbiota resulted from high exposure to infectious organisms, and prebiotics intake and/or differences in diet intake between Thai and Norwegian subjects could also explain the high density of CgA cells in Thai subjects.
While the CgA cell density was reduced in the colon of Norwegian IBS patients, the density in the rectum was not affected. These observations are in line with published data [Citation29,Citation32]. Similar to the Norwegian IBS patients, the density of CgA cells was unchanged in the rectum of Thai IBS patients; however, in contrast to the Norwegian IBS patients, the colonic CgA cell density in Thai patients was also unaffected. The abnormalities in the intestinal endocrine cells in Western IBS patients are (a) a reduced density of the total endocrine cells and (b) changes in the proportions of different endocrine cell types [Citation59]. It has been postulated that a reduced intestinal cell density is caused by abnormal stem cell clonogeny and differentiation progeny toward endocrine cells, and that the change in the proportions of endocrine cell types is caused by switching on and off of the production of certain hormones [Citation59]. The absence of abnormality in the colonic endocrine cell density in Thai IBS patients suggests that the pathophysiology of IBS in these patients is different from that in the Norwegians
Several hypotheses have been put forward to explain the differences between Asians and Western patients with IBS, including a hygiene hypothesis model and differences in the intestinal microbiota and diet [Citation60–62]. In the hygiene model, Gwee speculated that the high exposure to infectious agents at childhood in Asia results in the intestine being colonized with favorable bacteria and the development of broad immune tolerance [Citation61]. Ghoshal et al. [Citation60] emphasized the role of differences in intestinal bacteria flora that might result from a high exposure to infectious organisms and to differences in diet, particularly the prebiotics intake. Gonlachanvit proposed that the typical high-carbohydrate, high-fiber, low-fat, and low-meat-protein Asian diet could explain the differences in the manifestation of IBS between Asia and Western countries [Citation2]. He suggested further that since rice and vegetables are the main source of carbohydrates and fibers in Asia and that rice is completely absorbed in the small intestine, this would have an important impact on IBS symptoms. However, there are other factors that could contribute to the low prevalence of IBS in Asia. For example, it could be due to a cultural difference in healthcare-seeking behavior, since it has been reported that Hispanics are less likely than non-Hispanics to seek healthcare for bowel complaints and Hispanics are also more likely to self-medicate with folk remedies to maintain good bowel function [Citation43]. Other possible factors are education and social status as well as a low rate of IBS diagnosis by Western-trained medical practitioners [Citation63].
Disclosure statement
The authors have no conflicts of interest to declare.
Additional information
Funding
References
- El-Salhy M. Recent developments in the pathophysiology of irritable bowel syndrome. World J Gastroenterol. 2015;21:7621–7636.
- Gwee KA, Lu CL, Ghoshal UC. Epidemiology of irritable bowel syndrome in Asia: something old, something new, something borrowed. J Gastroenterol Hepatol. 2009;24:1601–1607.
- Hoseini-Asl MK, Amra B. Prevalence of irritable bowel syndrome in Shahrekord, Iran. Indian J Gastroenterol. 2003;22:215–216.
- Shah SS, Bhatia SJ, Mistry FP. Epidemiology of dyspepsia in the general population in Mumbai. Indian J Gastroenterol. 2001;20:103–106.
- Ghoshal UC, Abraham P, Bhatt C, et al. Epidemiological and clinical profile of irritable bowel syndrome in India: report of the Indian Society of Gastroenterology Task Force. Indian J Gastroenterol. 2008;27:22–28.
- Han SH, Lee OY, Bae SC, et al. Prevalence of irritable bowel syndrome in Korea: population-based survey using the Rome II criteria. J Gastroenterol Hepatol. 2006;21:1687–1692.
- Kwan AC, Hu WH, Chan YK, et al. Prevalence of irritable bowel syndrome in Hong Kong. J Gastroenterol Hepatol. 2002;17:1180–1186.
- Husain N, Chaudhry IB, Jafri F, et al. A population-based study of irritable bowel syndrome in a non-Western population. Neurogastroenterol Motil. 2008;20:1022–1029.
- Xiong LS, Chen MH, Chen HX, et al. A population-based epidemiologic study of irritable bowel syndrome in South China: stratified randomized study by cluster sampling. Aliment Pharmacol Ther. 2004;19:1217–1224.
- Chang FY, Lu CL, Chen TS. The current prevalence of irritable bowel syndrome in Asia. J Neurogastroenterol Motil. 2010;16:389–400.
