Abstract
Background: Ustekinumab (UST), a human anti-IL12/23p40 monoclonal antibody, has been approved for treatment of Crohn’s Disease (CD) since the end of 2016. This nationwide noninterventional, retrospective chart review explored real-life data in patients receiving UST to provide guidance in UST treatment in the era of increasing prevalence of CD.
Methods: The study assessed UST treatment patterns such as dosing frequency, concomitant medication and persistence in 48 CD patients commencing UST therapy in 12 Finnish hospitals during 2017. Clinical remission and response rates were explored using a modified Harvey–Bradshaw index (mHBI) and endoscopic response via the simple endoscopic score for Crohn’s disease (SES-CD) as proportions of patients at week 16 and at the end of follow-up.
Results: Forty patients (83%) continued UST-treatment at the end of follow-up. At week 16, clinical response and endoscopic healing was observed, where data were available; mHBI decreased from 9 to 3 (p = .0001) and SES-CD from 12 to 3 (p = .009). Clinical benefit was achieved by 83% (19/23) at week 16 and by 76% (16/21) at the end of follow-up. The proportion of patients using corticosteroids decreased from 48% to 25% at week 16 and to 13% at the end of the follow-up.
Conclusion: UST showed to be effective and persistent, inducing short-term clinical benefit and endoscopic response in this real-life nationwide study of CD patients. Significant corticosteroid tapering in patients with highly treatment refractory and long-standing CD was observed.
Introduction
Crohn’s Disease (CD) is a chronic, incurable inflammatory bowel disease (IBD) that can lead to irreversible damage of the intestine and disability. Current therapies aim for a deep and prolonged remission with the goal of preventing complications and halting the progressive course of disease [Citation1]. The treatment of CD with thiopurines and methotrexate is often insufficient or limited by adverse effects [Citation2]. The use of monoclonal antibody against TNF has improved treatment outcomes remarkably [Citation3], but still a considerable number of patients either fail to respond or lose response over time [Citation4]. For these patients with refractory disease, medical agents with other modes of action than TNF-inhibition provide new and promising treatment options.
Ustekinumab (UST) is a fully human monoclonal IgG1k antibody directed against the p40 subunit of interleukin (IL)-12 and IL-23. The treatment with UST has been reported to induce and maintain remission in patients with CD in randomised controlled phase II (CERTIFI) and III clinical trials (UNITI 1 and 2, IM-UNITI) [Citation5–8]. The efficacy of UST has been proven in both TNF-inhibitor naïve and in TNF-inhibitor experienced patients, with a better response in bio-naïve patients [Citation5].
At the end of 2016 UST was approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of moderate or severe CD [Citation9,Citation10], using intravenous induction and subcutaneous maintenance with either every 8- (for nonresponders) or every 12-week dosing (EMEA/H/C958/X/49) [Citation9]. The dosing regimen with one intravenous dose and subsequent subcutaneous dosing differs from the dosing of other biologics used for the treatment of CD. This dosing regimen is based on large clinical trials with highly selected patient populations [Citation5]. Prior to UST approval for CD patients, access to UST outside of clinical trials was limited only to compassionate use programs. Therefore, in previously published open-label studies, only subcutaneous administration of UST, available due to its previous approval for the treatment of psoriasis, with a variety of different dosing regimens was used [Citation11–18]. Only recently, a prospective real-life study of intravenous induced UST-treated patients was published [Citation19].
Although the efficacy and safety of UST has been shown in clinical trials, more knowledge on patient outcomes with the approved dosing regimen [Citation20] in real-life settings is needed to help guide clinicians treating CD patients. In current study, we describe our experience with UST in a real-life nationwide cohort of CD patients in Finland.
Patients and methods
Study population and data collection
The FINUSTE study, including data from 12 Finnish hospitals, was a nationwide retrospective observational, noninterventional patient chart review on adults (≥18 years) diagnosed with CD (International Classification of Diseases, 10th revision; ICD-10: K50 all subclasses), and who initiated intravenous UST treatment during 2017. The data were collected from health records in an electronic standardized health questionnaire by the local gastroenterologist at each hospital. UST treated patients were included regardless of their previous treatments with conventional therapies or biological agents. Patients diagnosed with ulcerative colitis or IBD-unclassified (ICD-10: K51 all subclasses) or other specified noninfective gastroenteritis and colitis (ICD-10: K52 all subclasses) were excluded.
