Abstract
Objectives: Liver transplantation in hepatocellular cancer (HCC) is curative only for a selection of patients. Commonly used criteria are mostly based on tumor size and number. However, patients within criteria do have tumor recurrences after transplantation and patients outside criteria are excluded even though some could benefit from transplantation. The tumor marker alpha fetoprotein (AFP) is associated with poor outcome and has already been reported to improve selection. We investigated the hypothesis that AFP level combined with traditional selection criteria could ameliorate the selection accuracy for liver transplantation in HCC.
Materials and methods: A retrospective national cohort study in 336 patients who had liver transplantation for HCC in Sweden 1996–2014.
Results: AFP cut-off levels of 20, 100, 1000 and >1000 ng/mL stratified both survival and tumor recurrence, with estimated 5-year survival rates of 74, 61, 49 and 31%, respectively. A simple score, combining three risk levels according to Milan and UCSF fulfillment with three levels of AFP, increased predictive accuracy. A high score identified 35 at-risk patients with estimated post-transplant 5-year survival rate of only 29% compared to 50% for 76 patients excluded by UCSF. More patients were within the combined score cut-off compared to within UCSF, but 5-year survival was similar, 67% versus 66%.
Conclusion: AFP combined with traditional selection criteria ameliorates the selection accuracy for liver transplantation in HCC.
Introduction
With the introduction of the Milan criteria (MC) in 1996, tumor size and number became the corner-stone for transplantation selection in hepatocellular cancer (HCC) patients [Citation1]. Expanded alternatives have been proposed, with the UCSF criteria [Citation2] among the more common.
In Sweden, the waiting list for liver transplantation used to be relatively short and selection criteria quite generous. In 2008, the UCSF criteria were adopted and later recommended in the Swedish National Guidelines and validated for Swedish settings in 2012 [Citation3]. In recent years, the practice of down-staging patients, originally outside UCSF, has become more common.
However, tumor recurrences in patients within criteria and long-term survival for patients outside criteria urge for improved selection precision. Though many risk factors for post-transplant tumor recurrences have been described, only pre-transplant data are truly useful for selection.
The tumor marker alpha fetoprotein (AFP) is associated with poor outcome [Citation4–7] and has gained popularity as a selection instrument for liver transplantation [Citation8]. Different cut-off levels have been proposed for both patient exclusion and inclusion in combination with other criteria [Citation9–14].
The objectives of this study were to evaluate whether high AFP should exclude patients from transplantation within traditional criteria, and whether low AFP levels could allow more liberal criteria with respect to tumor size and number.
Methods
This is a total population-based retrospective cohort study in HCC patients who underwent liver transplantation in Sweden 1996–2014. The inclusion criteria were a pre-transplant diagnosis of HCC, confirmed in postoperative histopathology in patients over 18 years. Patients were identified in local operation and transplant registries. Liver transplantation is performed in two centers in Sweden – Stockholm and Gothenburg. Before 2010 Uppsala performed a few, included in the Stockholm registry. Pre-transplant HCC diagnosis was checked as well as histopathology reports to confirm the HCC diagnosis. Most data were collected by the same investigator (M. E.), except data from Stockholm 1996 to 2007, which was retrieved from a registry. The latest follow-up data were collected in early 2016.
A total of 336 patients were included, after excluding 53 patients with unknown HCC diagnosis before the transplantation (). Patients were defined as recurrence-free if clinically well and showing no sign of tumor on available radiology.
Before 2008, few patients in Sweden received neoadjuvant anti-tumor treatment while on the waiting list, except 46 patients in a negative randomized study on systemic neoadjuvant doxorubicin [Citation15]. Since then, locoregional anti-tumor treatments have been increasingly used to prevent tumor progression and drop-out from the waiting list. Also, tumor down-staging for transplantation has become an option for additional patients initially outside accepted criteria. Only one measure for AFP and tumor parameters were registered in the old cohort (1996–2007), while AFP values and radiologic measures both before and after neoadjuvant treatment were collected when available in the later cohort (2008–2014). In the analysis of the entire cohort, the pre-transplant AFP value closest to the transplantation was used together with measures reflecting largest viable pre-transplant tumor burden and the corresponding fulfillment of the Milan and UCSF criteria.
