?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective: The effectiveness of golimumab in Crohn’s disease (CD) is largely unknown as it is not approved for the treatment of the disease. We aimed to identify the population of CD patients treated with golimumab in Sweden, to assess the effectiveness of golimumab (defined as the drug retention rate), and to identify predictors of drug discontinuation.
Methods: Patients with CD who received at least one injection of golimumab were identified through the Swedish National Quality Registry for Inflammatory Bowel Disease, which includes prospectively collected clinical information. Cox regression models were used to identify predictors of golimumab discontinuation.
Results: The study cohort involved 94 patients of whom the majority (96.8%) had previously discontinued at least one anti-tumour necrosis factor (anti-TNF) agent. The drug retention rate at 12 weeks was 85.1%. Predictors of golimumab discontinuation at 12 weeks were previous surgery (adjusted HR = 7.52, 95% CI: 1.12–50.36), concomitant corticosteroid use at baseline (adjusted HR = 5.70, 95% CI: 1.13–28.68) and female sex (adjusted HR = 6.59; 95% CI: 1.04–41.62). The median duration of follow-up was 89 (IQR: 32–158) weeks. The drug retention at the most recent follow-up was 35.1%. Predictors of golimumab discontinuation at the most recent follow-up were corticosteroid use at baseline (adjusted HR = 2.60, 95% CI: 1.17–5.79) and female sex (adjusted HR = 2.24; 95% CI: 1.19–4.23).
Conclusion: Patients with CD treated with golimumab were a treatment-refractory group. Despite this, more than one-third of the patients appeared to have had clinical benefit after a median follow-up of more than 1.5 years.
Introduction
Crohn’s disease (CD) causes chronic inflammation in the gastrointestinal tract, giving rise to symptoms such as diarrhoea, abdominal pain and weight loss [Citation1]. The disease course is characterized by intermittent flares and periods of remission. Conventional treatment options such as corticosteroids and immunomodulators are often used as first-line therapy at diagnosis. For patients who have an inadequate response, lose their response, or cannot tolerate conventional treatment, biologics may be used to induce and maintain remission [Citation2]. The anti-tumour necrosis factor (anti-TNF) agents, infliximab and adalimumab, were the first biologics to be approved for the treatment of CD. More recently, biological therapies with novel mechanisms of action, such as the integrin blocker vedolizumab and the p40 inhibitor ustekinumab, have been introduced [Citation2–4]. The introduction of anti-TNFs has dramatically improved treatment outcomes in patients with CD. However, about one-third of the patients do not respond to a specific anti-TNF agent and some patients lose their response over time or develop intolerance [Citation5,Citation6], indicating the need to identify alternative treatment options in these patients. The efficacy of specific anti-TNF agents may differ at the level of the individual, and patients who do not benefit from a particular anti-TNF agent may respond to another [Citation7].
The fully human anti-TNF agent golimumab may be of great interest in this respect, since its effectiveness has been demonstrated in the treatment of the other main type of inflammatory bowel disease (IBD), ulcerative colitis [Citation8–10]. However, its possible benefit in CD patients remains largely unknown. To our knowledge, the use of golimumab in CD has only been described in two small case series [Citation11,Citation12] and two retrospective studies from tertiary centres [Citation13,Citation14]. Off-label use of drugs is not prohibited in Sweden, and golimumab may have been used for the treatment of patients with CD. The Swedish National Quality Registry for IBD (SWIBREG) includes information on more than 42,000 IBD patients and can be used to generate accurate real-world evidence using prospectively recorded data.
Our aim was to assess the clinical effectiveness of golimumab, to identify predictors of treatment failure in CD, and to determine the characteristics of patients receiving the drug using data obtained from SWIBREG.
