Abstract
Objectives: Resistance-associated substitutions (RASs) may impair treatment response to direct-acting antivirals (DAA) in hepatitis C virus (HCV) treatment. We investigated the effects of baseline NS3-RASs (Q80K and R155K) and clinically relevant NS5A-RASs in patients with HCV genotype (GT) 1a infection on treatment outcome, with or without resistance-based DAA-treatment. This multi-center study was carried out between 2014 and 2016.
Patients/methods: Treatment in the intervention group (n = 92) was tailored to baseline resistance. Detection of NS3-RAS led to an NS5A-inhibitor-based regimen and detection of NS5A-RAS to a protease-inhibitor regimen. Patients without baseline RAS in the intervention group and all patients in the control group (n = 101) received recommended standard DAA-treatment.
Results: The sustained virologic response rates (SVR) in the intervention and control groups were 97.8% (90/92) and 93.1% (94/101), respectively (p = .174). A trend toward higher SVR-rate in cirrhotic patients (p = .058) was noticed in the intervention group compared to the control group with SVR-rates 97.5% (39/40) and 83.3% (35/42), respectively. All patients with baseline NS3 (Q80K/R155K) or NS5A-RASs in the intervention group achieved SVR with personalized resistance-based treatment. In the control group, five patients with Q80K or R155K at baseline were treated with simeprevir + sofosbuvir and treatment failed in two of them. Furthermore, one of three patients who failed ledipasvir + sofosbuvir treatment had NS5A-RASs at baseline.
Conclusions: In line with the findings of the OPTIMIST-2 trial for Q80K and the EASL-guidelines 2016 for NS5A-RASs, baseline RASs appeared to have an impact on treatment outcome albeit a statistical significance was not observed in this low-prevalence population.
Introduction
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease, liver cirrhosis, hepatocellular carcinoma (HCC) and liver failure [Citation1]. Globally, the prevalence of viremic HCV infection is estimated to be around 1.1%, corresponding to 64–103 million actively infected individuals [Citation2]. In Sweden and Norway, about 0.4–0.5% of the population is infected with HCV, i.e., approximately 45,000 and 20,000 individuals, respectively [Citation3–5]. HCV is classified into seven genotypes (GT) and several subtypes [Citation6]. In Sweden, the most common GT is 1a, followed by 3a [Citation7], while in Norway GT 3a is the most common, followed by 1a (personal communication Gutteberg T).
The development of direct-acting antiviral agents (DAAs) has led to major advances in the treatment of HCV infection, with substantially higher sustained virologic response (SVR) rates, shorter treatment duration and fewer side effects than previous interferon-based treatment. Currently, four classes of DAAs are available targeting three nonstructural proteins in HCV; NS3/4A protease inhibitors (PI), NS5A inhibitors and nucleoside and non-nucleoside inhibitors of the NS5B RNA-dependent RNA polymerase (RdRP) [Citation8]. Treatment with different combinations of these potent DAAs with or without ribavirin has made it possible to obtain SVR rates of above 95% in the majority of patients with chronic hepatitis C (CHC).
HCV displays a pronounced genetic heterogeneity due to the lack of proofreading activity of the RdRP and the rapid turnover rate in HCV replication [Citation9]. In the resulting HCV quasispecies, resistance-associated substitutions (RASs) can emerge under the selective pressure of treatment with DAAs [Citation10], but may also occur prior to treatment, i.e., baseline resistance [Citation9]. Depending on their frequency within the HCV quasispecies and the level of resistance conferred, baseline RAS can contribute to treatment failure in the presence of other negative predictive factors such as advanced stages of liver fibrosis, previous treatment and suboptimal treatment [Citation8].
These methods available for detecting RASs are the Sanger sequencing and the next-generation sequencing (NGS) methods. The Sanger sequencing method carries a 20% cutoff level for detecting RASs in the viral population compared to 1% with the NGS method. However, the general consensus is to recommend a cutoff level of 10–20%, for detecting RASs within the HCV quasispecies, in order to be of clinical relevance in predicting treatment failures [Citation11].