The study was approved at the Regional Ethical board at Gothenburg University (ref 934-14 and 915-15).
Statistics
Statistics were calculated using Stata 15.1 [Citation16] and SPSS v24. Five-year overall survival rates (5yOS) were estimated by the Kaplan–Meier survival function. Cumulative incidence of recurrence was estimated by use of competing risk methodology [Citation17]. Time was measured from date of liver transplantation to the date of recurrence or date of death or end of follow-up. Death without preceding recurrence was regarded as a competing event. The stcompet macro for Stata was used for the estimation of the cumulative incidence. Flexible parametric survival models (Royston–Parmar models) were used to estimate the continuous prognostic effect of AFP (ng/mL) for mortality and recurrence after transplantation. The macro stpm2 for Stata was used for these calculations [Citation18,Citation19].
Uni- and multivariable Cox regression including preoperative variables was performed for death and recurrence, and continuous variables were categorized. Significant and near-significant factors in the univariate analyses were included in the multivariate analyses, avoiding overlapping factors. Models included AFP, pre-transplant treatment, child class, age, blood group, era, gender and either Milan/UCSF criteria fulfillment, tumor number/largest tumor diameter or total tumor diameter. A last model included combined score instead of AFP and tumor parameters.
Results
At follow-up, 137 (40.8%) subjects had died and 79 (23.5%) had tumor recurrence, while recurrence status was unknown in 9 (2.7%). Mean time from transplantation to last follow-up among living persons was 5.3 years.
Baseline characteristics are presented in . In the 136 patients who underwent liver transplantation 1996–2007, etiology of liver disease was not registered. In the 200 patients who underwent liver transplantation 2008–2014, etiology of cirrhosis was hepatitis C alone or in combination with other etiology in 63%, hepatitis B in 11%, alcohol in 32%, NASH in 6% and autoimmune or primary biliary liver disease in 5%. In eight patients, HCC recurrence after a former liver resection was the transplantation indication.
Table 1. Demographics.
MC were fulfilled in 61% according to pretreatment radiology, while UCSF criteria were fulfilled in 76%. In the recent cohort (2008–2014), neoadjuvant treatment was given in 110 (55%), of which 26 were outside UCSF criteria before treatment start. The most common treatment was TACE, alone (n = 63) or combined with other treatments (n = 19).
Microscopic vascular invasion was described in 116 explanted livers (41%, n = 286). In one patient with pre-transplant HCC diagnosis and neoadjuvant treatment, only necrosis and no viable tumor was seen in the explant histology. Mixed hepatocellular tumors with cholangiocellular differentiation were seen in five cases and with sarcomatous differentiation in two cases.
Risk factors for death and tumor recurrence
In multivariable Cox regression analyses, AFP, criteria fulfillment and time period were significant predictors of both 5-year mortality and recurrence (, model 1a and 1b). In additional multivariable models (not shown) including either tumor number and largest diameter or total tumor diameter instead of criteria fulfillment, AFP remained a significant predictor of both death and recurrence. Combined score was a significant predictor of both death and recurrence (, model 2a and 2b) in a separate multivariable analysis.
Table 2. Multivariable cox regression analyses of 5-year survival and tumor recurrence including AFP and traditional criteria separately.
Table 3. Multivariable Cox regression analyses of mortality and tumor recurrence including AFP and traditional criteria in a combined score.
Survival
Kaplan–Meier-estimated 5-year overall survival (5yOS) was 62% in the entire cohort, 52% in the old cohort (1996–2007) and 70% in the new (2008–2014). Estimated 5-year recurrence-free survival was 61% and disease-specific survival was 74% in the entire cohort. In the 79 patients with recurrent tumor, median time from transplantation to tumor recurrence was 336 d (range 41–3150).
Survival depending on traditional criteria category
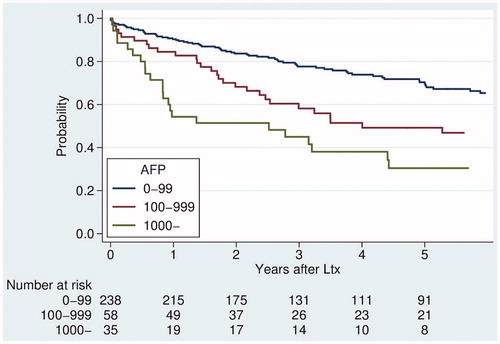
MC stratified survival with 5yOS of 51% outside and 70% inside. Seventy-six patients were outside UCSF, with a 5yOS of 50% compared to 66% for 257 patients who fulfilled UCSF. 5yOS and 5-year recurrence incidence depending on traditional criteria category (both Milan and UCSF criteria fulfillment) are presented in and , last column to the left.
