Abstract
Objective: Approximately 20–30% of all colorectal cancer (CRC) cases may have a familial contribution. The family history of CRC can be prominent (e.g., hereditary colorectal cancer (HCRC)) or more moderate (e.g., familial colorectal cancer (FCRC)). For family members at risk, colonoscopic surveillance is a well-established method to prevent both HCRC and FCRC, although the evidence for the exact procedures of the surveillance is limited. Surveillance can come at a high price if individuals are frequently examined, as this may result in unnecessary colonoscopies in relation to actual risk for CRC. This study analyses the cost-effectiveness of a surveillance programme implemented in the Northern Sweden Health Care Region.
Methods: The study includes 259 individuals prospectively recorded in the colonoscopic surveillance programme registry at the Cancer Prevention Clinic, Umeå University Hospital. We performed a cost–utility analysis with a contrafactual design: we compared observed costs and loss of quality-adjusted life years (QALYs) due to CRC with the surveillance programme to an expected outcome without surveillance. The main measure was the incremental cost-effectiveness ratio (ICER) between surveillance and non-surveillance. Scenario analysis was used to explore uncertainty.
Results: The ICER between surveillance and non-surveillance in the base model was 3596€/QALY. The ICER varied from −4620€ in the best-case scenario to 33,779€ in the worst-case scenario.
Conclusion: Colonoscopic surveillance is a very cost-effective method to prevent HCRC and FCRC compared to current thresholds for cost-effectiveness and other cancer preventive interventions.
Introduction
Approximately 20–30% of patients with colorectal cancer (CRC) have close relatives who have had the same disease [Citation1–3]. However, only 3–5% of CRC patients have a dominant inheritance, such as a known monogenetic hereditary colorectal cancer (HCRC) syndrome. Most family histories of CRC are less dominant and often referred to as familial colorectal cancer (FCRC) [Citation1–3]. The lifetime risk for CRC can be up to 80% in HCRC, compared to 10–30% in FCRC [Citation1–3].
The most common HCRC is Lynch syndrome, which is caused by inherited alterations of DNA mismatch repair (MMR) genes [Citation1]. In families with HCRC, genetic testing can often detect mutations in MMR genes and identify carriers at risk for CRC. In FCRC, no genetic tests are available as the aetiology most likely is multifactorial and not monogenic [Citation1–4]. Consequently, in a family with FCRC, the risk for CRC is entirely based on family history, and carriers at risk for cancer cannot be distinguished from non-carriers.
Colonoscopic surveillance is a well-established method [Citation1,Citation2,Citation5,Citation6] for HCRC and FCRC prevention, although the scientific evidence on how to design an optimal surveillance programme is limited. There are different opinions and guidelines on how extensive a familial clustering of CRC must be to justify surveillance, at what age to start surveillance, and the length of examination intervals [Citation5–10].
If individuals in a surveillance programme have unnecessary or too frequent colonoscopies in relation to their true risk for CRC, the preventive effect may come at a high price. The disadvantages of unnecessary or too frequent colonoscopies could be harmful side effects or a high cost compared to the health benefits. Consequently, cost-effectiveness studies of colonoscopic surveillance should be an important method to both analyse and optimise surveillance.
There are a few cost-effectiveness studies published on colonoscopic surveillance for individuals with inherent risk for CRC [Citation11–15]. These studies are all based on simulation models and not on the outcome of a real cohort undergoing surveillance. We recently performed a prospective study that found a high cancer preventive effect of a colonoscopic surveillance programme for HCRC and FCRC in the Northern Sweden Health Care Region [Citation9]. This study investigates whether this surveillance programme was a cost-effective method for preventing CRC.
Methods
Population and study design
The study population was individuals recorded in the colonoscopic surveillance registry at the Cancer Prevention Clinic, Umeå University Hospital, between 1 January 1995 and 1 September 2012. All study subjects were prospectively recorded and defined as HCRC or FCRC ().