Materials and methods
The National Quality Registry for IBD in Sweden
SWIBREG was started in 2005, and today all Swedish IBD centres except four provide information for the registry. Both children and adult patients are included, and the number of patients enrolled is steadily increasing. Currently, more than 42,000 IBD patients have been included, corresponding to >60% of the prevalent IBD population in Sweden [Citation15]. The coverage is higher for some groups of IBD patients, such as those who received biologic treatments. The registry holds prospectively recorded information on diagnosis, the Montreal Classification, laboratory parameters including C-reactive protein (CRP), surgery, medication, reason for drug discontinuation and smoking status. The reasons for drug discontinuation include the following criteria: lack of or loss of response (termination because of primary non-response or secondary loss of response), intolerance (discontinuation due to intolerance, including infusion reactions), or other reasons including patient’s request and pregnancy [Citation16,Citation17]. The validity of diagnoses in the registry has been shown to be high [Citation18].
Study population
Patients with CD who received at least one injection of golimumab were identified through SWIBREG. Data on clinical characteristics (including Montreal Classification and smoking status), previous medical treatment, and surgery at initiation of golimumab treatment, biochemical activity and demographic characteristics, were extracted from the registry on 13 February 2017. Surgery was defined as any CD-related surgery except perianal surgery and endoscopic balloon-dilatation.
Outcome measures
To assess the primary objective, we used drug retention rates as a proxy for clinical effectiveness. The primary outcomes were defined as drug retention rate at end of induction therapy (12 weeks) and at the most recent follow-up. The secondary outcome was to identify predictors of drug discontinuation at 12 weeks and at the most recent follow-up. Further, we investigated the possible effect of golimumab on inflammatory activity defined as change in concentration of CRP/high sensitivity CRP at 12 weeks and at the most recent follow-up compared with the baseline level.
Reasons for drug discontinuation were evaluated based on the criteria used in SWIBREG. Data at baseline and at 12 weeks were used, if measured within 4 weeks before or after those time points.
Statistical analysis
Continuous variables are presented as median with interquartile range (IQR). The one-sample z-test was used to investigate whether the observed proportion of men treated with golimumab differed from the hypothesized proportion of 50%. Kaplan–Meier curves were used to illustrate the duration of golimumab treatment. Drug retention rates were defined as the proportion of patients, who remained on treatment at specific occasions. Changes in biochemical activity between baseline and 12 weeks as well as the most recent follow-up, were analysed using the Wilcoxon matched-pairs signed-rank test. For CRP levels below the lower limit of detection (LOD), the values were substituted with LOD/2 [Citation19]. Univariate and multivariate Cox proportional hazard regression models were used to identify predictors of golimumab discontinuation at 12 weeks and at the most recent follow-up. The proportional hazard assumption was tested visually or using the statistical significance of time-dependent covariates. The variables sex, age at baseline, disease location from the Montreal Classification at baseline, perianal disease, smoking status (current smoker or non-smoker), previous surgery, concomitant treatment with corticosteroids or immunomodulators at baseline, more than one previous anti-TNF therapy and CRP at baseline were included in the models. CRP values at baseline were divided in quarters of the distribution. To specifically examine the impact of previous failure of anti-TNF therapy, patients who were previously exposed to an anti-TNF agent were identified. The difference in proportions of patients who stopped golimumab treatment because of poor treatment response was compared by the Chi-square test, stratified by reason for discontinuation of the last anti-TNF agent. All tests were two-tailed and p-values <.05 were considered to be statistically significant. SPSS version 22 (IBM Corp., Armonk, NY) was used to perform the statistical analysis.
Ethical considerations
The study was approved by the Regional Ethics Board in Linköping, Sweden (2016/339-32).
Results
Patients
Altogether, 100 golimumab-treated CD patients were identified in SWIBREG. One patient was excluded due to conflicting data on the specific IBD diagnosis in SWIBREG. Five other patients were excluded due to a lack of follow-up data. Thus, the final study cohort consisted of 94 patients with CD who had been treated with golimumab. The demographic and clinical characteristics at baseline are summarized in . The median age at initiation of golimumab treatment was 33.5 (26.0–48.3) years, and 40 (42.6%) of the patients were men (p = .15). The great majority of the patients (96.8%) had a previous treatment failure with at least one anti-TNF agent and 36.2% had undergone CD-related surgery.