The polymorphism Q80K, a naturally occurring amino acid substitution in the viral NS3 region, is mainly present in HCV GT 1a and is associated with reduced susceptibility to the NS3/4A protease inhibitor simeprevir, as indicated by the COSMOS and the first OPTIMIST studies [Citation12,Citation13]. The prevalence of baseline Q80K varies geographically and is reported to be 48% in the USA [Citation14], compared to 5.7–15.2% in Sweden [Citation7,Citation15], and 4.8% in Norway [Citation15].
The NS3 amino acid substitution R155K confers resistance to PI and is frequently observed in GT 1a-infected patients who failed to achieve SVR after treatment with boceprevir, telaprevir or simeprevir [Citation10]. R155K can also be present in <1% of GT 1a PI treatment-naïve patients [Citation16].
In current clinical practice, it is more important to consider baseline RASs in NS5A than NS3 for GT 1a. Many RASs confer a very high fold resistance, e.g., Y93H/N (>1000 fold), with GT 1a in in vitro replicon assays [Citation8]. It should be noted that NS5A RASs at positions 30 and 31 confer a medium to high resistance, but these in vitro resistance profile might not be high enough to be of clinical importance for current approved NS5A inhibitors (with exception of elbasvir and ledipasvir). Nevertheless, these NS5A RASs are rarely found as baseline polymorphisms in GT 1a patients, i.e., in 2–5% of DAA treatment-naïve patients when using the population-sequencing method. However, EASL and AASLD guidelines recently recommended NS5A baseline testing for GT 1a prior to treatment with elbasvir and that testing should also be considered in treatment-experienced patients before treatment with ledipasvir regimens [Citation17,Citation18].
At the start of the study, there were no available guidelines regarding baseline resistance testing. The aim of this real-life study was initially to investigate the impact of Q80K, subsequently also including R155K and NS5A RASs, on treatment outcome in GT 1a infected patients treated with DAAs, and to evaluate the resultant economic consequences. Known factors influencing treatment outcome were evaluated. The study was conducted during 2014–2016 when simeprevir plus sofosbuvir combination was a recommended alternative to NS5A inhibitor based regimes.
Patients and methods
Patients diagnosed with chronic HCV GT 1a infection from Uppsala, Gävle and Tromsø received resistance-based treatment (intervention group) and from Örebro, Falun and Bodö received treatment according to the national guidelines [Citation19,Citation20] without previous resistance testing (control group). They were consecutively included in this real-life, open-label, non-randomized Nordic multi-center study from 1 April 2014 to 30 June 2015 (Sweden) and 26 January 2016 (Norway). During this period, baseline NS3 resistance testing (Q80K and R155K) of HCV GT 1a was performed routinely for the intervention group. In January 2015, when the fixed combination ledipasvir plus sofosbuvir was approved in Sweden and Norway, analysis of NS5A RAS was introduced and was performed in the intervention group, approximately 40% of 92 patients in the intervention group. The NS5A RASs considered important by us at that time were Q30E/H/R, L31M and Y93C/H/N. All these RASs were indicated by the HCV drug development advisory group to be clinically relevant with a > 100 fold increase in resistance toward ledipasvir [Citation10].
Recommended treatment, according to the National Boards [Citation19,Citation20], was, therefore, given to patients without baseline RASs in the intervention group and to all patients in the control group. For patients in the intervention group with Q80K or R155K mutation, the treatment was amended to a NS5A inhibitor-based regimen. In case of baseline NS5A RAS, treatment with a protease inhibitor-based regimen i.e., simeprevir plus sofosbuvir was considered. Presence of baseline NS3 RASs were analyzed for all patients in the control group retrospectively, whereas baseline NS5A analysis in the control group was only done (also retrospectively) for those that had failed ledipasvir plus sofosbuvir treatment.
Resistance analyses for emerging RASs were performed in all non-responders at the time of relapse; NS3A analysis in simeprevir failures and NS5A analysis in patients with failure after the treatment with NS5A inhibitor-based regimen.