Data were collected at baseline, at week 16 (±4 weeks) and at the end of follow-up (April 30, 2018). Collected baseline data on clinical characteristics and demographics were: age, sex, smoking status, height, weight, diagnosis, year of diagnosis, history of bowel surgery, comorbidities (psoriasis, ankylosing spondylitis, hidradenitis suppurativa), age at diagnosis, disease location and behaviour according to the Montreal classification, and clinically relevant medication for CD. As a part of the clinical routine follow-up haemoglobin, leukocytes, platelets, albumin, serum C-reactive protein (CRP) and faecal calprotectin (fCal) were assessed and these data were collected retrospectively at baseline, around week 16 and at the end of follow-up. For fCal values considered as normal were <100 μg/g of stool [Citation21]. If the investigators encountered notations related to adverse events possibly, probably or very likely attributed to UST while reviewing the patient charts within the scope of the information required by the protocol, they were instructed to report these separately and directly to Janssen-Cilag Oy due to the pharmacovigilance obligations of drug manufacturers.
Outcome measures
The analyzed UST treatment patterns included doses at induction and dosing interval during the maintenance phase of treatment. The proportion of patients discontinuing UST treatment, the reason for discontinuing and the use of concomitant drugs were determined. The percentage of patients without concurrent corticosteroid medication and the percentage of patients using UST monotherapy was calculated.
Short-term clinical outcome was evaluated using clinical and endoscopic disease activity scores. For assessment of clinical disease activity, a modified Harvey–Bradshaw index (mHBI) excluding findings in abdominal palpation–variable was used [Citation22], as omitting the abdominal palpation has previously shown not to make any difference [Citation23]. Clinical remission was defined as mHBI ≤4 points and clinical response as an mHBI reduction of ≥3 points from baseline [Citation24]. Clinical benefit was determined as the proportion of patients in remission and/or with response. The simple endoscopic score for Crohn’s disease (SES-CD) served for assessment of endoscopic CD activity [Citation25].
Statistical analysis
The descriptive findings were reported as mean and standard deviation (SD) for continuous variables, median and interquartile range (IQR) for laboratory measures and clinical outcomes and as proportions for categorical variables. In addition, changes over time in fCal, CRP, mHBI and SES-CD were described. The results were reported for patients still using UST at each time point. In the analyses, UST use was considered to continue if the date of UST discontinuance was less than eight weeks from the assessment time point. The significance of the observed changes was tested with the test of proportions for categorical variables and Wilcoxon matched-pairs signed-rank test for changes in clinical outcomes and laboratory measurements. Results having a p-value lower than .05 were considered statistically significant. All analyses were performed with Stata MP 14 statistical software (StataCorp 2015, Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Ethical considerations
The study was registered in the European Union electronic Register of Post-Authorization Studies (EU PAS Register, EUPAS 24728). The study protocol was reviewed by the ethics committee of Tampere University Hospital (No: R18055) and approved by the local register holders.
Results
Patient characteristics
Fifteen Finnish hospitals identified as centers using intravenous UST induction for CD treatment were invited to participate in this study. Of these, 12 hospitals participated, including three university and nine central hospitals with a geographically representative coverage of Finland (). The study population consisted of 48 patients. Patient characteristics and treatment history are presented in and , respectively. According to the Montreal classification of disease behaviour, 29% had luminal, 52% stricturing and 19% penetrating disease. Approximately one third of patients had also perianal CD.
Figure 1. Map presenting study participants at represented hospital district in Finland including the number of patients treated with ustekinumab.
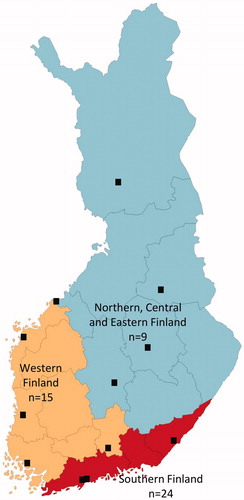
Table 1. Baseline patient characteristics and phenotype.