Table 1. Baseline demographics and clinical characteristics, according to the Montreal classification, of patients with Crohn’s disease who were treated with golimumab.
Clinical effectiveness at 12 weeks
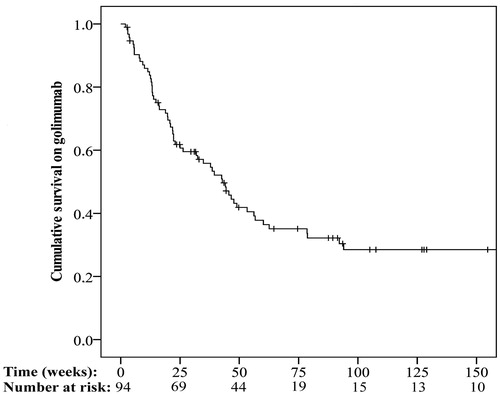
The golimumab retention rate at 12 weeks was 85.1% (). A total of 78 patients were treated for at least 12 weeks. Of these, information on CRP at baseline and at 12 weeks was available for 55 individuals (71%). Among these 55 patients, the median CRP decreased from 6.0 (1.8–16.0) mg/L to 4.4 (1.5–17.0) mg/L at 12 weeks (p = .53).
Predictors of drug discontinuation at 12 weeks
Some 14 patients discontinued treatment within 12 weeks. Predictors of golimumab discontinuation at 12 weeks are presented in . Female sex (adjusted HR = 6.59, 95% CI: 1.04–41.62; p = .05), previous surgery (adjusted HR = 7.52, 95% CI: 1.12–50.36; p = .04), and concomitant treatment with corticosteroids (adjusted HR = 5.70, 95% CI: 1.13–28.68; p = .04) were associated with an increased risk of discontinuing golimumab treatment within 12 weeks. The patients were categorized into four quarters of the CRP concentration distribution at baseline. There was not a statistically significant difference when comparing each of the quarters of the CRP distribution for the association with drug discontinuation at 12 weeks; but when the quarter with the highest CRP values (≥16 mg/L) was compared to the three others, a statistically significantly higher risk was apparent (adjusted HR = 8.35, 95% CI: 1.88–37.05; p < .01).
Table 2. Predictors of drug discontinuation at 12 weeks.
Clinical effectiveness at the most recent follow-up
The median duration of follow-ups was 89 (IQR: 32–158) weeks. The golimumab retention at the most recent follow-up was 35.1%. Patients (n = 61) who discontinued golimumab stayed on the treatment for 22 (12–44) weeks. The reasons for discontinuation were poor treatment response (n = 45, 73.8%), intolerance (n = 11, 18%), or another explanation (n = 5, 8.2%). Of the 33 patients who remained on golimumab treatment until the end of the study period, information on CRP concentrations at baseline and at the most recent follow-up were available for 28 of them (85%). There was a significant decline in median CRP from 4.9 (1.2–15.5) mg/L to 2.0 (1.0–5.2) mg/L (p < .01).
Predictors of drug discontinuation at the most recent follow up
Some 61 patients discontinued treatment during the follow-up. Predictors of golimumab discontinuation at the most recent follow-up are presented in . Both female sex (adjusted HR = 2.24, 95% CI: 1.19–4.23; p = .01) and concomitant treatment with corticosteroids (adjusted HR = 2.60, 95% CI: 1.17–5.79; p = .02) were associated with an increased risk of discontinuing golimumab treatment at the most recent follow-up. In contrast with the outcome at week 12, no association was found between CRP concentration at baseline and treatment failure in the long-term. Being in the highest quarter of the CRP distribution (≥16 mg/L) did not predict the outcome (adjusted HR = 1.58, 95% CI: 0.78–3.18; p = .20).
Table 3. Predictors of drug discontinuation at the most recent follow-up.