- Karaman N, Turkay C, Yonem O. Irritable bowel syndrome prevalence in city center of Sivas. Turk J Gastroenterol. 2003;14:128–131.
- Celebi S, Acik Y, Deveci SE, et al. Epidemiological features of irritable bowel syndrome in a Turkish urban society. J Gastroenterol Hepatol. 2004;19:738–743.
- Masud MA, Hasan M, Khan AK. Irritable bowel syndrome in a rural community in Bangladesh: prevalence, symptoms pattern, and health care seeking behavior. Am J Gastroenterology. 2001;96:1547–1552.
- Quigley EM, Locke GR, Mueller-Lissner S, et al. Prevalence and management of abdominal cramping and pain: a multinational survey. Aliment Pharmacol Ther. 2006;24:411–419.
- Vandvik PO, Lydersen S, Farup PG. Prevalence, comorbidity and impact of irritable bowel syndrome in Norway. Scand J Gastroenterol. 2006;41:650–656.
- Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Digest Dis Sci. 1993;38:1569–1580.
- Saito YA, Schoenfeld P, Locke GR. 3rd. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–1915.
- Boekema PJ, van Dam van Isselt EF, Bots ML, et al. Functional bowel symptoms in a general Dutch population and associations with common stimulants. Neth J Med. 2001;59:23–30.
- Agreus L, Svardsudd K, Nyren O, et al. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995;109:671–680.
- Hillila MT, Farkkila MA. Prevalence of irritable bowel syndrome according to different diagnostic criteria in a non-selected adult population. Aliment Pharmacol Ther. 2004;20:339–345.
- Kay L, Jorgensen T, Jensen KH. The epidemiology of irritable bowel syndrome in a random population: prevalence, incidence, natural history and risk factors. J Intern Med. 1994;236:23–30.
- Thompson WG, Irvine EJ, Pare P, et al. Functional gastrointestinal disorders in Canada: first population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig Dis Sci. 2002;47:225–235.
- Boyce PM, Koloski NA, Talley NJ. Irritable bowel syndrome according to varying diagnostic criteria: are the new Rome II criteria unnecessarily restrictive for research and practice? Am J Gastroenterology. 2000;95:3176–3183.
- Mearin F, Badia X, Balboa A, et al. Irritable bowel syndrome prevalence varies enormously depending on the employed diagnostic criteria: comparison of Rome II versus previous criteria in a general population. Scand J Gastroenterol 2001;36:1155–1161.
- El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T, Irritable bowel syndrome: diagnosis, pathogenesis and treatment options. New York: Nova Science Publishers, Inc.; 2012.
- Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149.
- Wiedenmann B, Huttner WB. Synaptophysin and chromogranins/secretogranins – widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol. 1989;58:95–121.
- Deftos LJ. Chromogranin A: its role in endocrine function and as an endocrine and neuroendocrine tumor marker. Endocr Rev. 1991;12:181–187.
- El-Salhy M, Lomholt-Beck B, Hausken T. Chromogranin A as a possible tool in the diagnosis of irritable bowel syndrome. Scand J Gastroenterol. 2010;45:1435–1439.
- El-Salhy M, Gilja OH, Gundersen D, et al. Duodenal chromogranin a cell density as a biomarker for the diagnosis of irritable bowel syndrome. Gastroenterol Res Pract. 2014;2014:462856.
- El-Salhy M, Wendelbo I, Gundersen D. Reduced chromogranin A cell density in the ileum of patients with irritable bowel syndrome. Mol Med Rep. 2013;7:1241–1244.
- El-Salhy M, Mazzawi T, Gundersen D, et al. Chromogranin A cell density in the rectum of patients with irritable bowel syndrome. Mol Med Rep. 2012;6:1223–1225.
- El-Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012;18:5151–5163.
- El-Salhy M, Hatlebakk JG, Gilja OH, et al. Irritable bowel syndrome: recent developments in diagnosis, pathophysiology, and treatment. Exp Rev Gastroenterol Hepatol. 2014;8:435–443.
- El-Salhy M, Gundersen D, Gilja OH, et al. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. 2014;20:384–400.
- El-Salhy M, Seim I, Chopin L, et al. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed). 2012;4:2783–2800.
- Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491.
- Spiller R, Aziz Q, Creed F, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770–1798.
