Abstract
Objectives: Functional gastrointestinal (GI) symptoms, such as IBS (irritable bowel syndrome), have been suggested to be associated with autonomic neuropathy. We therefore examined associations between hemodynamic indices of autonomic control, functional GI symptoms and stress in a population-based cohort.
Methods and materials: The study included 2094 participants of the Malmö Offspring Study (mean age 40.6 ± 13.8 years, 53.9% women). 509 (24.3%) reported having GI symptoms the last 2 weeks, without having organic GI disease, and 347 subjects (16.6%) reported IBS. Office and ambulatory 24-h systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate were measured. Associations between hemodynamic parameters and abdominal pain, diarrhea, constipation, bloating and flatulence, vomiting and nausea and psychological well-being according to the visual analog scale for IBS (VAS-IBS), and stress, were performed by Spearman’s correlation test and linear regression models.
Results: Subjects who reported GI symptoms had lower office supine and standing DBP and lower 24 h SBP and DBP compared with those without GI symptoms. Regarding specific symptoms, diarrhea was correlated with 24-h measurements of SBP (rs = 0.197), DBP (rs = 0.173) and heart rate (rs = 0.134). Subjects with the most severe diarrhea had higher 24-h SBP (125.2 vs. 119.0 mmHg; p = .038), DBP (74.0 vs. 69.0 mmHg; p = .033) and heart rate (74.5 vs 71.1 beats/minute; p = .048), after adjustments for confounders, compared to the other. There were no associations between other GI symptoms, IBS, stress and hemodynamic alterations.
Conclusion: Functional diarrhea was associated with hemodynamic indices of sympathetic activation, supporting a possible role of the autonomic nervous system in diarrhea.
Introduction
Functional gastrointestinal disorders (FGID) are chronic diseases with unknown etiology that affect millions of people worldwide. The most prevalent manifestation of FGID is irritable bowel syndrome (IBS) [Citation1]. It has been demonstrated that IBS is strongly related to stress, psychological disorders [Citation2] and visceral hypersensitivity [Citation3]. The enteric nervous system (ENS) is a part of the autonomic nervous system (ANS), which constitutes the anatomical and physiological basis for correlations between visceral hypersensitization and autonomic dysfunction [Citation4]. Pain circuits are conducted through afferent parasympathetic and sympathetic neurons to corticolimbic structures and other brain centers modulating pain signals [Citation5]. The ANS governs the most important life-supporting and adaptive functions in humans. Dysfunction of the ANS is probably best seen in cardiovascular autonomic disorders, including clinical manifestations of disturbed heart rhythm and blood pressure regulation, such as orthostatic hypotension and postural orthostatic tachycardia syndrome (POTS) [Citation6,Citation7]. Impaired autonomic control of the gastrointestinal (GI) system is usually more discrete [Citation8]. Some conditions with primary autonomic failure exhibit any prominent cardiovascular and GI symptoms, e.g. diabetes mellitus [Citation9], Parkinson’s disease [Citation10] or primary autonomic failure [Citation11]. However, in the less pronounced or advanced forms of ANS impairment, the cardiovascular and GI symptoms may coexist, but be milder and more difficult to identify [Citation12].
The autonomic dysfunction previously described in IBS may rather be a secondary response to peripheral and/or central hypersensitization [Citation4,Citation13,Citation14], than of primary, etiological importance. On the other hand, genetic studies have shown associations between IBS and gene variants of locus 9q31.2, including IKBKAP [Citation15], gene variants found in patients with familial dysautonomia, who also express a high prevalence of GI symptoms [Citation16].
We hypothesized that autonomic dysfunction in FGID would show GI symptoms in parallel with disturbed hemodynamic parameters. To study this, office and ambulatory blood pressure and heart rate measurements, as well as the orthostatic response, were compared with the occurrence and severity of functional GI symptoms in a population-based study cohort of Malmö Offspring Study (MOS). The primary aim of the present study was to compare hemodynamic parameters in subjects with and without functional GI symptoms. Secondary aims were to relate the hemodynamic parameters to the degree of specific GI symptoms and psychological well-being, and presence of self-reported chronic stress.
Material and methods
The study was performed in accordance to the Helsinki declaration and approved by the Regional Ethics Review Board at Lund University (2012/594, Date of approval 05/12/2012). All subjects gave their written, informed consent before entering the study.