Table 1. Classification of family history for definition of hereditary colorectal cancer (HCRC) and familial colorectal cancer (FCRC).
Individuals at risk for HCRC were examined every second year from the age of 25 and for FCRC every fifth year from 5 to 10 years before the age of the first diagnosed case in the family. Genetic counselling and testing was done at the Cancer Prevention Clinic, and the colonoscopies were performed at local hospitals. From the registry, we gathered data on sex, age at first colonoscopy, number of examinations, mismatch repair status and colonoscopic findings or complications during the study period.
As in the previous study with the same cohort, we had no control group (i.e., no group without surveillance). Thus, we calculated the expected number of CRC cases anticipated within the cohort, if it had not been surveilled. Details of the CRC estimations are given in the earlier study [Citation9]. First, we performed a cohort analysis based on age, sex and calendar year matched CRC incidence rates for the general population in Sweden [Citation9]. Then, as proposed by Dowe-Edwin et al. [Citation7], we applied three sets of relative risk estimations (lowest, best and highest) for individuals with inherited risk for CRC. To compare observed vs. expected cases of CRC, two-tailed standard incidence ratios (SIR) with 95% confidence intervals were calculated according to Byar’s formula. The best-set estimation of expected cases of CRC without surveillance was used to construct a base model for the cost-effectiveness analysis.
Model for cost-effectiveness analysis and outcome measures
The cost-effectiveness of surveillance during the study period was examined by a cost–utility analysis with a contrafactual design. This means ‘real’ costs and effects of surveillance were compared with an estimate of expected costs and effects without any surveillance in the same cohort ().
Figure 1. Model for cost–utility analysis comparing colonoscopic surveillance vs. non-surveillance and calculations of incremental cost effectiveness ratio (ICER).
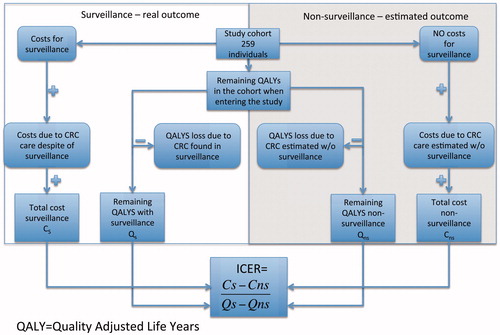
The costs for the competing alternatives – surveillance vs. non-surveillance – were calculated in a societal perspective, a viewpoint that includes costs both for the health care sector and all other sectors in society. The effects or gains of surveillance were calculated by transforming the difference in CRC cases (effect) into remaining quality-adjusted life years (QALYs) for the two competing alternatives. The summary measure of cost-effectiveness of this study is the incremental cost-effectiveness ratio (ICER) between surveillance and non-surveillance ().
ICER enables comparisons between different health care interventions and assessment of the cost-effectiveness in relation to the so-called threshold values. In Sweden, all treatments with an ICER less than 10,900€/QALY are considered very cost-effective, less than 54,300€/QALY are considered general acceptable, and over 54,300€/QALY are considered interventions to be undertaken only if the disease is very severe [Citation16]. All analyses were performed in IBM® Statistics SPSS® version 20 and 22 (SPSS Inc., Chicago, IL) and Microsoft© Excel©.
Calculation of QALYs
The remaining QALYs in the cohort were calculated based on the year the study subjects had their first surveillance colonoscopy (i.e., when they entered the study). The calculations were based on Swedish age-sex-specific data on expected remaining lifetime and health-related quality of life (HRQoL) [Citation17,Citation18] (Supplementary Table A). Due to the few cases of CRC, a simplified model was used to calculate losses of QALYs due to CRC.