- El-Salhy M, Sandstrom O, Nasstrom E, et al. Application of computer image analysis in endocrine cell quantification. Histochem J. 1997;29:249–256.
- Eiden LE. Is chromogranin a prohormone?. Nature. 1987;325:301
- Buffa R, Mare P, Gini A, et al. Chromogranins A and B and secretogranin II in hormonally identified endocrine cells of the gut and the pancreas. Basic Appl Histochem. 1988;32:471–484.
- Buffa R, Capella C, Fontana P, et al. Types of endocrine cells in the human colon and rectum. Cell Tissue Res. 1978;192:227–240.
- El-Salhy M, Danielsson A, Stenling R, et al. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–419.
- Bishop AE, Pietroletti R, Taat CW, et al. Increased populations of endocrine cells in Crohn’s ileitis. Virchows Arch A Pathol Anat Histopathol. 1987;410:391–396.
- El-Salhy M, Mazzawi T, Umezawa K, Gilja OH. Enteroendocrine cells, stem cells and differentiation progenitors in rats with TNBS-induced colitis. Int J Mol Med 2016;38:1743–1751.
- El-Salhy M, Umezawa K. Treatment with novel AP-1 and NF-kappaB inhibitors restores the colonic endocrine cells to normal levels in rats with DSS-induced colitis. Int J Mol Med 2016;37:556–564.
- El-Salhy M, Gundersen D, Hatlebakk JG, et al. Chromogranin a cell density as a diagnostic marker for lymphocytic colitis. Dig Dis Sci. 2012;57:3154–3159.
- El-Salhy M, Lomholt-Beck B, Gundersen TD. High chromogranin A cell density in the colon of patients with lymphocytic colitis. Mol Med Rep. 2011;4:603–605.
- Egger M, Beer AG, Theurl M, et al. Monocyte migration: a novel effect and signaling pathways of catestatin. Eur J Pharmacol. 2008;598:104–111.
- Feistritzer C, Mosheimer BA, Colleselli D, et al. Effects of the neuropeptide secretoneurin on natural killer cell migration and cytokine release. Regul Pept. 2005;126:195–201.
- Khan WI, Ghia JE. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol. 2010;161:19–27.
- Margolis KG, Gershon MD. Neuropeptides and inflammatory bowel disease. Curr Opin Gastroenterol. 2009;25:503–511.
- Bampton PA, Dinning PG. High resolution colonic – amanometry–what have we learnt? – a review of the literature 2012. Curr Gastroenterol Rep. 2013;15:328.
- Ameri P, Ferone D. Diffuse endocrine system, neuroendocrine tumors and immunity: what’s new?. Neuroendocrinology. 2012;95:267–276.
- Farzi A, Reichmann F, Holzer P. The homeostatic role of neuropeptide Y in immune function and its impact on mood and behaviour. Acta Physiol (Oxf). 2015;213:603–627.
- El-Salhy M, Hausken T. The role of the neuropeptide Y (NPY) family in the pathophysiology of inflammatory bowel disease (IBD). Neuropeptides. 2016;55:137–144.
- Mazzawi T, El-Sahy M, Lied G, Gilja OH, Hatlebakk JG, Hausken T. Effect of fecal transplantation on the symptoms and duodenal endocrine cells in patients with irritable bowel syndrome. UEG J. 2016;4:677.
- Mazzawi T, Hausken T, Gundersen D, et al. Normalization of large intestinal endocrine cells following dietary management in patients with irritable bowel syndrome. Exp Biol Med. in press.
- El-sahy M, Hausken T, Gilja OH, et al. The possible role of gastrointestinal endocrine cells in the pathophysiology of irritable bowel syndrome. Exp Rev Gastroenterol Hepatol. 2016;11:139–148.
- Ghoshal UC, Shukla R, Ghoshal U, et al. The gut microbiota and irritable bowel syndrome: friend or foe? Int J Inflam. 2012;2012:151085.
- Gwee KA. Irritable bowel syndrome in developing countries – a disorder of civilization or colonization?. Neurogastroenterol Motil. 2005;17:317–324.
- Gonlachanvit S. Are rice and spicy diet good for functional gastrointestinal disorders? J Neurogastroenterol Motil. 2010;16:131–138.
- Zuckerman MJ, Guerra LG, Drossman DA, et al. Health-care-seeking behaviors related to bowel complaints. Hispanics versus non-Hispanic whites. Dig Dis Sci. 1996;41:77–82.