Study participants
The population-based Malmö Diet and Cancer Study (MDCS) consists of 28,098 participants, born between 1923 and 1950, and enrolled between 1991 and 1996 [Citation17]. Later, 6103 individuals were randomly selected and constituted the Malmö Diet and Cancer cardiovascular cohort (MDC-CV). Children and grand-children to subjects in MDC-CV were invited to participate in the Malmö Offspring Study (MOS). Between March 2013 and April 2017, a total of 2644 subjects have attended the MOS program (participation rate, 46.7%). Subjects who reported any organic GI disease, i.e. inflammatory bowel disease (n = 30; 14 with Crohn’s disease, 21 with ulcerative colitis and five with both conditions) or celiac disease (n = 24) were excluded. Of the remaining 2590 subjects, data according to the questionnaires were available for 2094 subjects, who formed the current study population.
Study design
Subjects were invited to an anthropometric and clinical examination including measurement of weight (kg) and height (m) in light in-door clothing, waist and hip circumference (cm), systolic (SBP) and diastolic (DBP) blood pressure (mmHg) (Omron® automatic reading, after 10 min supine rest and 5 min standing, mean of two readings) and pulse rate (beats/min) at the Clinical Research Unit (CRU), Skåne University Hospital, Malmö. Subjects with acute disease were rescheduled to a later time point. The occurrence of orthostatic hypotension (OH), defined as an orthostatic decrease in SBP ≥ 20 mmHg and/or DBP ≥ 10 mmHg after 3 min [Citation18], was recorded. In addition to office measurements, subjects performed 24-h ambulatory measurements of SBP, DBP and heart rate using the TensioMed Arteriograph 24 device (TensioMed™ Ltd, Hungary). Blood pressure as well as heart rate was measured every 15 min during daytime (06.00–22.00) and every 30 min during the night (22.00–06.00). Subjects were excluded from analyses if more than 30% of the blood pressure recordings were missing. At the same day as the above measurements were performed, all participants were asked to complete a study questionnaire including questions on sociodemographic factors, lifestyle factors and medical history, and the Visual Analog Scale for Irritable Bowel Syndrome (VAS-IBS) with questions about GI symptoms.
Data on prevalent cardiovascular comorbidities at the time of the examination were retrieved from four registers, as previously described in detail (Supplementary Material) [Citation19]. Coronary artery disease (CAD) was defined as fatal or non-fatal myocardial infarction, death from ischemic heart disease, coronary artery bypass grafting or percutaneous coronary intervention. The prevalence of diabetes was retrieved from the questionnaire or a total of 15 registers (Supplementary Material).
Questionnaires
Study questionnaire
This web-based questionnaire included questions on sociodemographic factors, lifestyle factors, medical history and self-perceived chronic stress during the past year. To assess self-reported IBS according to ROME-III criteria [Citation20], participants were asked in the questionnaire ‘Have you several times during a month suffered from abdominal pain related to un-regular bowel habits which is called IBS?’ If the participants answered yes, they were referred to as having IBS.
Visual analog scales for irritable bowel syndrome
The participants were asked ‘Have you experienced bowel symptoms during the last 2 weeks?’ If they answered ‘yes’ to this question, they were encouraged to complete the VAS-IBS. This questionnaire is validated for estimation of the most common non-organic, functional GI symptoms experienced during the previous 2 weeks [Citation21]. VAS-IBS has been further validated for estimation of symptoms over time [Citation22]. The symptoms addressed on a scale from 0–100 mm are abdominal pain, diarrhea, constipation, bloating and flatulence, vomiting and nausea, psychological well-being and intestinal symptoms’ influence on daily life, where 0 represents a complete lack of problems and 100 represents severe problems. The item psychological well-being has been found to strongly correlate to positive and negative aspects of psychological well-being, anxiety in close relations, self-esteem and coping skills [Citation23]. The scales were inverted from the original version [Citation21]. VAS-IBS is similar to Irritable Bowel Syndrome-Symptom Severity Scale [Citation24], and share most scales, except that VAS-IBS separates bowel habits into diarrhea and constipation [Citation21].