We made an approximation of stage-specific survival rates based on data from the Swedish Colorectal Cancer Registry (SCRCR) 2007–2011 [Citation19]: Stages I and II – 100% survival; Stage III – 50% deceased on year five after one year of terminal care; and Stage IV – 100% deceased after one year of palliative care. Stage distribution at CRC diagnosis was also based on data from the SCRCR. The stage-specific losses of QALYs due to CRC were derived from studies by Hees et al. and Ness et al. [Citation20,Citation21] (Supplementary Table B). Colon and rectal cancer was not separated. The impact on HRQoL from each surveillance colonoscopy procedure is negligible according to previous studies and was not included [Citation22,Citation23].
Costs
Detailed model input (parameter values) for costs is given in Supplementary Tables C and D. The costs are mostly based on tariffs from Swedish county councils’ collaborations and national guidelines for CRC Care. The principle for the tariffs is to estimate the cost on the basis of resources used, and these tariffs are used to make agreements on actual costs. These tariffs are thus scrutinised and accepted by both providers and purchasers.
Costs for genetic counselling, testing, and maintaining a registry for families with HCRC and FCRC were derived from the budget of the Cancer Prevention Clinic in Umeå during the study period. To investigate the cost-effectiveness of the surveillance programme from a population perspective, the costs from the Cancer Preventive Clinic includes all referred families due to possible inherited CRC, not just the individuals who were recommended surveillance. All costs are given in Euros at the price level of December 2015 (1€≈ 9.2 SEK).
Sensitivity analysis
We constructed worst- and best-case scenarios to examine the stability of the base-case results. The worst-case scenario for cost-effectiveness of colonoscopic surveillance is a low incidence of CRC (i.e., a low risk for CRC) in the study population without surveillance in combination with high costs for surveillance and low costs for CRC Care. Consequently, the best-case scenario is a high incidence of CRC without surveillance in combination with low costs for surveillance and high costs for CRC Care. Input data in the base-case model, which are varied in the sensitivity analysis, are given in .
Ethics
The Regional Ethical Review Board in Umeå approved the study and all study subjects gave their informed consent to be included in the registry.
Results
Outcome of surveillance and estimations of CRC without surveillance
In this study, 259 individuals (two excluded due to missing data) from 118 families participated in surveillance. The participants had a total of 597 colonoscopies. Mean age for their first colonoscopy was 53 years (24–79) and 61% (159/259) were females (). No complications requiring surgical intervention were reported as the result of a colonoscopy.
Table 2. Baseline data and outcome of surveillance for the study cohort.
In the surveillance programme, one case (a 70-year-old female) was diagnosed with CRC (Stage III) although 9.5 cases were expected according to the best estimate for CRC without surveillance (). The standardised incidence ratio (SIR) of CRC – observed vs. expected cases – based on the best estimates is 0.11 (95%CI 0.0014–0.5857), indicating a significant reduction in CRC due to surveillance [Citation9]. Mean age of the expected cases of cancer was 62 years.
Differences in QALYs and costs, surveillance vs. non-surveillance
Surveillance saved 64.8 QALYs (1.7%) in the study population compared to non-surveillance (). Surveillance was 378,047€ more costly than non-surveillance for the health care sector, whereas non-surveillance had 145,010€ in higher costs due to loss of production. In total, the net cost for surveillance was 233,038€ ( and ).
Table 3. Estimations on expected cases of CRC in the study cohort without surveillance.
Table 4. Calculations on differences in quality-adjusted life years (QALYs), surveillance vs. non-surveillance.
Table 5. Costs for the health care sector and loss of production, surveillance vs. non-surveillance.
Main outcome and sensitivity analysis
The ICER, comparing surveillance and non-surveillance, was in the base case 3596€/QALY (233,038/64.8). In the sensitivity analysis, the ICER varied from −4620€ in the best-case scenario to 33,779€ in the worst-case scenario.
Discussion
Colonoscopic surveillance is a cost-effective method for hereditary and familial colorectal cancer prevention. Compared to current thresholds for cost-effectiveness of health care interventions in Sweden, the ICER in our study is low in the base-case and moderate in the worst-case scenario. The negative ICER in the best-case scenario means that surveillance, with these assumptions, saves both lives – e.g., QALYs – and money.