Notably, there was a difference in the reason for termination of golimumab between those who had stopped their last previous anti-TNF because of poor treatment response and those who had stopped due to intolerance or other reasons. Among patients who had stopped their last anti-TNF because of poor treatment response, 32/38 (84%) terminated golimumab due to lack or loss of response. Whereas the corresponding figure in those who had stopped their last anti-TNF treatment because of intolerance or other reasons was 11/21 (52%, p < .01).
Discussion
Using data from the National Quality Registry for IBD in Sweden, we have examined the clinical effectiveness of golimumab, for the first time using a national cohort of CD patients, based on prospectively recorded information. Although almost all of the patients (96.8%) had undergone anti-TNF therapy previously, 35.1% of the patients continued golimumab treatment with a median follow-up period of 89 (IQR: 32–158) weeks. The clinical effectiveness was supported by evidence of reduced inflammatory activity. Concomitant use of corticosteroids at baseline was identified as an independent risk factor for drug discontinuation in both the short term and the long term. There was an indication that female sex may also be associated with a greater likelihood of drug discontinuation. In addition, previous surgery, and a high concentration of CRP at baseline were associated with an increased risk of drug discontinuation in the short term, although the low number of events may have impaired the validity of the results in the short term.
There is an unmet need for new treatment options in patients with CD who show an inadequate response, lose their response, or are intolerant of existing treatment alternatives [Citation20]. Two large randomized, placebo-controlled, double-blind trials have assessed the efficacy of golimumab in ulcerative colitis [Citation9,Citation10], but its possible efficacy in CD remains largely unproven. Off-label use is not prohibited in Sweden, and by extracting prospectively recorded data from the national quality registry we identified a national cohort of 94 patients with CD who had been treated with golimumab. To our knowledge, there have only been four previous reports on golimumab treatment in CD. In 2016, Merras-Salmio et al. [Citation11] described the use of golimumab in six paediatric patients with CD; four of them discontinued treatment during follow-up. Another report, presented as an abstract at the Digestive Disease Week congress, described the clinical effectiveness of golimumab in nine patients with CD who had failed treatment with other biologics [Citation12]. Eight of the patients had clinical benefit after a median follow-up of 17 months (range: 3–24 months). The experience of golimumab in 45 patients at a single tertiary centre in Canada suggested that golimumab might indeed be effective in CD [Citation14]. High clinical response rates were observed at both 12 weeks (78%) and 52 weeks (81%), but interpretation of the results was complicated because the study was based on retrospective assessment of the medical records and because the clinical response was not measured using a validated instrument. Notably, the authors excluded all patients who discontinued golimumab before 3 months, which probably produced selection bias. Recently, Martineau et al. [Citation13] published a retrospective study of 115 CD patients, who had been recruited at 12 referral centres in France. The observed drug retention rate at 52 weeks was higher in the French cohort (67.1%) than the corresponding rate in our cohort (41%). This was despite the two cohorts being similar in sex distribution, disease location, behaviour, and in that both cohorts appear to represent a treatment-refractory group of patients, where most of them had previously been exposed to other anti-TNF agents. However, patients with a follow-up of less than 6 weeks or who had had less than two golimumab administrations were excluded from the French study, which might indicate the possibility of selection bias. Also, 33% of their patients were diagnosed with spondyloarthropathy, and the main reason for initiation of golimumab treatment might therefore been arthritis rather than active CD. Clinical response was defined by the Harvey–Bradshaw index, but data were available for only 38 of the 115 French patients, which further indicates that the results of the French study should be interpreted with some caution. In contrast to our study, information on CRP values was missing during follow-up in more than half of the French patients; the patients in our cohort had shorter median disease duration, lower median CRP levels, less perianal involvement, fewer had previous surgery, and a lower proportion were active smokers, which may explain the results if our patients tended to have a better outcome.
