Abstract
Background and aim: Progression to fibrosis in non-alcoholic fatty liver disease (NAFLD) is associated with an increased risk of liver-related events, overall mortality and possibly metabolic comorbidities. Our aim was to determine if non-invasive fibrosis scoring systems can predict the future risk of diabetes mellitus, cardiovascular disease (CVD), chronic kidney disease (CKD), liver-related events and overall mortality.
Methods: Patients with biopsy-proven NAFLD 1978 to 2006 were identified from a computerised register in Malmö, Sweden. Medical records were scrutinised in detail to collect data from inclusion to endpoint (death or end of 2016). Non-invasive fibrosis scoring systems (FIB-4-index, NAFLD fibrosis score (NFS), APRI and BARD score) were calculated and the scores classified into three risk categories (low, intermediate and high risk for advanced fibrosis). Chronic kidney disease was evaluated using the CKD-EPI equation.
Results: One hundred and forty-four patients with biopsy-proven NAFLD were included, with a mean age of 53.2 years and a mean follow-up time of 18.8 years. At inclusion, 18% had advanced fibrosis. NFS was the only score that could predict the future risk of all included outcomes with fairly good accuracy (Area-under-ROC curve). Multivariate-adjusted hazard ratios revealed that both the intermediate and high-risk category of FIB-4-index and NFS could significantly predict metabolic outcomes. All four scoring systems significantly predicted overall mortality in the high-risk category.
Conclusions: Non-invasive fibrosis scoring systems, especially NFS and FIB-4-index, can be used to identify patients at risk of future liver-related events, overall mortality, metabolic comorbidities and CKD.
Introduction
Non-alcoholic fatty liver disease (NAFLD), currently the most common chronic liver disease in the Western world, is associated with aspects of the metabolic syndrome. Insulin resistance seems to be the link between NAFLD and the metabolic syndrome. In parallel with the increasing prevalence of obesity, the prevalence rate of NAFLD is also increasing and is now around 20% in Europe [Citation1]. The histological spectrum ranges from simple steatosis (non-alcoholic fatty liver, NAFL), via non-alcoholic steatohepatitis (NASH) with hepatocyte injury and inflammation, to fibrosis and cirrhosis. The disease is highly heterogeneous, with uncertainties regarding the natural history of the disease and with difficulties in predicting progressors to end-stage liver disease. A subgroup of patients with NAFL can progress to advanced fibrosis, influenced by environmental and genetic factors [Citation2]. Predictors of moderate to severe fibrosis include older age, male sex, increased aspartate aminotransaminase (AST) and alanine aminotransferase (ALT) levels, diabetes mellitus and hypertension [Citation3]. Few studies have included paired biopsies to evaluate progression rate. In one study, 42% had progression of fibrosis after a median follow-up time of 6.6 years, which was significantly associated with diabetes mellitus [Citation4]. Progression to fibrosis is associated with increased mortality compared to the general population, especially from cardiovascular disease (CVD), but also liver-related mortality. Fibrosis is the strongest predictor of mortality in these patients [Citation5,Citation6].
Liver biopsy or elastography (including magnetic resonance elastography or transient elastography) are different methods to evaluate the level of fibrosis. However, for practical and economic reasons several non-invasive scoring systems have been developed to evaluate fibrosis. These scoring systems incorporate simple laboratory test and clinical parameters. The most commonly used are the FIB-4-index [Citation7], NAFLD fibrosis score (NFS) [Citation8], Body Mass Index, AST/ALT ratio, Diabetes score (BARD) [Citation9] and AST/platelet ratio index (APRI) [Citation10]. Numerous previous studies have validated these tests and they accurately distinguish between patients with no fibrosis and significant fibrosis, typically with a higher negative predictive value (NPV) than positive predictive value (PPV) [Citation11–13]. One previous study showed that these non-invasive scoring systems can be used to predict future liver-related events and mortality in NAFLD patients [Citation14].
Considering the global epidemic of NAFLD there is a need for a simple diagnostic tool for early identification of the sub-group of patients who are at risk of future progression to fibrosis, development of liver-related complications and increased risk of mortality, and also associated metabolic comorbidities.
The aim of our study was to determine if non-invasive fibrosis scoring systems could identify NAFLD patients at risk of future metabolic comorbidities, liver-related events and overall mortality.