Ribavirin was added at the responsible medical doctor`s (MD) discretion, mainly due to the presence of cirrhosis.
The inclusion criteria were: infection with HCV GT 1a; ≥18 years of age; informed consent; and treatment according to Swedish and Norwegian consensus recommendations as well as completed treatment course (per-protocol). Patients included were either treatment-naive or treatment-experienced to interferon-based therapy, including triple therapy containing the first generation NS3 protease-inhibitors boceprevir or telaprevir. Patients previously treated with other DAAs were excluded.
Liver elasticity (kPa) was measured with FibroScan® 502 (Echosens, Paris, France) (Swedish study sites) and FibroScan® 402 (Norwegian study sites) by experienced nurses or doctors. For patients who had undergone a liver biopsy, the Metavir score was recorded [Citation21]. Presence of cirrhosis was determined by FibroScan >12.5 kPa or Metavir score 4 in liver biopsy. Child-Pugh score was estimated from available information on the level of liver elasticity [Citation22], biochemical results and ultrasound. Patient data were extracted from the medical records by the responsible MD at each study site, anonymized and transferred to a joint database.
SVR was defined as undetectable HCV RNA 12 weeks after the end of treatment. Non-SVR was regarded as a viral breakthrough (a negative viral load nadir followed by a positive HCV RNA level during therapy), or viral relapse (non-detectable viral load at the end of treatment followed by an increase in HCV RNA level after therapy).
Laboratory methods
The Clinical Microbiology laboratory at the University Hospital, Uppsala, performed the resistance analysis of RASs (baseline and emerging). A nested PCR method, followed by Sanger sequencing (population sequencing, cutoff 20%) method was adopted for the NS3 resistance analysis. The pan-genotypic NS3 resistance method has been described elsewhere [Citation7]. In brief, RNA extraction from the samples was done using the BioMerieux NucliSENS® easyMAG™ system (bioMérieux, Marcy-l'Étoile, France). cDNA was synthesized from RNA template with the SuperScript™ III Reverse Transcriptase (Invitrogen™, Thermo Fisher, Waltham, MA, USA) using random hexamers. First round PCR and nested PCR were performed with in-house primers targeting parts of the NS3 region using the Taq PCR Master Mix (Qiagen, Hilden, Germany). The integrity of the nested PCR products was verified by agarose-gel electrophoresis. PCR-positive samples were purified using QIAquick® PCR Purification Kit (QIAgen, Hilden, Germany). All protocols used were performed according to the manufacturer’s instructions. The purified products were sequenced by the Sanger sequencing method using the same primers used in the nested PCR. The HCV NS3 sequences were analyzed using SeqScape® Software version 2.6 (Applied Biosystems, Foster City, CA). The NS3 sequence of GT 1a H77 strain was used as a reference template. The mutations were interpreted as relevant NS3 RASs by comparing with RASs reported in the literature [Citation10,Citation23]. The NS5A resistance analysis was performed with the same method and is described elsewhere [Citation24].
HCV RNA titer quantification was performed at the Department of Clinical Microbiology, University Hospital, Uppsala, Sweden and at the Department of Microbiology and Infection Control, University Hospital of North Norway, Tromsø, Norway using Roche COBAS® AmpliPrep/TaqMan® HCV Quantitative Test version 2.0 with a LOQ of 15 IU/mL (Roche Molecular Systems Inc., Branchburg, NJ).
Outcomes
The primary objective was to study the treatment efficacy in the intervention group compared to the control group, with respect to the proportion of patients achieving SVR. Secondary objectives included to determine (1) the proportion of patients with baseline NS3 (Q80K and R155K) RASs, (2) the proportion of patients with baseline NS3 and NS5A RASs experiencing viral breakthrough or relapse, (3) the proportion of patients with baseline NS3 RASs not experiencing viral breakthrough or relapse and (4) to compare total expenditures (treatment and baseline analysis costs) per capita in the two study groups.