The ability to predict clinical response to a specific drug is becoming increasingly important with the number of existing treatment options, and is essential for the effective implementation of personalized medicine. Results from our analysis using Cox regression suggest that previous surgery, concomitant treatment with corticosteroids at baseline and female sex are associated with a higher risk of not responding to induction therapy with golimumab, that is, discontinuation of the drug at 12 weeks. However, the ability to predict the sustained clinical benefit of a drug over a long period might be more relevant in a chronic disease such as CD. Only, concomitant use of corticosteroid at baseline and female sex were identified as predictors of golimumab discontinuation in the longer term. The extent to which these clinical characteristics are predictive for golimumab treatment specifically or are general markers of a poor treatment response to medication can be questioned. Several studies have tried to identify factors that are predictive of a response to anti-TNF in IBD, although the results have been, at least in part, inconsistent [Citation21]. In accordance with our findings, previous surgery, and a high CRP level at initiation of treatment have previously been found to be associated with a poor response to anti-TNF treatment [Citation21–23]. The biological mechanism underlying the difference in drug retention rates in males and females is not known. A similar difference has been reported in IBD patients receiving vedolizumab, and in CD patients treated with adalimumab or infliximab [Citation24]. In theory, some women may discontinue treatment due to pregnancy, but in the present study, none of the patients stopped treatment for this reason. In contrast to our findings, Martineau et al. [Citation13] found an association between concomitant use of immunosupressors and a decreased risk of drug discontinuation. Interestingly, an association between reason for discontinuation of golimumab and cause of termination of last previous anti-TNF was observed. Patients who stopped golimumab because of lack or loss of response, had more frequently terminated their last previous anti-TNF due to the same reason, compared to those who stopped golimumab because of intolerance or other reasons.
A major strength of this study was that we included all the golimumab-treated patients with CD in the whole of Sweden, as documented in the National Quality Registry for IBD, irrespective of which hospital they were treated at. This design probably enhanced the generalisability of the study. The study was also strengthened by the fact that data were reported prospectively to the national registry (SWIBREG). In contrast to a randomized clinical trial, where the duration is predetermined by the protocol, follow-up varied between the patients in our study. This may question the generalisability of some of our findings, and it might be difficult to apply our results from the most recent follow-up to other patient populations. We used drug retention rates as a proxy for clinical effectiveness. In theory, patients who continue treatment probably have a sustained clinical benefit from the treatment; otherwise, treatment would be terminated. This may have introduced selection, since outcomes such as CRP level were only assessed in patients who continued treatment. In contrast, our cohort represents a treatment-refractory group, and therefore it is possible that some patients continued treatment even with a minor response only, simply because they had few other medical options. The study was limited by the absence of any detailed information on the Crohn’s Disease Activity Index (CDAI) or an endoscopic index, such as the Crohn’s Disease Endoscopic Index of Severity (CDEIS), recorded at standard time points during follow-up. SWIBREG does not collect information on specific adverse drug reactions represents another limitation of the study, since data on the safety of golimumab could not be reported. Another restraint was that we had to exclude five patients from analysis due to missing data. Few events were observed during the first 12 weeks, which may have impaired the validity of our findings in the short term.
Conclusion
In conclusion, the CD patients treated with golimumab in this national cohort, representing a variety of care contexts, were a treatment-refractory group. Despite this, more than one-third of the patients appeared to have sustained clinical benefit after a median follow-up of more than 1.5 years, since they continued their golimumab treatment. Concomitant treatment with corticosteroids at baseline was identified as a potential predictor of poor response to both induction therapy and long-term treatment with golimumab. Our results show that golimumab may have a valid role in the treatment of CD and indicate the need for randomized clinical trials.
Acknowledgments
We thank Malin Olsson, SWIBREG registry coordinator (at Linköping University Hospital), for assistance in the extraction of data from SWIBREG. Furthermore, we are grateful to all the patients and to the medical staff for entering information into SWIBREG. Also, we thank Anders Magnuson whose expertise greatly assisted the research.