Table 2. Treatment history and concomitant drug use at baseline.
Of 48 patients, 46 (96%) had been treated with at least one biologic drug before initiation of UST. Of these 46 patients, 34 (71%) had experienced two or three prior biologic agents. depicts drug treatment history of the patients in more detail.
The most common reason to initiate UST treatment was nonresponse to previous biologic treatment (90%). Other reasons were side effects of prior biologic drugs (31%) or intolerability or inefficacy of immunomodulators (40%). In less than 10% of patients the reported reasons for choosing UST were psoriasis, cardiomyopathy, or unspecified contraindications to TNF-inhibitors.
Dosing and treatment persistence
UST intravenous induction dose was administered according to the EU SmPC 6 mg/kg in 130 mg steps and mean UST dose was 5.6 mg/kg (SD 0.8). All 48 patients received at least one 90 mg subcutaneous dose of UST following the intravenous induction. A vast majority (46 patients, 96%) received the first subcutaneous dose 8 weeks after intravenous induction whereas the remaining two patients received that dose 12 weeks after the intravenous induction.
After induction with one intravenous and one subcutaneous UST dose, most patients (n = 42, 88%) continued UST maintenance treatment (). Of these 42 patients, 29 (69%) received UST 90 mg subcutaneously every 8 weeks and 13 (31%) 90 mg subcutaneously every 12 weeks. Due to insufficient response, 5 patients (12%) needed shortening of the dosing interval either to 8, 6 or 4 weeks. This dose adjustment occurred on average 8 months (median 5.5 months) after treatment initiation.
Figure 2. Flow-chart of ustekinumab (UST) treatment in the FINUSTE study population. A total of 48 Crohn’s disease patients received intravenous (iv) UST treatment at induction. Six patients (12.5%) lacked primary response or discontinued for other reasons. Forty-two patients (87.5%) continued UST treatment at 16 weeks. Two patients (4.2%) discontinued treatment due to lack of response after 16 weeks. Forty patients (83.3%) maintained UST at the end of follow-up, including 5 UST dose intensified patients and 35 not intensified.
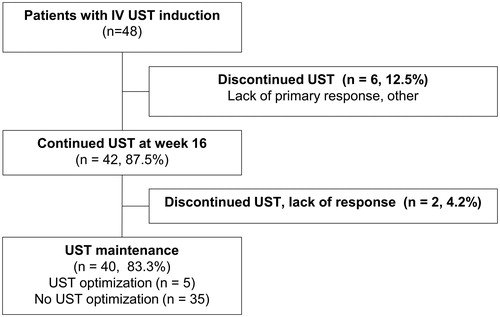
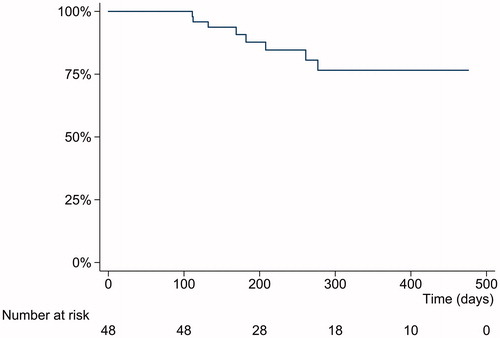
At the end of the follow-up, 40 patients (83%) persisted on UST therapy ( and ). The reasons for discontinuation of UST were lack of response, infection, pregnancy and possible allergic reaction with unclear association to UST ().
All patients were followed for at least 16 weeks and were, therefore, assessed at the 16 week timepoint. End of follow-up data were available for 37 patients and 32 of them were assessed at the end of follow-up based on continued UST use.
Concomitant drugs
At baseline, 35 patients of 48 (73%), and at 16 weeks, 27 patients of 48 (56%) used concomitant drugs for treatment of CD (, ). At the end of follow-up, concomitant medication occurred in 15 patients of those 32 with continued UST use (47%). Subsequently, the proportion of patients receiving UST as monotherapy doubled from induction to the end of follow-up.