Statistical analyses
Data distribution was evaluated by visual inspection of histograms. Study population characteristics (age, sex, current smoking, BMI, self-reported chronic stress, CAD and diabetes) and hemodynamic measurements (supine and standing SBP/DBP, supine heart rate, 24-h ambulatory mean of SBP, DBP and heart rate) were compared with the occurrence of self-reported IBS and functional GI symptoms by linear regression, with the dichotomous variables (0/1) denoting IBS and/or GI symptoms as the independent variables. The hemodynamic parameters were further compared with GI symptoms in adjusted linear models including age, sex, current smoking and self-reported chronic stress as covariates. Pearson’s Chi2 test was used for testing of dichotomous variables and Mann–Whitney U-test for group comparisons.
A composite VAS-variable was constructed to assess the combined severity of all five types of GI symptoms (‘VAS-total’; range 0–500). Quartiles of VAS-total (independent) were related to hemodynamic measurements in linear regression entering hemodynamic parameters as continuous variables, adjusted for age, sex, current smoking and self-reported chronic stress.
To test the correlation between the hemodynamic variables and specific types of GI symptoms and psychological well-being, Spearman’s rank correlation test was used. Significant findings were further explored by associating the quartiles of reported VAS (range 0–100; independent variable) with hemodynamic measurements (dependent) in linear regression models, adjusted for age, sex, current smoking and self-reported chronic stress.
All analyses were performed using IBM SPSS Statistics version 25 (SPSS Inc., Chicago, IL, USA). All tests were two-sided. p < .05 was considered statistically significant.
Results
Population characteristics
A total of 509 subjects (24.3%) reported that they had had GI symptoms during the last 2 weeks and 347 subjects (16.6%) reported that they suffered from IBS. There was a strong correlation between the presence of IBS and the presence of GI symptoms during the last 2 weeks (Pearson’s correlation (Phi) = 0.536; p < .001). Subjects who reported GI symptoms were slightly younger and more often women, were more often current smokers and reported chronic stress to a greater degree, whereas BMI and the prevalence of diabetes and CAD did not differ, in relation to those who did not report any symptoms (). The use of drugs with potential effects on the ANS were only sporadically used (Beta blockers were used by 61 subjects [2.9%], angiotensin-converting-enzyme inhibitors/angiotensin II receptor blockers by 105 [5.0%], and selective serotonin reuptake inhibitors [SSRI] by 80 subjects [3.8%]).
Table 1. Study population characteristics.
Relations between hemodynamic parameters and reported symptoms
There were no statistically significant alterations in hemodynamic parameters between IBS and non-IBS subjects after adjustment for confounders (p > .05). In contrast, subjects who reported GI symptoms during the past 2 weeks had lower SBP and DBP, both according to office measurements in the supine and in the standing position and during ambulatory 24-h measurements. The lower values of DBP in supine and standing position, and mean SBP and DBP during ambulatory 24-h measurements, remained significant also in the models including adjustments for age, sex, current smoking and chronic stress (). The mean heart rate during 24 h was higher in subjects who reported GI symptoms. However, the significance was attenuated after additional adjustment for current smoking and chronic stress ().
Table 2. The relation between hemodynamic measurements and reported GI symptoms.
There were no associations between hemodynamic parameters and the total reported GI symptom burden after adjustments for confounders (Supplementary Table 1).
Relations between 24-h measurement of hemodynamic parameters and specific symptoms
A negative correlation was observed between supine office SBP and the severity of constipation, bloating and flatulence, vomiting and nausea and psychological well-being (inverted), i.e. higher SBP was associated with less severe symptoms, in the group of subjects reporting any kind of GI symptom (n = 509) ().
Table 3. Spearman’s correlation between office measurement of hemodynamic parameters and specific symptoms.
A total of 142 subjects (27.9%) reported diarrhea being their most predominant symptom. For the 24-h measurements, there were concordant positive correlations between both SBP, DBP and heart rate and the severity of diarrhea. Excluding the subjects with diabetes (n = 15) did not alter the correlations (data not shown). A higher heart rate was also correlated with more pronounced symptoms of vomiting and nausea ().
In accordance with the correlation analyses, the 24-h hemodynamic measurements were higher with increasing severity of diarrhea after adjustment of confounders (, Supplementary Table 2). Excluding all subjects with self-reported diabetes (n = 15) resulted in a slight attenuation of the adjusted associations between 24-h heart rate and diarrhea (p = .056), whereas all other associations remained statistically significant (data not shown).