Subjects and methods
Study population
Patients were retrospectively selected from a computerised liver biopsy register at Skåne University Hospital in Malmö, Sweden, between 1978 and 2006. Malmö, with ∼250,000–300,000 inhabitants at this time, is the third largest city in Sweden. All biopsies were undertaken for clinical reasons, i.e., elevated liver function tests or pathological findings on imaging. The register includes clinical and histological diagnoses assessed by hepatologists and pathologists, and laboratory tests at the time of biopsy. Liver biopsies were routinely stained at the local pathology laboratory and were read by a pathologist. The stage of fibrosis (stage 0 to stage 4) was recorded based on the scoring system proposed by Batts-Ludwig [Citation15,Citation16]. Hepatitis C (HCV) was retrospectively excluded in all subjects who underwent biopsy 1978 through 1989, before the availability of reagents for detecting HCV antibodies [Citation17]. Assessment of alcohol intake as a cause of liver disease was done by a hepatologist, including review of patients’ medical records, laboratory tests and histological features. Patients with biopsy-proven primary NAFLD without liver disease of other aetiologies were included in the study.
Hospital medical records were scrutinised in detail to collect all available data from inclusion (time of liver biopsy) until endpoint (death or end of 2016). The intention was to find anthropometrics, metabolic diagnoses including type 2 diabetes mellitus, hypertension (medication or blood pressure ≥140/90 mm Hg) and CVD (ischaemic heart disease i.e., angina pectoris, myocardial infarction, and ischaemic stroke), and diagnosis of cirrhosis and liver-related events (hepatocellular cancer (HCC), ascites, encephalopathy and variceal bleeding) any time during the entire study period, and to exclude secondary causes of NAFLD. Body mass index (BMI) was calculated and BMI 25–30 kg/m2 was defined as overweight and ≥30 kg/m2 as obesity. Chronic kidney disease (CKD) was evaluated using the CKD-EPI formula, where an estimated glomerular filtration rate (eGFR)<60 mL/min/1.73 m2 is defined as CKD [Citation18].
If medical records could not be retrieved, if follow-up time was less than one year, or if emigration or unknown vital status was present, patients were excluded from statistical analyses.
Non-invasive fibrosis scoring systems
Four validated non-invasive scoring systems were calculated using the original equations [Citation7–10]. FIB-4-index: age (years) × AST (U/L)/platelets (×109/L)×√ALT (U/L). NFS: −1.675 + 0.037 × age (years)+0.094 × BMI (kg/m2)+1.13 × impaired fasting glucose or diabetes (yes = 1, no = 0)+0.99 × AST/ALT ratio – 0.013 × platelets (×109/L) – 0.66 × albumin (g/dL). APRI: ((AST (U/L)/upper limit of normal)/platelets (×109/L))×100. BARD score: Scale 0–4, BMI ≥28 kg/m2 = 1 point, AST to ALT ratio ≥0.8 = 2 points, Diabetes Mellitus = 1 point.
The FIB-4-index, NFS and APRI scores were classified into three risk categories (low, intermediate and high) according to cut-points described in the original publications. They are 1.30 and 2.67 for FIB-4-index; −1.455 and 0.676 for NFS; and 0.5 and 1.5 for APRI. In the original BARD only two categories (low risk: 0–1 points, and high risk: 2–4 points) were described. To compare against the above-mentioned scores we, therefore, classified BARD as low: 0–1 points, intermediate: 2 points and high risk: 3–4 points.
Statistics
Statistical analyses were performed using SPSS software (IBM SPSS Statistics version 24). Categorical data are presented as number (percentage), and continuous data as mean ± standard deviation (SD) and median (IQR, interquartile range). The chi-square test calculated the differences in outcome (diabetes mellitus, CVD, CKD, liver-related events and overall mortality) between the three risk categories (low, intermediate and high-risk of advanced fibrosis) of the non-invasive fibrosis scoring systems. The Kruskal–Wallis’ test calculated differences in fibrosis score between fibrosis stage 0, 1–2 and 3–4. ROC curves separately for the four fibrosis scoring systems were created, and Area-under-ROC curve (AUROC) calculated for all included outcomes. The Kaplan–Meier method was used to construct crude unadjusted survival curves, with log-rank test for comparison, between the three risk categories of the different fibrosis scoring systems. Cox regression models (univariate and multivariate) calculated the association between fibrosis score and outcomes, with estimates presented as hazard ratios (HRs). The Cox regression models were adjusted for variables not already included in the equations, including fibrosis stage. A p-value of <.05 was considered statistically significant.
Ethical considerations
The study was approved by the Ethics Committee at Lund University (2016/518).