Figure 4. Concomitant drug use during ustekinumab treatment for Crohn’s disease (CD) at baseline, at 16 weeks, and at end of follow-up. Given are the total number of patients still using UST and the proportion of patients for the relevant CD treatment at specific time period. In parenthesis are the numbers of patients with available data. *Indicates statistically significant difference compared to baseline; p < .05.
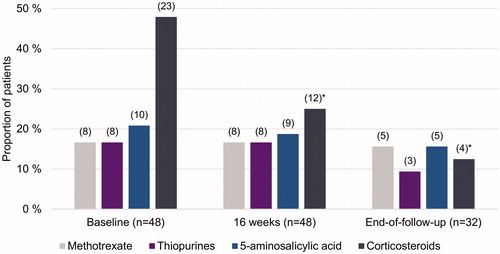
Importantly, after UST initiation the use of corticosteroids decreased from 48% (23/48 patients) to 25% (12/48 patients) in 16 weeks. By the end of follow-up, the use of corticosteroids in the study population had decreased significantly (p = .001; 4/32 patients, 13%) and 88% of those patients continuing UST therapy were steroid-free ().
Clinical effectiveness
Clinical outcome
Modified HBI decreased significantly from median of 9 (IQR 3–13; n = 35) at baseline to 3 (IQR 1–7) at 16 weeks (n = 24, p = .0001) and to 4 (IQR 0–8) at the end of follow-up (n = 21, p = .001; , ).
Figure 5. Box and whiskers plot showing upper-lower extreme and median changes in (a) the Simple Endoscopic Score for Crohn’s disease (SES-CD), (b) modified Harvey–Bradshaw index (mHBI), (c) faecal calprotectin (fCal) and (d) C-reactive protein (CRP), at baseline, at 16 weeks and at end of follow-up of Crohn’s disease patients treated with ustekinumab. In parenthesis are the numbers of patients with available data at each timepoint. *Indicate statistically significant difference compared to baseline; p < .05.
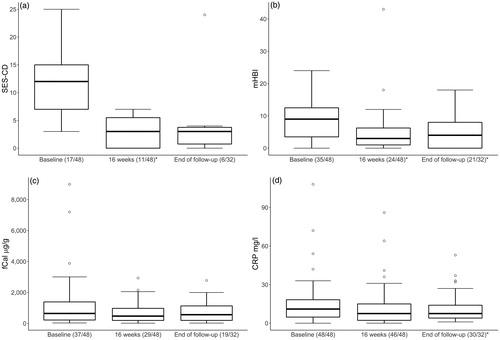
Table 3. Clinical parameters during the study follow-up among UST users: median, (IQR, 25th–75th percentile).
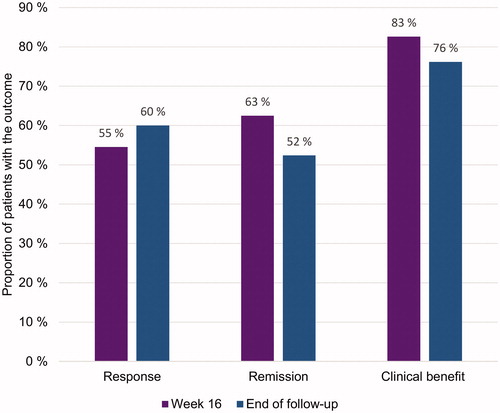
At 16 weeks, 63% of patients (15/24), and at the end of follow-up 52% (11/21 patients) were in clinical remission. Furthermore, clinical response was achieved by 55% (12/22) of patients at week 16 and by 60% (12/20) at the end of follow-up. Clinical benefit, defined as either a response or remission, was achieved by 83% (19/23) at week 16 and by 76% (16/21) at the end of follow-up ().
Biological markers
The results of measured laboratory tests at baseline, around 16 weeks and at the end of the follow-up are presented in . Compared to baseline, there was a statistically significant decrease in median serum CRP at the end of follow-up (). Furthermore, compared to baseline, blood leukocyte count decreased significantly around 16 weeks. Modest changes in median fCal from baseline (643 μg/g; IQR 219–1390) to 16 weeks (472 μg/g; IQR 194–974) or end of follow up (561 μg/g; IQR 118–1150) failed to reach statistical significance (, ).