Figure 1. The relationship between the severity of diarrhea and 24-hour hemodynamic measurements. The reported severity of diarrhea among subjects with gastrointestinal symptoms (n = 509) in relation to 24-hour systolic blood pressure (blue bars), diastolic blood pressure and heart rate . P-values indicate significance in mean differences between VAS diarrhea quartile four and combined quartiles one to three, using linear regression including age, sex, smoking and self-reported chronic stress as covariates. Q: quartile; VAS: visual analogue scale; bpm: beats per minute.
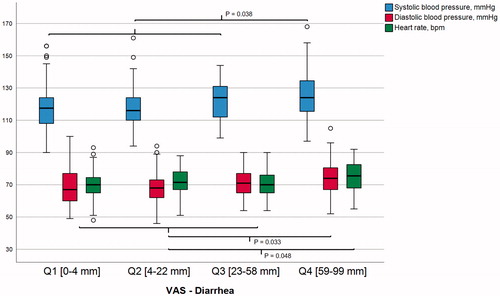
Subjects who reported chronic stress had more severe symptoms of diarrhea (median VAS 30 vs 18.5 mm; Mann–Whitney U-test; p < .001). Moreover, subjects reporting chronic stress had lower office SBP (mean SBP 114.1 vs 117.5 mmHg; p = .014), whereas there were no significant differences in DBP, resting heart rate and the 24-h measurements between subjects with and without self-reported chronic stress (p > .05). Including chronic stress as an additional covariate in the regression models did not substantially change the associations between the hemodynamic measurements and diarrhea (Supplementary Table 2).
Despite showing significant correlations with supine SBP and 24-h heart rate, respectively (), the symptoms of constipation, bloating and flatulence, vomiting and nausea and psychological well-being did not demonstrate any significant linear associations with the corresponding hemodynamic measurements in the adjusted model (data not shown).
Discussion
In this population-based study, participants who reported any kind of functional GI symptoms during the past 2 weeks had lower systolic and diastolic blood pressure than those who had no symptoms. However, subjects with more severe diarrhea had higher 24-h values of SBP and DBP, as well as higher mean heart rate, regardless of potential confounders such as smoking and self-reported chronic stress, compared to those with less diarrhea. In contrast, no differences in hemodynamic parameters were found when comparing IBS versus non-IBS subjects.
Our results agree with a previous review, which showed that IBS patients from the general population, when studied as a whole group, did not differ in heart rate variability compared to healthy controls [Citation25], which may be explained by differences in psychological and somatic symptoms among IBS subjects who seek medical care versus non-consulters [Citation26]. However, several studies have shown signs of autonomic dysfunction in IBS, with differences according to subgroups of IBS [Citation4,Citation13,Citation14,Citation25], disease duration [Citation27], or severity of symptoms [Citation28]. Although apparently demonstrating conflicting results, most studies have shown increased sympathetic and decreased parasympathetic activity, the same pattern as in the organism’s response to stress [Citation8,Citation25]. Our findings of a close relationship between functional diarrhea and hemodynamic signs of sympathetic activation are in line with previous studies [Citation14].
Since our study is cross-sectional, the signs of cardiovascular autonomic dysfunction in patients with severe functional GI symptoms may be primary or secondary to the GI disease, with several possible explanations. First, autonomic neuropathy secondary to GI symptoms may be an effect of the peripheral and/or central hypersensitization associated with functional GI disorders [Citation3], with aggravated ANS responses, due to afferent signals from the GI tract being dispersed to amygdala, hippocampus, insula, cingulate cortex and other brain centers modulating pain signals [Citation5,Citation29]. Second, a primary autonomic dysfunction such as present in genetic diseases [Citation16], Parkinson’s disease [Citation10] or diabetes mellitus [Citation9], may act as a causal agent disturbing both afferent and efferent pathways between the gut and brain. We cannot exclude the possibility that subclinical primary or secondary autonomic pathology, not clearly detected at baseline screening, was present in individuals with increased diarrhea susceptibility. Third, the elevated sympathetic and decreased parasympathetic activity may be an expression of body’s stress responses [Citation8]. Stress, anxiety and affective disorders are closely related to IBS [Citation2], which was also observed in the present study. The alterations of GI motility, abdominal pain and discomfort may be the expression of life stress and poor psychological well-being leading to GI symptoms [Citation8]. Although the sympathetic nervous system exerts inhibitory effects on the GI motility, chronic or prolonged stress has been shown to alter normal neural circuits and neurotransmitter release, which may be responsible for the propulsive impact on the GI motility [Citation30]. Interestingly, chronic stress was clearly associated with increased severity of diarrhea in our cohort, but did neither associate with blood pressure nor heart rate during the 24-h measurements, and did not attenuate the associations between hemodynamic parameters and increased severity of diarrhea. Thus, the observed associations between hemodynamic parameters and increased diarrhea propensity are unlikely to be mediated by chronic psychological stress. Consequently, we hypothesize that dehydration due to diarrhea may create physiological stress to which the organism responds with increased sympathetic tonus, elevated blood pressure and heart rate.