Results
Patient selection
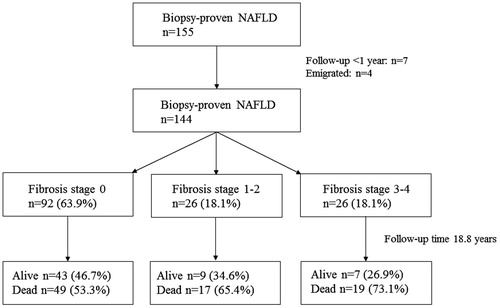
In all, 155 patients with biopsy-proven primary NAFLD between 1978 and 2006 were initially included. Out of the 155 patients, 11 were lost in follow-up, therefore 144 were analysed further for this study (). Mean age at biopsy was 53.2 years, mean follow-up time 18.8 years and the majority of patients were men (). At inclusion, 63.9% (n = 92) had simple steatosis histologically and 18.1% (n = 26) had advanced fibrosis (stage 3–4). At follow-up, a total of 16.7% (n = 24) of the entire cohort had been diagnosed with cirrhosis, and of these, the majority had advanced fibrosis at baseline (75%, n = 18). In addition, 5.6% (n = 8) of the entire cohort had been diagnosed with hepatocellular cancer (HCC) and 2.8% (n = 4) had a liver transplant.
Table 1. Patients’ characteristics at baseline and follow-up.
Non-invasive fibrosis scoring systems
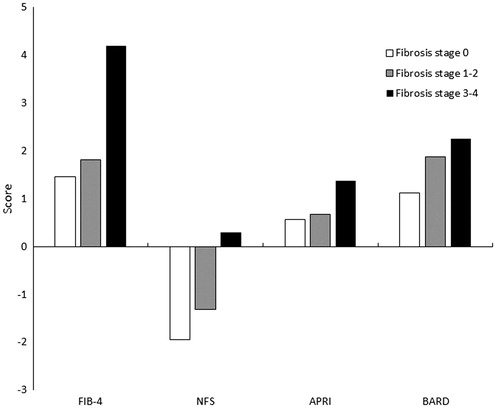
There were significant differences in all four fibrosis scoring systems between fibrosis stage 0, 1–2 and 3–4 (). In accordance with previous studies, all four scoring systems had a higher NPV than PPV, meaning that they perform better in excluding advanced fibrosis. FIB-4-index had the highest NPV (91%) and APRI the highest PPV (71%). The AUROC for FIB-4-index, NFS and APRI acceptably predicted advanced fibrosis with values between 0.81–0.86, for BARD 0.69 (data not shown).
Metabolic complications
At inclusion 73.6% (n = 106) were overweight, the most common metabolic disorder in this group (). In addition, 45.8% (n = 66) had been diagnosed with hypertension, 22.2% (n = 32) with diabetes mellitus and 11.8% (n = 17) with cardiovascular disease. Patients with advanced fibrosis histologically (stage 3–4) had significantly higher prevalence of diabetes mellitus at inclusion compared to those with fibrosis stage 0–2 (Chi-square test, p = .007). Information and laboratory tests regarding hyperlipidaemia were scarce.
NFS was the only score when calculating AUROC, that significantly predicted all included non-hepatic complications at follow-up (diabetes mellitus, CVD and CKD), with moderately good accuracy ().
Table 2. Area under the ROC curve for non-invasive scoring systems in predicting outcomes (AUROC ± SE (95% CI)).
Multivariate-adjusted Cox regression analyses showed that both the intermediate and high-risk category of FIB-4-index and NFS significantly predicted all included non-hepatic complications (). Only the high-risk group of APRI could predict these complications and for BARD the results were not significant.
Table 3. Multivariate-adjusted Hazard Ratios (HR) for metabolic complications at follow-up according to risk categories in fibrosis scoring systems (HR (95% CI), p-value).
Liver disease
In all, 13.9% (n = 20) of patients had liver-related events at follow-up, the most common complication being ascites in 9% (n = 13) of patients. The AUROC showed that FIB-4-index, NFS and APRI, but not BARD, significantly predicted liver-related events with moderately good accuracy (). Multivariate-adjusted Cox regression analyses revealed significantly higher hazard ratios in the high-risk category of FIB-4-index, NFS and APRI in predicting liver-related events ().
Table 4. Multivariate-adjusted Hazard Ratios (HR) for overall mortality and liver-related events according to risk categories in fibrosis scoring systems (HR (95% CI), p-value).