Figure 2. Overall survival after transplantation for HCC, stratified by traditional selection criteria; Milan (MC) and UCSF.
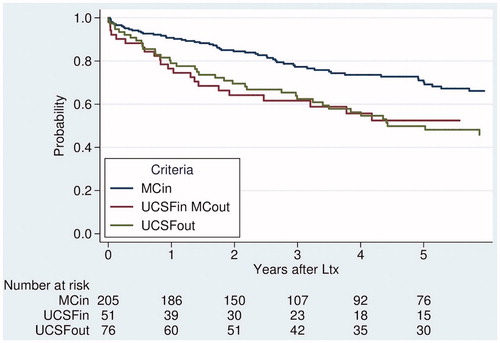
Table 4. Five-year survival and tumor recurrence rates stratified both by traditional criteria (vertically) and by AFP-level (horizontally), with each score subgroup separately (summed score in parentheses).
Survival depending on AFP level
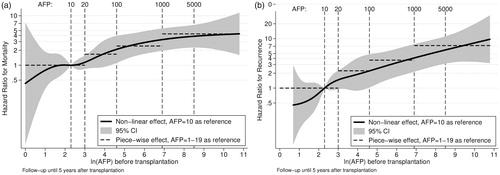
Hazard ratios for each AFP level with respect to death () and recurrence () were plotted, respectively. No natural cut-off was identified, but rather a successive risk increase from normal levels to about 1000 ng/mL.
Figure 3. (a) The prognostic value of AFP for predicting mortality after transplantation for HCC. (b) The prognostic value of AFP for predicting tumor recurrences after transplantation for HCC.
Like traditional selection criteria, the pre-transplant AFP level (categorized) stratified post-transplant survival and recurrence rate ( and , bottom row). AFP-level was prognostic even at low levels, with 5-year recurrence rate of 12% in 159 patients with AFP-level 0–19 ng/mL (5yOS 74%) compared to 26% in 79 patients with AFP-level 20–99 ng/mL (5yOS 61%).
Combining traditional criteria and AFP
A successive risk increase with higher AFP was seen within each criteria category. Similarly, traditional criteria discriminated survival within each AFP category (). To ameliorate prediction, a score was created, combining AFP and criteria category with three risk levels each (low risk 0 points, intermediate risk 1 point and high risk 2 points, ). The sum of the respective points (AFP + criteria) yielded a combined score (0–4 points). The score cut-off was set to exclude patients with at least one “high” risk and one “intermediate” risk factor. Survival rates based on this combined score are presented in . Survival and recurrence rates based on score with cut-off (excluding score 3 and 4) are presented in . Competing risks (cumulative incidence of tumor recurrences and of deaths without recurrence) for patients inside and outside UCSF are presented in , and based on score cut-off in .
Figure 5. Overall survival after transplantation for HCC, stratified by score combining traditional selection criteria and AFP.
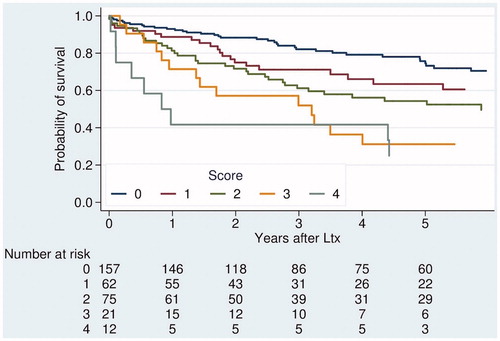
Figure 6. (a) Cumulative incidence of competing events, recurrence and death, after transplantation for HCC, stratified by UCSF. (b) Cumulative incidence of competing events, recurrence and death, after transplantation for HCC, stratified by score combining traditional selection criteria and AFP.