A few earlier studies have examined the cost-effectiveness of colonoscopic surveillance or colonoscopic screening based on family history [Citation11–15]. All these studies conclude colonoscopy is a cost-effective method to prevent inherent CRC. Unfortunately, it is difficult to compare the ICER in these studies with our study due to differences in aim, methodology (on-going surveillance vs. simulation) and setting (time and location). Compared to other cancer preventive interventions, the ICER for colonoscopic surveillance in this study is lower or similar ().
Figure 3. Comparison of incremental cost effectiveness ratios (ICERs) for different cancer preventive or screening health care interventions and threshold for low-cost interventions according to the Swedish National Board of Health and Welfare (SNBHW).
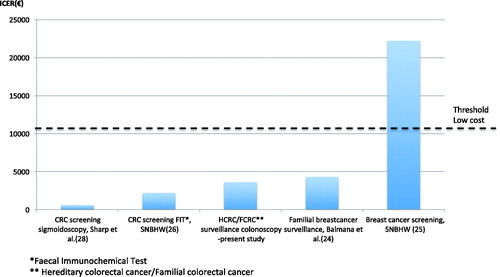
A Spanish study from 2004 by Balmana et al. examined the cost-effectiveness of surveillance for familial breast cancer and found ICERs that resembled ours; however, since their estimated costs are now over ten years old, their ICERs might be underestimated [Citation24]. More recently, the Swedish National Board of Health and Welfare (SNBHW) published cost-effectiveness evaluations of breast and colorectal cancer screening in the general population. Biannually screening for breast cancer with mammography for women age 50–69 years has a moderate ICER according to threshold values in Sweden [Citation25]. CRC screening is a low-cost intervention using biannual faecal immunochemical test (FIT) for individuals 50–74 years old [Citation26]. FIT, however, is not a valid alternative for surveillance of inherited CRC due to a low detection rate for premalignant adenomas. This is especially true since inherited CRC has an accelerated adenoma–carcinoma sequence compared to the slower oncogenesis in sporadic CRC [Citation27]. Sigmoidoscopy can detect premalignant adenomas and a single examination at age 60 is very cost-effective way to screen for CRC in the general population [Citation28]. However, since most hereditary CRC cases are found proximal of the splenic flexure, sigmoidoscopy is not an option in surveillance for HCRC or FCRC.
Strengths and limitations
To our knowledge, this is the first cost-effectiveness study on colonoscopic surveillance based on an on-going programme. Our study analyses the cost-effectiveness of maintaining a surveillance programme for a defined population; all the individuals in this study had suspected FCRC or HCRC and lived in the Northern Sweden Health Care Region where they were referred to the Cancer Prevention Clinic in Umeå. Furthermore, costs include genetic testing and counselling for all referred individuals and not just costs for those who were recommended for surveillance.
The reliability of CRC findings is high as all study subjects were linked to the Regional Cancer Registry. Nevertheless, in the absence of a control group, we had to estimate expected cases of CRC without surveillance. A control group with no surveillance would have been ethically impossible since our material includes HCRC individuals with up to 80% lifetime risk for CRC. We consider cohort analysis, including best available data on relative risk for HCRC/FCRC, as the most plausible way to evaluate a surveillance programme.
The relative risks for HCRC/FCRC in our cohort are based on a study from 2005 by Dowe-Edwin et al [Citation7]. The estimations correspond to an average lifetime risk for CRC in about 40% in the best estimate for HCRC families. More recent studies suggest the risk for CRC in the most common HCRC syndrome – Lynch – is variable from 46% to 10%, depending on the associated MMR gene [Citation29]. The MMR gene associated with the lowest risk for CRC is PMS-2, which was not tested during our study period. A large proportion of unknown PMS-2 carriers might cause an overestimation of both the expected cases of CRC in the cohort and the cost-effectiveness of surveillance.