Survival
At follow-up, 59% (n = 85) patients had died. The most common primary causes of death were CVD in 39%, non-hepatic malignancies in 18%, infectious disease in 16% and end-stage liver disease including HCC in 11%.
The unadjusted cumulative survival probability using the Kaplan-Meier method comparing the risk categories (low vs. intermediate, low vs. high and intermediate vs. high-risk), showed that all four non-invasive scoring systems significantly predicted overall mortality in the high-risk group (FIB-4: p < .001, <.001 and .003. NFS: p < .001, <.001 and <.001. APRI: p = .905, <.001 and .001. BARD: p = .001, <.001 and .036).
The AUROC showed that FIB-4-index and NFS seem to predict overall mortality better than the other scoring systems ().
Multivariate-adjusted hazard ratios for overall mortality were significantly different between the three risk categories for FIB-4-index and NFS (). For APRI only the highest risk category was significant and for BARD no significant difference was found.
Discussion
In this prospective study of biopsy-proven NAFLD, non-invasive fibrosis scoring systems significantly predicted future liver-related events and overall mortality, as previously shown, and also future metabolic comorbidities including diabetes mellitus, cardiovascular disease and chronic kidney disease.
The strengths of this study is a long-term follow-up, since NAFLD in many cases is a disease with a slow progression rate. All included patients had biopsy-proven NAFLD, which validates the use of the non-invasive fibrosis scoring systems. Extensive review of patients’ medical records, including both inpatient and outpatient visits, enabled us to find information regarding metabolic complications and chronic kidney disease which in several cases would not have been found in a register.
Guidelines recommend the use of non-invasive fibrosis scoring systems in established NAFLD to identify patients for specialist referral [Citation1]. They can be used as a screening tool to identify patients in need of liver biopsy, i.e., in the intermediate risk group [Citation19]. Our results indicate that, even after adjusting for fibrosis stage, patients in both the intermediate and high-risk category of FIB-4-index and NFS run an increased risk of future diabetes mellitus, CVD and CKD, and an increased risk of overall mortality, most commonly from CVD. This indicates that patients in the intermediate and high-risk category, despite fibrosis stage, should regularly be examined with the intention to find metabolic complications and chronic kidney disease.
Progression of NAFLD to advanced fibrosis increases the risk of liver-related events and overall mortality [Citation5,Citation6]. Patients with NAFLD have an increased risk of incident diabetes mellitus compared to individuals without NAFLD, when adjusting for potentially confounding factors, a risk that seems higher in advanced liver disease [Citation20]. NAFLD is also clearly associated with an increased risk of CVD morbidity and mortality, independent of established CVD risk factors, although it is not entirely clear if this risk increases further in more advanced liver disease [Citation2,Citation21]. Previous studies have shown that non-invasive fibrosis scoring systems can predict future liver-related events and overall mortality [Citation14,Citation22]. They can also predict prevalent CKD [Citation23,Citation24]. One study with a shorter follow-up time showed a significant correlation between a high score of NFS and FIB-4-index, and incident diabetes mellitus, cardiovascular and cerebral disease [Citation25]. We could confirm the significant correlation between the scores and incident diabetes mellitus, as well as with cardiovascular disease (data not shown). However, no previous study has investigated the use of non-invasive fibrosis scoring systems in predicting the future risk of metabolic comorbidities and CKD.
There are, however, several limitations to this study. NAFLD and fibrosis stage were only assessed at baseline, as was the calculation of the non-invasive fibrosis scoring systems. We had, therefore, no information regarding fibrosis progression or changes of the scores over time. Among all the patients who were diagnosed with cirrhosis during follow-up, 25% did not have advanced fibrosis at inclusion, probably reflecting the long follow-up time. Between 3–9% with low-risk scores developed liver-related events at follow-up. Only calculating these scores once will, therefore, lead to a misclassification of the future risk. The results are therefore probably an underestimate of the predictive capacity of these scoring systems.
Inclusion of patients in our study was between 1978 and 2006, and end-point death or end of 2016. This results in various follow-up time, and there were significant differences in age at baseline and follow-up time between patients who died and those still alive (data not shown). Both NFS and FIB-4-index takes age into account, which could be an explanation why these two scoring systems performed better in the ROC analyses. In the multivariate regression analyses, we adjusted for age when calculating adjusted HR for APRI and BARD.
In line with a previous study, NFS and FIB-4-index seem to perform better regarding liver-related events and total mortality [Citation14]. In that study, 51% had advanced fibrosis, compared to 18% in our study, which is higher than in the general NAFLD population. Therefore, the results cannot be directly extrapolated to a primary care setting.