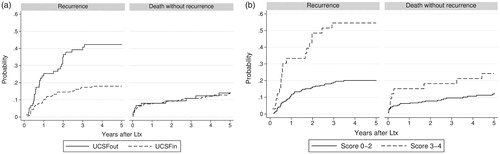
Table 5. Five-year survival and tumor recurrence rates stratified by combined score.
If score cut-off was applied to the whole cohort (294 out of 327), a similar estimated post-transplant 5yOS (67%) to patients within the UCSF (66%, n = 257 out of 332) was seen. Compared to the 203 patients within Milan with a 5yOS of 70% (tumor recurrence rate 15%), the 91 patients outside Milan, but within score, had a 5yOS of 59% (tumor recurrence rate 32%). For the subgroups with score 2, 5yOS was poor in the entire cohort (). In the new cohort (2008–2014), results were generally better and 5yOS rates for the subgroups with score 2 were 77%, NA and 70%, respectively.
Neoadjuvant anti-tumor treatment in the cohort transplanted 2008–2014
The subgroup with pre-transplant treatment (n = 110) was compared to the untreated in the new cohort 2008–2014 (). Traditional criteria did not discriminate well in the treated subgroup, neither based on imaging before neoadjuvant treatment nor after. Post-treatment-pre-transplant AFP of 100–999 ng/mL, however, was a significant risk factor for mortality (HR 4.84 [1.90–12.34]) and recurrence (HR 5.60 [1.93–16.23]) (univariate analysis), while pretreatment AFP did not reach significance. Corresponding to the importance of AFP, combined score discriminated more than traditional criteria in the pretreated subgroup, but not as much as in the untreated group. Irrespective of initial criteria fulfillment, patients with post-treatment AFP less than 100 ng/mL had a good outcome (5yOS 84%), while post-transplant 5yOS was 33% for patients with post-treatment AFP >100 ng/mL and 23% for 29 patients with missing AFP values.
Table 6. Five-year survival and tumor recurrence rates in patients who underwent liver transplantation 2008–2014, stratified by combined score in the entire group and in the pretreated and non-treated subgroups.
Discussion
Pre-transplant AFP level was an independent risk factor for tumor recurrence and death in this Swedish total population cohort of liver transplanted HCC patients, which is concordant with previous studies [Citation7,Citation20–25]. Compared to radiologic parameters, AFP has the advantage of not being observer-dependent, which supports the use of AFP for selection.
Searching for the optimal AFP level, we analyzed the hazard ratio for death and recurrence for the logarithm of continuous AFP level and found no significant cut-off level. The continuous risk increase for recurrence and death with higher AFP makes the use of several levels of AFP logical. Even near-normal levels (10 and 20 ng/mL) of AFP were discriminative. The literature is inconsistent weather AFP-slope is superior to single AFP-values, and we had a limited number of AFP measures. In patients with several AFP measurements, we chose the value after neoadjuvant treatment just before liver transplantation, which had a better predictive value compared to measures before neoadjuvant treatment, similar to previous studies [Citation26–29].
Several authors have proposed selection models with integrated AFP [Citation13,Citation14,Citation23,Citation30,Citation31], sometimes combined with other biological markers [Citation9,Citation22]. Single AFP cut-off levels have been used, either for inclusion of additional patients outside criteria with low AFP levels [Citation10,Citation30,Citation32] or for exclusion of patients within criteria, but with high AFP levels [Citation23,Citation33].
An ameliorated prediction was seen when combining the Milan criteria with pre-transplant AFP levels and other biomarkers [Citation22]. Combinations of tumor size and number with different levels of AFP have also been implemented for outcome prediction [Citation23,Citation24,Citation34,Citation35]. The importance of AFP to improve accuracy was described in a small study, where an AFP-cut-off was added in an extended selection group with preserved post-transplant survival corresponding to the score 1 subgroup outside Milan in our cohort [Citation36]. In a French model, AFP and tumor factors were weighted based on hazard ratios in multivariate analyses in a derivation set, and then tested in a validation set [Citation23]. This model has been validated in other reports [Citation37,Citation38]. When applied to our cohort, the French score included fewer patients for transplantation (out of 328 evaluable patients, 225 within the AFP-model had 5yOS of 69% compared to 49% in the excluded group). A recently suggested selection model based on the large MetroTicket database is congruent with our data, though more restrictive as it excluded all patients with AFP >1000 ng/mL [Citation35].