Statistics
The null hypothesis of this study was that the SVR rate is equal in the intervention and control groups. The basic statistical computing was done in Microsoft® Excel® 2013 (Microsoft Office professional plus 2013, Microsoft Corporation) and in Statistical Package for Societal Sciences (SPSS version 24, SPSS Inc., Chicago, IL). The Fisher`s exact test was used to test the differences between groups (small expected cell count). A two-tailed p value <.05 was considered significant.
Ethics
The regional committee of medical research ethics Committee in Uppsala (Dnr: 2013/185 and Dnr: 2013/185/1) and the Data Protection Official at The University Hospital of Northern Norway (Nr. 0574) approved the study. All participants received written information and the opportunity to withdraw from the study.
Results
Patient baseline characteristics
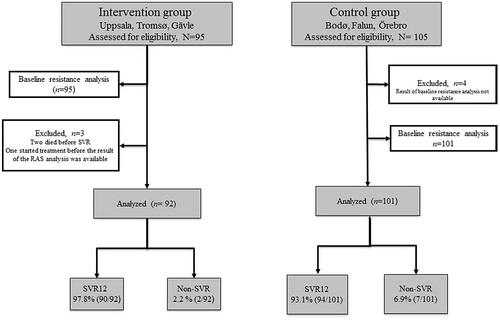
In total, 200 patients were assessed to be eligible for the study. Samples from 196 patients were available for baseline resistance analyses (95 in the intervention group and 101 analyzed retrospectively in the control group). In the intervention group, three patients were omitted from further analyses; two of them died before the time of evaluation for SVR and one patient started treatment before the result of the baseline resistance analysis was available. Thus, week 12 follow-up data were obtained from 193 patients; 92 in the intervention group and 101 in the control group ().
Figure 1. Flowchart of patients included in the study. Baseline resistance testing in the control group was performed retrospectively.
Demographic and baseline clinical characteristics are provided in . The majority of patients were treatment-naïve and male. The median age was 56 years. The distribution of patients with cirrhosis and baseline NS3 RASs (Q80K and R155K) in the intervention and the control groups were similar. The majority of cirrhotic patients were Child-Pugh A.
Table 1. Patient demographics and baseline characteristics.
shows treatment characteristics. The proportion of patients treated with simeprevir was higher in the intervention group compared to the control group. Most of the patients received treatment for 12 weeks and ribavirin was added to a minority.
Table 2. Treatment characteristics.
Efficacy and baseline RASs
The overall prevalence of baseline Q80K and R155K polymorphisms was 7.1% (14/196) and 5.2% (10/196), respectively. The prevalence of Q80K in Sweden and Norway was 7.0% (11/158) and 10.5% (4/38), respectively. The prevalence of R155K in Sweden and Norway was 4.4% (7/158) and 7.9% (3/38), respectively. Prevalence data of NS5A RASs could not be specified since the mandatory screening of baseline NS5A RASs was not started until January 2015, and done only for a part of the intervention group. However, three patients with baseline NS5A RASs were found: two in the intervention group (M28T and Y93H), and one in the control group that harbored both M28A and Q30R.
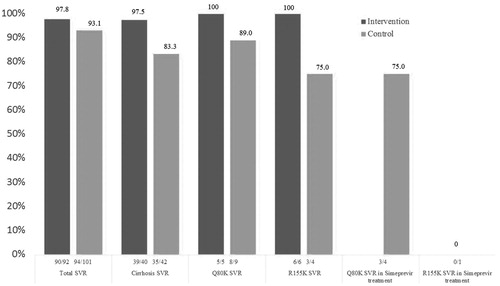
The SVR rate in the intervention group and the control group was 97.8% (90/92) and 93.1% (94/101), respectively (p = .174). A trend toward higher SVR rates in cirrhotic patients (p = .058) was noticed in the intervention group compared to the control group, 97.5% (39/40) and 83.3% (35/42), respectively (). Overall, liver cirrhosis was associated with a lower SVR12 rate compared to non-cirrhosis, 90 (74/82) and 99% (110/111), respectively (p = .005).