The observation that non-invasive scoring systems can predict these outcomes, despite adjusting for fibrosis stage, can only be explained by the variables included in the equations. Moderate to severe fibrosis is associated with older age, male sex, higher AST and ALT, diabetes mellitus and hypertension [Citation3]. These equations incorporate factors associated with fibrosis development (for example age, AST and ALT, diabetes mellitus), and also factors associated with cirrhosis (for example albumin and platelets). APRI includes fewer variables and BARD is a simple score with no variable reflecting cirrhosis. The prevalence of metabolic comorbidities also increases with older age and possibly in more advanced NAFLD.
Our primary intention was not to compare the different scoring systems. As mentioned above FIB-4-index and NFS performed similarly and better in the predictive capacity of the included outcomes. Although significant results were obtained, these scoring systems only predicted the included outcomes with moderately good accuracy regarding liver-related events and overall mortality, and only with fairly good accuracy regarding metabolic comorbidities. Calculating NFS and/or FIB-4-index at regular intervals, not only at baseline, will probably increase the predictive capacity. However, it indicates a possibility for an early assessment of NAFLD patients at risk of future complications. Future prospective studies involving a large group of NAFLD patients, comparing or combining different scoring systems, which could include more complex models with direct fibrosis markers, or creating a new score incorporating both factors associated with future fibrosis and cirrhosis, and regularly calculate these scoring systems over a longer period of time, are warranted. An ideal scoring system needs to be well-validated, easy to use, inexpensive and with a high predictive capacity of specific outcomes. Our results indicate that especially NFS and FIB-4-index seem to perform better than the other included scoring systems in predicting future complications, and can today be used in the assessment of NAFLD in a clinical setting, especially regarding future liver-related and overall mortality, and to some extent metabolic comorbidities. One suggested option is to monitor patients with scores indicating intermediate and high risk of fibrosis at closer intervals than patients with low risk. The recommended intervals for these categories are beyond the scope of this paper and needs to be established in future studies.
In conclusion, non-invasive fibrosis scoring systems, especially NFS and FIB-4-index, can with moderately good predictive capacity early identify NAFLD patients at risk of future liver-related events, overall mortality, and to some extent diabetes mellitus, cardiovascular disease and chronic kidney disease.
Acknowledgements
We would like to thank all the participating patients.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- European Association for the Study of the, L., D. European association for the study of, and O. European association for the study of, EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402.
- Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–344.
- Hossain N, Afendy A, Stepanova M, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(11):1224–1229.
- McPherson S, Hardy T, Henderson E, et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148–1155.
- Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554.
- Hagstrom H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265–1273.
- Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325.
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854.
- Harrison SA, Oliver D, Arnold HL, et al. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447.
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526.
- Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112.
- Demir M, Lang S, Nierhoff D, et al. Stepwise combination of simple noninvasive fibrosis scoring systems increases diagnostic accuracy in nonalcoholic fatty liver disease. J Clin Gastroenterol. 2013;47:719–726.
- Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649.
- Angulo P, Bugianesi E, Bjornsson ES, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782–789 e4.
- Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417.
- Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47:598–607.
- Verbaan H, Widell A, Lindgren S, et al. Hepatitis C in chronic liver disease: an epidemiological study based on 566 consecutive patients undergoing liver biopsy during a 10-year period. J Intern Med. 1992;232:33–42.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- Stal P. Liver fibrosis in non-alcoholic fatty liver disease - diagnostic challenge with prognostic significance. World J Gastroenterol. 2015;21:11077–11087.
- Mantovani A, Byrne CD, Bonora E, et al. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care. 2018;41:372–382.
- Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64.
- Unalp-Arida A, Ruhl CE. Liver fibrosis scores predict liver disease mortality in the United States population. Hepatology. 2017;66:84–95.
- Xu H-W, Hsu Y-C, Chang C-H, et al. High FIB-4 index as an independent risk factor of prevalent chronic kidney disease in patients with nonalcoholic fatty liver disease. Hepatol Int. 2016;10:340–346.
- Wijarnpreecha K, Thongprayoon C, Scribani M, et al. Noninvasive fibrosis markers and chronic kidney disease among adults with nonalcoholic fatty liver in USA. Eur J Gastroenterol Hepatol. 2018;30:404–410.
- Takahashi Y, Kurosaki M, Tamaki N, et al. Non-alcoholic fatty liver disease fibrosis score and FIB-4 scoring system could identify patients at risk of systemic complications. Hepatol Res. 2015;45:667–675.