Endoscopic healing
Endoscopic data and scoring of the SES-CD were available for a small subgroup of study patients (at baseline n = 17, at 16 weeks n = 11, at the end of follow-up n = 6). The median SES-CD decreased significantly from 12 (IQR 7–15) to 3 (IQR 0–6; p = .002) at 16 weeks. At the end of follow-up median SES-CD was 3 (IQR 0–4), but this change could not show statistical significance (p = .09, , ).
Adverse events
During the study period four cases of potential adverse events were reported. Half of them were mild (rash after infusion, nonspecific pain in mouth), resolved spontaneously and did not require UST withdrawal. In the remaining cases UST treatment was discontinued (abscess formation, a possible allergic reaction with unclear association to UST).
Discussion
In the current study, we present data from a nationwide multicentre cohort that represents the real-life clinical practice in the treatment of CD with UST in Finland. Patients had a long-standing disease with a complex phenotype and the most patients had failed several biological agents before initiation of UST. This highly treatment refractory cohort differs from the patients included in the phase III UNITI trials, in which the efficacy and safety of UST was demonstrated in patients with CD [Citation5]. Although, inclusion criteria for the UNITI-1 trial were nonresponse or unacceptable side-effects to TNF-inhibitors, all patients in the UNITI-2 trial were either bio-naïve or TNF-inhibitor experienced without failing, and in the IM-UNITI maintenance treatment trial less than half (44%) of the patients had a history of previous treatment with TNF-inhibitors [Citation5]. By contrast, the proportion of patients in our study failing prior biologic treatments is similar to that in the other real-life cohort studies [Citation11–19].
The majority of patients in our study benefited from UST treatment and continued therapy at the end of follow-up. Several retrospective single- and multicentre cohort studies have evaluated UST clinical efficacy in patients with CD but with highly variable dosing regimens before the approval of the current posology by EMA. Although, the administration route of UST has mainly been subcutaneous, the reported short-term clinical response rates in patients with CD have been between 39% and 84% and medium- and long-term rates between 60% and 78% [Citation11–18]. Furthermore, treatment persistence rates from 55% to 72% have been described in these real-life cohorts [Citation13,Citation18]. In a recently published prospective study using intravenous UST induction a clinical response rate of 40% at week 24 was observed in patients with CD [Citation19]. We observed comparable results to these studies with an overall UST persistence rate of 83% at the end of the follow-up and a clinical benefit in 76% of patients achieving either clinical response and/or being in clinical remission.
An important finding in our study was the significant reduction in the use of corticosteroids after the initiation of UST. At the end of follow-up, 88% of patients were steroid-free. In previously published cohort studies of CD patients, steroid-free remission rates vary largely from 25% to 95%, which could be due to the heterogeneity of dosing regimens and clinical endpoints [Citation13,Citation14,Citation16,Citation26,Citation27].
Further, in the current study, a significant reduction of endoscopic activity, measured by the validated SES-CD was observed at week 16 in a subgroup of patients with available endoscopic data. This is in line with the results of the endoscopic sub-study in the UNITI-studies, where mean SES-CD at week 8 decreased by 2.8 points after a single UST infusion and demonstrated an endoscopic response and remission rate of 21% and 8%, respectively [Citation7]. Other retrospective cohort studies have evaluated UST efficacy on mucosal healing and reported endoscopic response and remission rates between 55% and 82% and 20% and 39%, respectively [Citation13,Citation14,Citation16,Citation18,Citation26]. The only prospective real-life UST study to date with intravenous induction reported an endoscopic response rate of 21% and an endoscopic remission rate of 7% [Citation19]. The median SES-CD in our study remained stable at a low-level during the follow-up period, but a slight numerical increase in fCal was measurable. However, neither the SES-CD decrease at the end of follow-up nor the changes in fCal reached statistically significance.