Disclosure statement
SR and CE have received a research grant from the Swedish government’s agreement on medical training and research, and speaker’s fees from Takeda. LA has received lecturing fees from Takeda. OG has received consulting fees during the last 5 years from Ferring, Takeda, Viphor Pharma, AbbVie, and Jansen-Cilag. HH has been a consultant/on the advisory board for AbbVie, Takeda, Janssen, and Tillotts, and has given lectures for AbbVie, Takeda, Ferring, Tillotts, Falk Pharma, and Shire. PK has been a consultant/on the advisory board for AbbVie, Ferring, Otsuka, and Takeda, has given lectures for AbbVie, Ferring, Hospira, Otsuka, Takeda, and Vifor, and has been Principal Investigator for AbbVie, Amgen, Chemo-centryx, Celgene, Ferring, GSK, Jansen, MSD, Otsuka, Pfizer, Roche, and Takeda. JH has received consultant/lecture fees from AbbVie, Hospira, Medivir, Pfizer, RenapharmaVifor, Tillotts Pharma, Janssen, MSD, and Takeda, and grant support from Janssen, MSD, and Takeda. For the remaining authors, there are no conflicts of interests and no sources of funding.
References
- Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078.
- Gomollon F, Dignass A, Annese V, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohn's Colitis. 2017;11:3–25.
- Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohn's Colitis. 2013;7:827–851.
- Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2016;375:1946–1960.
- Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohn's Colitis. 2010;4:355–366.
- Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev. 2014;13:24–30.
- Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab. Ann Intern Med. 2007;146:829–838.
- Rutgeerts P, Feagan BG, Marano CW, et al. Randomised clinical trial: a placebo-controlled study of intravenous golimumab induction therapy for ulcerative colitis. Aliment Pharmacol Ther. 2015;42:504–514.
- Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85–95.
- Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:96–109.e1.
- Merras-Salmio L, Kolho KL. Golimumab therapy in six patients with severe pediatric onset Crohn disease. J Pediatr Gastroenterol Nutr. 2016;63:344–347.
- Ben-Bassat O, Iacono A, Irwin SP, et al. Tu1327a golimumab for treatment of moderate to severe anti-TNF refaractory Crohn's disease: open label experience. Gastroenterology. 2012;142:S-804.
- Martineau C, Flourie B, Wils P, et al. Efficacy and safety of golimumab in Crohn's disease: a French national retrospective study. Aliment Pharmacol Ther. 2017;46(11–12):1077–1084.
- Greener T, Boland K, Steinhart AH, et al. The unfinished symphony: golimumab therapy for anti-TNF refractory Crohn's disease. J Crohn's Colitis. 2018;12(4):458–464.
- Busch K, Ludvigsson JF, Ekstrom-Smedby K, et al. Nationwide prevalence of inflammatory bowel disease in Sweden: a population-based register study. Aliment Pharmacol Ther. 2014;39:57–68.
- SWIBREG: Swedish Inflammatory Bowel Disease Registry [cited 2018 April 13]. Available from: http://www.swibreg.se/
- Ludvigsson JF, Myrelid P. [Swibreg–a new version of national IBD registry]. Lakartidningen. 2009;106:3014–3015. Swedish.
- Jakobsson GL, Sternegard E, Olen O, et al. Validating inflammatory bowel disease (IBD) in the Swedish National Patient Register and the Swedish Quality Register for IBD (SWIBREG). Scand J Gastroenterol. 2017;52:216–221.
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51.
- Gordon JP, McEwan PC, Maguire A, et al. Characterizing unmet medical need and the potential role of new biologic treatment options in patients with ulcerative colitis and Crohn's disease: a systematic review and clinician surveys. Eur J Gastroenterol Hepatol. 2015;27:804–812.
- Lopetuso LR, Gerardi V, Papa V, et al. Can we predict the efficacy of anti-TNF-alpha agents? Ijms. 2017;18:1973.
- Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn's disease - algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30–51.
- Narula N, Kainz S, Petritsch W, et al. The efficacy and safety of either infliximab or adalimumab in 362 patients with anti-TNF-alpha naive Crohn's disease. Aliment Pharmacol Ther. 2016;44:170–180.
- Cosnes J, Sokol H, Bourrier A, et al. Adalimumab or infliximab as monotherapy, or in combination with an immunomodulator, in the treatment of Crohn's disease. Aliment Pharmacol Ther. 2016;44:1102–1113.