Figure 2. Sustained virologic response rates (SVR) in the intervention and control groups. SVR rates in the intervention group (dark grey bars) and the control group (light grey bars). The two bars to the right show SVR rates by simeprevir treatment in patients with baseline Q80K and R155K RAS in the control group.
NS3 RASs
In the intervention group, all patients with baseline Q80K (n = 5) and R155K (n = 6) were successfully treated with a regimen containing a NS5A inhibitor. In the control group, the SVR rates in patients with baseline Q80K and R155K were 89% (8/9) and 75% (3/4), respectively. Only five of 13 patients with such RASs at baseline were treated with simeprevir plus sofosbuvir in the control group. Notable, of this one in four (1/4) patients with Q80K RAS and one in one (1/1) with R155K RAS failed treatment ().
NS5A RASs
In the intervention group, all patients with baseline NS5A RASs (n = 2) were successfully treated. The patient with Y93H was treated with simeprevir plus sofosbuvir, whereas the patient with M28T was treated with ledipasvir plus sofosbuvir since the M28T was not considered a clinically relevant NS5A RAS. In the control group, three patients failed ledipasvir plus sofosbuvir treatment and one of these patients harbored a relevant NS5A RAS at baseline Q30R (together with M28A) (Supplementary Table S1).
In total, nine patients failed to achieve SVR, the reason for non-SVR was a viral relapse. In three of these nine patients, Q80K, R155K and Q30R one each, were detected at baseline (all in control group). Majority of the 10 observed baseline R155K was connected with prior boceprevir or telaprevir treatment failure, but in one patient, it was found as a natural polymorphism. Overall, patients with treatment failure were all male, had a median age of 56 years, 89% (8/9) of them had liver cirrhosis and most were treatment naïve (6/9) ().
Table 3. Clinical characteristics, baseline and emerging RASs (NS3 and NS5A) in the non-responders.
Supplementary Table S1 gives an overview of the patients with detected baseline NS3 RASs (Q80K and R155K) and NS5A RASs.
Economic implications
In 2014/2015, the cost of simeprevir plus sofosbuvir treatment for 12 weeks was 750,000 NOK/650,000 SEK. In 2015, the price for 12 weeks of ledipasvir plus sofosbuvir treatment was 500,000 NOK/400,000 SEK. Baseline NS3 RAS was detected retrospectively in two patients who experienced virological relapse after treatment with simeprevir plus sofosbuvir. Switching these two patients to a NS5A inhibitor-based regimen could possibly have reduced treatment costs and in addition, contributed to a best practice approach. The same trend occurred with the use of simeprevir plus sofosbuvir treatment for the patient with NS5A Q30R RAS at baseline. Thus, in the control group, there was an economic loss of 2.0 million NOK/1.7 million SEK compared to the intervention group where no patients with Q80K/R155K or clinically important NS5A RAS at baseline experienced non-SVR. In comparison, the baseline analysis costs (2000 NOK/SEK per analysis) for the 95 patients in the intervention group were less than 0.2 million NOK/SEK.
Discussion
In this real-life study conducted in Q2 2014 to Q1 2016, we found a low prevalence of baseline Q80K RAS in HCV GT 1a in Sweden and Norway (7.1%), which is in line with previous studies [Citation7,Citation15]. Liver cirrhosis was significantly associated with treatment failure. There were not enough GT 1a patients with Q80K RAS to detect a significant effect of baseline resistance-guided treatment on the SVR rate. However, our findings appear to agree with earlier studies. The COSMOS study in 2014 indicated lower SVR rates in GT 1a patients with baseline Q80K RAS compared to patients without Q80K at baseline [Citation13]. The OPTIMIST-2 study revealed lower SVR rates for GT 1a patients with cirrhosis and baseline Q80K (SVR 74%) compared to those without Q80K (SVR 92%) [Citation25].