However, since PMS-2 seems to be the most rare MMR gene variant (5–10%), undiagnosed PMS-2 carriers would probably be very few with a low impact on the CRC estimations [Citation29]. Another limitation is the small study population, although the statistical analysis indicates a significant reduction in CRC owing to surveillance.
We also chose to perform the sensitivity analysis as a scenario analysis instead of using a more complex probabilistic sensitivity analysis. A different sensitivity analysis would probably not change the study’s conclusion, as the ICER in the base case is far below the current threshold (≈ 54,300€) for moderate cost-effective interventions in Sweden [Citation7].
Improving surveillance in the future
As FCRC comprises individuals with a heterogeneous risk, many of these individuals might have colonoscopies too early or too often in relation to their true risk for CRC. There is growing evidence that five- or six-year examination intervals from the age of 45 are sufficient in most cases of FCRC [Citation7–9]. Findings at the individual’s first colonoscopy may also provide tools to customise future surveillance [Citation30]. FCRC patients with pre-malignant high-risk adenomas may have shorter intervals, and older individuals without alarming initial findings might not need continued surveillance [Citation7,Citation8].
The risk assessment in HCRC can also be improved. In HCRC, there are families who have a negative view of testing for known HCRC genes. Gene carriers cannot be discriminated from non-carriers, and all family members are recommended surveillance. Considering a lifetime perspective of one young non-carrier, 20–25 colonoscopies might be avoided.
In our study population, there is one larger family who fulfilled the Amsterdam criteria for Lynch syndrome, but who were negative for known Lynch genes. The family’s 22 members had 81 colonoscopies during the study period. If the gene that causes CRC in this family could be identified, all the non-carriers could be excluded from the surveillance programme. In theory, half of the family members should be non-carriers, and the potential savings are about 40 colonoscopies or 33,000€ during our limited study period.
To conclude, colonoscopic surveillance of HCRC every second year from the age of 25 and for FCRC every fifth year from 5 to 10 years before the age of the first diagnosed case in the family is a cost-effective intervention to prevent CRC. Enhanced genetic testing and customising surveillance on the basis of colonoscopic findings could further improve cost-effectiveness.
Supplementary_tables.pdf
Download PDF (84.5 KB)Acknowledgements
The authors would like to thank genetic counsellors Monica Emanuelsson and Elisabeth Stenman for managing the quality registry and assistant Lena Lundkvist for making budget figures available.
Disclosure statement
None of the authors have any conflicts of interest to state.
Additional information
Funding
References
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932.
- Stoffel EM, Kastrinos F. Familial colorectal cancer, beyond Lynch syndrome. Clin Gastroenterol Hepatol. 2014;12:1059–1068.
- National Cancer Institute: Genetics of Colorectal Cancer. 2017. [cited17.02.06]; Available from: https://www.cancer.gov/types/colorectal/hp/colorectal-genetics-pdq#section/all.
- Chubb D, Broderick P, Frampton M, et al. Genetic Diagnosis of High-Penetrance Susceptibility for Colorectal Cancer (CRC) is achievable for a high proportion of familial CRC by exome sequencing. JCO. 2015;33:426–432.
- Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666–689.
- Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739–750.
- Dove-Edwin I, Sasieni P, Adams J, et al. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. BMJ. 2005;331:1047
- Hennink SD, van der Meulen-de Jong AE, Wolterbeek R, et al. Randomized comparison of surveillance intervals in familial colorectal cancer. J Clin Oncol. 2015;33:4188–4193.
- Sjostrom O, Lindholm L, Tavelin B, et al. Decentralized colonoscopic surveillance with high patient compliance prevents hereditary and familial colorectal cancer. Fam Cancer. 2016;15:543–551.
- Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834.