The safety of UST treatment as measured by the number and severity of reported adverse events in our study resembles the good safety profile of UST observed in the clinical trials and other cohort studies both in patients with CD and those with psoriasis [Citation5,Citation12–19,Citation28]. Concomitant immunomodulators and corticosteroids can increase the risk of adverse events, especially infections [Citation2]. Recent data suggest that UST can be used as monotherapy as concurrent use of immunomodulators do not seem to effect immunogenity or remission efficacy of UST [Citation29,Citation30].
A major strength of this study is the nationwide inclusion of hospitals, covering almost all centres in Finland administrating UST intravenous induction during 2017. Also, a wide variety of collected data such as demographic factors, comorbidities, previous and concomitant CD medication, as well as biomarkers, were included in the study. Among the limitations of this study are the small patient number and incomplete data of the SES-CD, mHBI and fCal due to the retrospective design. Further limitations are the lack of data in UST trough levels or anti-drug antibodies and the missing information of UST efficacy on fistula healing.
In conclusion, the current real-life study presents evidence that UST is an effective treatment option in CD patients who have previously failed even several other biologic agents. Currently in Finland, UST is mainly used as a second- or third-line biologic medication for CD patients. Emerging data from real-life studies together with the increasing clinical experience with UST will probably influence treatment algorithms for CD in the future. In further studies, the potential benefit of UST dose-optimisation for mucosal healing needs to be evaluated.
Acknowledgement
The authors would like to thank Laura Hakala and Petri Mankinen for their expertise in the study management.
Disclosure statement
This study was supported by Janssen-Cilag Oy (Finland). A. Eberl has received research support from Janssen-Cilag and consulting fees from MSD, Pfizer and Takeda. T. Sipponen has received speaker fees from Abbvie, Ferring, Janssen-Cilag, MSD, Pfizer, Takeda and Tillotts Pharma; has received consulting fees from Hospira, Janssen-Cilag, Pfizer, Takeda and MSD; and has received a research grant from Janssen-Cilag and Takeda. T. Hallinen and E. Soini are employees and shareholders of ESiOR Oy, the company commissioned by Janssen-Cilag Oy to help perform this study. T. Hallinen and E. Soini declare no personal conflict of interest. C.G. af Björkesten has received consultant fees from Abbvie, MSD, Mylan, Ferring, Janssen-Cilag, Pfizer, Takeda, has received lecture fees from: Abbvie, MSD, Pfizer, Ratiopharm, has received congress fees from AbbVie, Ferring, Meda, MSD, Mylan, Tillotts. M. Kellokumpu has received consultant fees from Takeda. H. Nuutinen has received lecture fees from Pfizer, Janssen-Cilag, Sanofi, Ferring, MSD, Abbvie, Takeda. E. Hirsi has received lecture fees from Janssen-Cilag, Tillotts Pharma, Takeda. K. Utriainen has received lecture fees from Abbvie, Takeda. C. Wennerström, R. Nissinen, A. Borsi, M. Koivunen are all employees of Janssen-Cilag. The following authors had no conflict of interest to declare: M. Heikkinen, I. Koskinen, V. Moilanen, C. Nielsen, U.-M. Suhonen, I. Vihriälä, J. Tillonen.
References
- Torres J, Mehandru S, Colombel JF, et al. Crohn's disease. Lancet. 2017;389:1741–1755.
- Axelrad JE, Roy A, Lawlor G, et al. Thiopurines and inflammatory bowel disease: current evidence and a historical perspective. World J Gastroenterol. 2016;22:10103–10117.
- Lichtenstein GR, Yan S, Bala M, et al. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn's disease. Gastroenterology. 2005;128:862–869.
- Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn's disease—algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30–51.
- Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2016;375:1946–1960.
- Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med. 2012;367:1519–1528.
- Rutgeerts P, Gasink C, Chan D, et al. Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn's disease. Gastroenterology. 2018;155:1045–1058.
- Sandborn WJ, Rutgeerts P, Gasink C, et al. Long-term efficacy and safety of ustekinumab for Crohn's disease through the second year of therapy. Aliment Pharmacol Ther. 2018;48:65–77.