In the control group, only 30% of the patients were treated with simeprevir + sofosbuvir combination compared to 50% in the intervention group, possibly due to new treatment guidelines introduced in February (Sweden) and March (Norway) 2015, which recommended treatment with the fixed combination of ledipasvir plus sofosbuvir. Thus, these guidelines recommended NS5A inhibitor-based regimens for previous treatment failures of boceprevir/telaprevir, without regard to baseline resistance analysis. Of note, the only patient in the control group with Q80K at baseline that failed treatment was one out of four patients with such RAS that were treated with simeprevir plus sofosbuvir (i.e., SVR 75%). Furthermore, the single patient with baseline R155K that underwent simeprevir plus sofosbuvir treatment in the control group also failed to attain SVR.
Our study also indicated that baseline resistance analysis may have an impact on treatment outcome in patients with liver cirrhosis, but the difference was not statistically significant, possibly due to the low prevalence of baseline NS3 and NS5A RASs.
Currently, the NS3 inhibitor simeprevir is no longer in use due to the development of more effective DAA treatment regimens. As a result, the focus has switched to study baseline NS5A RASs in predicting the most effective DAA combination and treatment duration [Citation11]. Since 2016, NS5A baseline resistance analysis is mainly recommended before treatment of GT 1a with the NS5A inhibitor elbasvir, co-formulated with the NS3 inhibitor grazoprevir. However, it is also recommended to consider baseline analysis of the NS5A RASs for treatment-experienced GT 1a patients prior to treatment with ledipasvir plus sofosbuvir [Citation17,Citation18]. Therefore, it was relevant for this study, when conducted in 2015, to include also baseline NS5A analysis.
Although we cannot report any significant effect on baseline Q80K/R155K and NS5A resistance analyses on the treatment outcome, baseline resistance testing could have economic implications. However, today’s considerably lower drug expenditures per patient combined with recommended regimens that are less dependent on the preexisting RASs addressed in this study, made our economic calculations somewhat obsolete.
In clinical practice, the impact of HCV RASs will probably become less important with the availability of new effective DAAs. However, drug resistance can be a problem in the context of other negative predictors for treatment response like the presence of cirrhosis, suboptimal treatment duration and prior treatment [Citation8,Citation11], and new emerging mutations in the highly variable HCV genome may affect the current high SVR rates. It could be noted that the Q80K RAS was the most commonly observed baseline NS3 variant in the few failures in the POLARIS-2 trial [Citation26]. In patients with GT 1a treated with the pan-genotypic NS3 protease inhibitor voxilaprevir combined with sofosbuvir plus velpatasvir for eight weeks, the SVR rate was lower in patients with baseline Q80K compared to patients without (88 and 94%, respectively) [Citation26].
Conclusion
We found a low prevalence of NS3 Q80K RAS in HCV GT 1a in Norway and Sweden. In this real-life study, baseline resistance analyses for NS3 RAS (Q80K and R155K) and clinically relevant NS5A RASs could not statistically determine the treatment outcome, probably due to small sample sizes. Liver cirrhosis was the most important predictor of treatment failure. However, the results indicate an adverse effect of RAS Q80K preexistence on the treatment outcome with simeprevir plus sofosbuvir, findings that were published in the OPTIMIST-2 trial in 2016. Furthermore, the results are in line with the recommendations by EASL in 2016 that NS5A RASs at baseline appeared to have an impact on ledipasvir plus sofosbuvir treatment outcome. Personalized treatment with regard to baseline resistance analyses could thereby be important to find the most cost-effective treatment combinations/duration, both in a perspective of evidence-based healthcare delivery and in the case of the individual patient to avoid relapse and reducing the retreatment options.
Supplementary Table S1
Download MS Word (19.3 KB)Acknowledgments
A. L. received Clinical Research Support (ALF) from Uppsala County Council and Uppsala University. The authors would like to thank Navaneethan Palanisamy for grammatical assistance. Anders Bergqvist, Christina Öhrmalm and Kåre Bondeson are thanked for their help with the resistance analysis.
Disclosure statement
J.L. has received an unrestricted research grant from Medivir, but this company was not involved in any parts of this study. The other authors report no conflicts of interest.
Additional information
Funding
References
- Seeff LB. The history of the “natural history” of hepatitis C (1968–2009). Liver Int. 2009;29:89–99.
- Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–S57.
- Büsch K, Waldenström J, Lagging M, et al. Prevalence and comorbidities of chronic hepatitis C: a nationwide population-based register study in Sweden. Scand J Gastroenterol. 2017;52:61–68.
- Dalgard O, Jeansson S, Skaug K, et al. Hepatitis C in the general adult population of Oslo: prevalence and clinical spectrum. Scand J Gastroenterol. 2003;38:864–870.
- Cornberg M, Razavi HA, Alberti A, et al. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 2011;31:30–60.
- Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318–327.
- Palanisamy N, Danielsson A, Kokkula C, et al. Implications of baseline polymorphisms for potential resistance to NS3 protease inhibitors in Hepatitis C virus genotypes 1a, 2b and 3a. Antiviral Res. 2013;99:12–17.
- Sarrazin C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J Hepatol. 2016;64:486–504.
- Schneider MD, Sarrazin C. Antiviral therapy of hepatitis C in 2014: do we need resistance testing? Antiviral Res. 2014;105:64–71.
- Lontok E, Harrington P, Howe A, et al. Hepatitis C virus drug resistance–associated substitutions: state of the art summary. Hepatology. 2015;62:1623–1632.
- Wyles DL. Resistance to DAAs: when to look and when it matters. Curr Hiv Aids Rep. 2017;14:229–237.
- Kwo P, Gitlin N, Nahass R, et al. Simeprevir plus sofosbuvir (12 and 8 weeks) in hepatitis C virus genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology. 2016;64:370–380.
- Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756–1765.
- Lenz O, Verbinnen T, Fevery B, et al. Virology analyses of HCV isolates from genotype 1-infected patients treated with simeprevir plus peginterferon/ribavirin in Phase IIb/III studies. J Hepatol. 2015;62:1008–1014.
- Sarrazin C, Lathouwers E, Peeters M, et al. Prevalence of the hepatitis C virus NS3 polymorphism Q80K in genotype 1 patients in the European region. Antiviral Res. 2015;116:10–16.
- Bartels DJ, Sullivan JC, Zhang EZ, et al. Hepatitis C virus variants with decreased sensitivity to direct-acting antivirals (DAAs) were rarely observed in DAA-naive patients prior to treatment. J Virol. 2013;87:1544–1553.
- AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. [www.hcvguidelines.org]. 2017. Available from: https://www.hcvguidelines.org/
- EASL guidelines 22 Sept. 2016. www.easl.eu/medias/cpg/HCV2016/Summary.pdf
- Stockholm: Janusinfo. 2017 [cited 2017 Jul 4]. Available from: http://www.janusinfo.se/Documents/Nationellt_inforande_av_nya_lakemedel/Hepatit-C-161215.pdf
- Faglig Veileder for Utredning og Behandling av Hepatitt C: Den Norske Legeforening. 2014 [cited 2018 Jan 8]. Available from: http://legeforeningen.no/PageFiles/246436/Veileder%20sept%202014.pdf
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology. 1996;24:289.
- Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350.
- Sarrazin C, Dvory-Sobol H, Svarovskaia ES, et al. Prevalence of resistance-associated substitutions in HCV NS5A, NS5B, or NS3 and outcomes of treatment with ledipasvir and sofosbuvir. Gastroenterology. 2016;151:501–512.
- Lindström I, Kjellin M, Palanisamy N, et al. Prevalence of polymorphisms with significant resistance to NS5A inhibitors in treatment-naive patients with hepatitis C virus genotypes 1a and 3a in Sweden. Infect Dis. 2015;47:555–562.
- Lawitz E, Matusow G, DeJesus E, et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: A phase 3 study (OPTIMIST-2). Hepatology. 2016;64:360–369.
- Jacobson IM, Lawitz E, Gane EJ, et al. Efficacy of 8 weeks of sofosbuvir, velpatasvir, and voxilaprevir in patients with chronic HCV infection: 2 phase 3 randomized trials. Gastroenterology. 2017;153:113–122.