In Sweden, criteria for transplantation have been generous due to a relatively balanced waiting list [Citation39]. The aim of this study was not to exclude additional patients, but rather to increase selection precision by identifying those without a fair chance of benefiting from transplantation and those outside traditional criteria where transplantation would give a reasonable chance of cure. We think this aim was supported by the score system used in this paper. The score exclusion group had a high rate of tumor recurrence (54% compared to 20%, and ). The discriminative pattern of the suggested score was consistent in the different time cohorts, although the cut-off was best adapted to the more recent cohort 2008–2014, since this recent cohort more reflects our current practice. However, as numbers were small and follow-up shorter, we preferred to focus this paper on results for the entire cohort.
This study is limited by the size of the cohort and by the fact that the inclusion period was very long (18 years). It is however, a total population study, which could permit generalization within Sweden. The retrospective design also limits the validity, and there might be a risk of underestimation of tumor recurrences; for that reason, we also chose to report overall survival, although recurrence rate is a more tumor-specific measure.
A strength was that the discriminative ability of AFP and the increased accuracy with the proposed selection score were consistent in the different subgroups divided by time periods and by neoadjuvant treatment, despite the limitations above.
Though AFP was more significant for outcome in this study compared to traditional criteria, it is important to remember that this cohort was already selected with respect to size and number, but not to AFP, which makes their relative importance difficult to compare and makes it uncertain to generalize results for patients outside UCSF criteria.
The suggested combined score takes advantage of the continuous risk increase of AFP and simultaneously relies on the solid experience with traditional criteria and stays congruent with other reports. It excluded the subgroup within UCSF with the worst prognosis, related to a high AFP in favor of a subgroup outside UCSF with low AFP and a better prognosis in this cohort. Therefore, this score could be purely viewed as a simplified way of combining separate risk factors, which could permit quick and easy clinical implementation.
In conclusion, pretreatment AFP is a significant independent prognostic factor for survival and recurrence after liver transplantation in Swedish HCC patients. The addition of easily available AFP to traditional criteria ameliorates selection. High AFP should exclude some patients from transplantation who are within traditional criteria, and low AFP levels can identify patients with good outcome despite tumor size and number outside traditional criteria.
Acknowledgements
The authors thank Per Sangfelt, Uppsala, for complementary data.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693.
- Yao F, Ferrell L, Bass N, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403.
- Söderdahl G, Sternby Eilard M, Rizell M. [Selection criteria decisive in hepatocellular carcinoma]. Lakartidningen. 2012;109:1750–1753.
- Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101:524–532.
- Zhang N, Gu J, Yin L, et al. Incorporation of alpha-fetoprotein(AFP) into subclassification of BCLC C stage hepatocellular carcinoma according to a 5-year survival analysis based on the SEER database. Oncotarget. 2016;7:81389–81401.
- Yang SL, Liu LP, Yang S, et al. Preoperative serum α-fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg. 2016;103:716–724.
- Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl. 2013;19:634–645.
- Lai Q, Iesari S, Melandro F, et al. The growing impact of alpha-fetoprotein in the field of liver transplantation for hepatocellular cancer: time for a revolution. Transl Gastroenterol Hepatol. 2017;2:72.
- Lee JH, Cho Y, Kim HY, et al. Serum tumor markers provide refined prognostication in selecting liver transplantation candidate for hepatocellular carcinoma patients beyond the Milan criteria. Ann Surg. 2016;263:842–850.
- Takada Y, Tohyama T, Watanabe J. Biological markers of hepatocellular carcinoma for use as selection criteria in liver transplantation. J Hepatobiliary Pancreat Sci. 2015;22:279–286.
- Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: hangzhou experiences. Transplantation. 2008;85:1726–1732.
- Lei JY, Wang WT, Yan LN. Hangzhou criteria for liver transplantation in hepatocellular carcinoma: a single-center experience. Eur J Gastroenterol Hepatol. 2014;26:200–204.
- Grąt M, Kornasiewicz O, Lewandowski Z, et al. Combination of morphologic criteria and alpha-fetoprotein in selection of patients with hepatocellular carcinoma for liver transplantation minimizes the problem of posttransplant tumor recurrence. World J Surg. 2014;38:2698–2707.
- Hameed B, Mehta N, Sapisochin G, et al. Alpha-fetoprotein level >1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20:945–951.