- Olsen KR, Bojesen SE, Gerdes AM, et al. Cost-effectiveness of surveillance programs for families at high and moderate risk of hereditary non-polyposis colorectal cancer. J Inter Tech Health Care. 2007;23:89–95.
- Ouakrim DA, Boussioutas A, Lockett T, et al. Cost-effectiveness of family history-based colorectal cancer screening in Australia. BMC Cancer. 2014;14:261.
- Vasen HF, van Ballegooijen M, Buskens E, et al. A cost-effectiveness analysis of colorectal screening of hereditary nonpolyposis colorectal carcinoma gene carriers. Cancer. 1998;82:1632–1637.
- Ramsey SD, Wilschut J, Boer R, et al. A decision-analytic evaluation of the cost-effectiveness of family history-based colorectal cancer screening programs. Am J Gastroenterol. 2010;105:1861–1869.
- Ladabaum U, Ferrandez A, Lanas A. Cost-effectiveness of colorectal cancer screening in high-risk Spanish patients: use of a validated model to inform public policy. Cancer Epidemiol Biomarkers Prev. 2010;19:2765–2776.
- Socialstyrelsen. Hälsoekonomiskt underlag, Nationella riktlinjer för tjock- och ändtarmscancer 2014. [Health economic evidence, National guidelines for colorectal cancer 2014]. 2014. [cited 16.06.01]; Available from: https://www.socialstyrelsen.se/SiteCollectionDocuments/nr-cancer-halsoekonomiskt-underlag-tjock-andtarmscancer.pdf. Swedish.
- Burström K. Hälsorelaterad livskvalitet i Stockholms län 2002. [Health related quality of life in Stockholm 2002]. Stockholm: Stockholms läns landsting; 2006.
- Statistics Sweden: 2016. [cited16.01.01]; Available from: http://www.scb.se/sv_
- Kodeda K, Nathanaelsson L, Jung B, et al. Population-based data from the Swedish Colon Cancer Registry. Br J Surg. 2013;100:1100–1107.
- Ness RM, Holmes AM, Klein R, et al. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol. 1999;94:1650–1657.
- van Hees F, Saini SD, Lansdorp-Vogelaar I, et al. Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology. 2015;149:1425–37.
- Niv Y, Bogolavski I, Ilani S, et al. Impact of colonoscopy on quality of life. Eur J Gastroenterol Hepatol. 2012;24:781–786.
- Kapidzic A, Korfage IJ, van Dam L, et al. Quality of life in participants of a CRC screening program. Br J Cancer. 2012;107:1295–1301.
- Balmana J, Sanz J, Bonfill X, et al. Genetic counseling program in familial breast cancer: analysis of its effectiveness, cost and cost-effectiveness ratio. Int J Cancer. 2004;112:647–652.
- Socialstyrelsen: Värdet av populationsbaserad screening för bröstcancer – hälsoekonomisk analys [Screening for breast cancer - health economic analysis]; 2013. [cited 16.09.01]; Available from: https://www.socialstyrelsen.se/SiteCollectionDocuments/nr-screening-brostcancer-halsoekonomi.pdf. Swedish.
- Socialstyrelsen: Tjock- och ändtarmscancer, screening med test av blod i avföringen [Colorectal cancer, screening with faecal blood tests]; 2013. [cited16.09.01]; Available from: http://www.socialstyrelsen.se/SiteCollectionDocuments/screening-tjockandtarmscancer-halsoekonomi.pdf. Swedish.
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595.
- Sharp L, Tilson L, Whyte S, et al. Cost-effectiveness of population-based screening for colorectal cancer: a comparison of guaiac-based faecal occult blood testing, faecal immunochemical testing and flexible sigmoidoscopy. Br J Cancer. 2012;106:805–816.
- Møller, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2017;66:464–472.
- Forsberg AM, Hagel E, Jaramillo E, et al. Predicting outcome in colonoscopic high-risk surveillance. Anticancer Res. 2015;35:4813–4819.