- European Commission Approves Stelara® [Ustekinumab] for treatment of adults with moderately to severely active Crohn’s disease. Beerse (Belgium): Johnson & Johnson Media Center; 2016.
- FDA Approves STELARA® [Ustekinumab] for treatment of moderate to severe Crohn’s disease. New York (NY): Crohn's & Colitis Foundation of America; 2016.
- Harris KA, Horst S, Gadani A, et al. Patients with refractory crohn's disease successfully treated with ustekinumab. Inflamm Bowel Dis. 2016;22:397–401.
- Khorrami S, Ginard D, Marin-Jimenez I, et al. Ustekinumab for the treatment of refractory Crohn's disease: the Spanish experience in a large multicentre open-label cohort. Inflamm Bowel Dis. 2016;22:1662–1669.
- Wils P, Bouhnik Y, Michetti P, et al. Long-term efficacy and safety of ustekinumab in 122 refractory Crohn's disease patients: a multicentre experience. Aliment Pharmacol Ther. 2018;47:588–595.
- Ma C, Fedorak RN, Kaplan GG, et al. Clinical, endoscopic and radiographic outcomes with ustekinumab in medically-refractory Crohn's disease: real world experience from a multicentre cohort. Aliment Pharmacol Ther. 2017;45:1232–1243.
- Kopylov U, Afif W, Cohen A, et al. Subcutaneous ustekinumab for the treatment of anti-TNF resistant Crohn's disease—the McGill experience. J Crohns Colitis. 2014;8:1516–1522.
- Greenup AJ, Rosenfeld G, Bressler B. Ustekinumab use in Crohn's disease: a Canadian tertiary care centre experience. Scand J Gastroenterol. 2017;52:1354–1359.
- Wils P, Bouhnik Y, Michetti P, et al. Subcutaneous ustekinumab provides clinical benefit for two-thirds of patients with Crohn's disease refractory to anti-tumor necrosis factor agents. Clin Gastroenterol Hepatol. 2016;14:242.e1-2–250.e1-2.
- Ma C, Fedorak RN, Kaplan GG, et al. Long-term maintenance of clinical, endoscopic, and radiographic response to ustekinumab in moderate-to-severe Crohn's disease: real-world experience from a multicenter cohort study. Inflamm Bowel Dis. 2017;23:833–839.
- Verstockt B, Dreesen E, Noman M, et al. Ustekinumab exposure-outcome analysis in Crohn's disease only in part explains limited endoscopic remission rates. J Crohns Colitis. [Epub ahead of print] .
- Summary of product characteristics, SmPC. Janssen Pharmaceutical Companies 2016. Available from: http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/STELARA-pi.pdf
- Sipponen T, Kolho KL. Fecal calprotectin in diagnosis and clinical assessment of inflammatory bowel disease. Scand J Gastroenterol. 2015;50:74–80.
- Best WR. Predicting the Crohn's disease activity index from the Harvey-Bradshaw index. Inflamm Bowel Dis. 2006;12:304–310.
- af Bjorkesten CG, Nieminen U, Turunen U, et al. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn's disease. Scand J Gastroenterol. 2012;47:528–537.
- Vermeire S, Schreiber S, Sandborn WJ, et al. Correlation between the Crohn's disease activity and Harvey-Bradshaw indices in assessing Crohn's disease severity. Clin Gastroenterol Hepatol. 2010;8:357–363.
- Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512.
- Battat R, Kopylov U, Bessissow T, et al. Association between ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2017;15:1427.e2–1434.e2.
- Soufflet N, Boschetti G, Roblin X, et al. Concentrations of ustekinumab during induction therapy associate with remission in patients with Crohn's disease. Clin Gastroenterol Hepatol. [Epub ahead of print].
- Papp K, Griffiths C, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844–854.
- Abedokun OJ, Xu Z, Gasink C, et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn's disease. Gastroenterology. 2018;154:1660–1671.
- Gosh S, Kramer BC, Gasink C, et al. Long-term efficacy of ustekinumab with or without concomitant immunosuppressants for Crohn’s disease: results from IM-UNITI long-term extension through 2 years. J Crohn's Colitis. 2019;13:S459–S460.