- Söderdahl G, Bäckman L, Isoniemi H, et al. A prospective, randomized, multi-centre trial of systemic adjuvant chemotherapy versus no additional treatment in liver transplantation for hepatocellular carcinoma. Transplant Int. 2006;19:288–294.
- StataCorp. Statistical software. College Station, TX: StataCorp LLC; 2017.
- Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18: 695–706.
- Royston P, Parmar MKB. Flexible proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Statist Med. 2002;21:2175–2197.
- Royston P, Lambert PC. Flexible parametric survival analysis using stata [Elektronic resource]: beyond the Cox model. College Station, TX: StataCorp.; 2011.
- Chen CL, Concejero AM. Liver transplantation for hepatocellular carcinoma in the world: the Taiwan experience. J Hepatobiliary Pancreat Sci. 2010;17:555–558.
- Ciccarelli O, Lai Q, Goffette P, et al. Liver transplantation for hepatocellular cancer: UCL experience in 137 adult cirrhotic patients. Alpha-foetoprotein level and locoregional treatment as refined selection criteria. Transpl Int. 2012;25:867–875.
- Chaiteerakij R, Zhang X, Addissie BD, et al. Combinations of biomarkers and milan criteria for predicting hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 2015;21:599–606.
- Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986–994.e3; quiz e14-5.
- The MetroTicket Calculator. The Metroticket Project; 2016. Available from: http://www.hcc-olt-metroticket.org/#project
- Hakeem AR, Young RS, Marangoni G, et al. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther 2012;35:987–999.
- Merani S, Majno P, Kneteman N, et al. The impact of waiting list alpha-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma. J Hepatol. 2011;55:814–819.
- Lai Q, Inostroza M, Rico Juri JM, et al. Delta-slope of alpha-fetoprotein improves the ability to select liver transplant patients with hepatocellular cancer. HPB (Oxford). 2015;17:1085–1095.
- Giard J-M, Mehta N, Dodge JL, et al. Alpha-fetoprotein slope >7.5 ng/ml per month predicts microvascular invasion and tumor recurrence after liver transplantation for hepatocellular carcinoma. Transplantation. 2018;102:816–822.
- Graąt M, Krasnodeębski M, Patkowski W, et al. Relevance of pre-transplant a-fetoprotein dynamics in liver transplantation for hepatocellular cancer. Ann Transplant. 2016;21:115–124.
- Furukawa H, Shimamura T, Suzuki T, et al. Liver transplantation for hepatocellular carcinoma: the Japanese experience. J Hepatobiliary Pancreat Sci. 2010;17:533–538.
- Lai Q, Levi SGB, Lerut J. Selection tool alpha-fetoprotein for patients waiting for liver transplantation: how to easily manage a fractal algorithm. World J Hepatol. 2015;7:1899–1904.
- Takada Y, Uemoto S. Liver transplantation for hepatocellular carcinoma: the Kyoto experience. J Hepatobiliary Pancreat Sci. 2010;17:527–532.
- Toso C, Kneteman N, Shapiro J, et al. The estimated number of patients with hepatocellular carcinoma selected for liver transplantation using expanded selection criteria. Eur Soc Organ Transpl. 2009;22:869–875.
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43.
- Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154:128–139.
- Grat M, Wronka KM, Stypułkowski J, et al. The Warsaw proposal for the use of extended selection criteria in liver transplantation for hepatocellular cancer. Ann Surg Oncol. 2017;24:526–534.
- Pinero F, Tisi Baña M, Cristinade Ataide E, et al. Liver transplantation for hepatocellular carcinoma: evaluation of the alpha-fetoprotein model in a multicenter cohort from Latin America. Liver Int. 2016;36:1657–1667.
- Notarpaolo A, Layese R, Magistri P, et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol. 2017;66:552–559.
- Melum E, editor. The Nordic Liver Transplant Registry (NTLR): annual report 2016 [Internet]. Aarhus: Scandiatransplant; 2016 [cited 2018 May 15]. Available from: http://www.scandiatransplant.org/members/nltr/TheNordicLiverTransplantRegistryANNUALREPORT2015.pdf
- Giesinger JM, Kieffer JM, Fayers PM, et al. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol. 2016;69:79–88.