Independent of primary or secondary associations, neither the present nor previous studies support that a great part of the IBS population has developed the disease because of a primary autonomic dysfunction [Citation8,Citation25]. Familial dysautonomia often presents itself with dysphagia, esophageal dysmotility and vomiting [Citation16]. Thus, the symptoms are not similar to classical functional GI symptoms [Citation1]. Impaired baroreflex pathways have been found in familial dysautonomia [Citation31], which is not supported in our cohort with a very low prevalence of orthostatic hypotension. The found association between gene mutations of IKBKAP and self-reported IBS may reflect that many patients with GI symptoms are not properly examined [Citation15], and could have other diagnoses of autonomic dysautonomia with secondary GI symptoms [Citation9–11].
The strength of this study is the large cohort of subjects from the general population, and knowledge of type and severity of each GI symptom. On the other hand, when performing population-based studies, the data is less validated than when including subjects at a health care center. Another strength is the adjustment for confounders, and that linear regression analysis was only calculated for significant correlations in the Spearman’s correlation test. To record specific symptoms on a VAS scale is important, since registration of symptoms or not is not enough [Citation28]. By calculation of all GI symptoms together, alterations found to be associated with specific symptoms may be equalized. Furthermore, different definitions of IBS over time complicates comparisons between studies [Citation32].
The limitation of the present study is that we do not know whether the self-reported IBS or functional GI symptoms represent subjects who have been visiting a physician with exclusion of severe dysmotility or other organic disorders. With the close access to health care in Sweden, one can assume that most patients with severe symptoms have been in contact with a health care center. Another limitation is that we have not performed autonomic nerve function tests on the subjects, but only measured standard hemodynamic parameters. Performing specific autonomic tests and compare the results according to the outer quartiles of GI symptom severity, would add valuable insights into autonomic function among the current subjects.
Conclusion
Stratification according to specific symptoms revealed strong associations between functional diarrhea and hemodynamic parameters, with more severe symptoms of diarrhea associating with higher blood pressure and heart rate measured over 24 h. Our results support a role of the autonomic nervous system with sympathetic activation in patients with functional diarrhea. Future studies of autonomic neuropathy and GI symptoms should focus on type and severity of each symptoms.
Supplemental Material
Download PDF (110.7 KB)Supplemental Material
Download MS Word (13.2 KB)Acknowledgements
The authors want to acknowledge all the staff at the Clinical Research Unit, Skåne University Hospital, Malmö, for inclusion of patients in the current study and Anders Dahlin, who retrieved data from the registers.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
All data can be obtained from the authors upon request.
Additional information
Funding
References
- Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393–1407.
- Shah E, Rezaie A, Riddle M, et al. Psychological disorders in gastrointestinal disease: epiphenomenon, cause or consequence? Ann Gastroenterol. 2014;27(3):224–230.
- Icenhour A, Witt ST, Elsenbruch S, et al. Brain functional connectivity is associated with visceral sensitivity in women with Irritable Bowel Syndrome. Neuroimage Clin. 2017;15:449–457.
- Liu L, Liu BN, Wang M, et al. Visceral and somatic hypersensitivity, autonomic cardiovascular dysfunction and low-grade inflammation in a subset of irritable bowel syndrome. J Zhejiang Univ Sci B. 2014;15(10):907–914.
- Fukudo S. Stress and visceral pain: focusing on irritable bowel syndrome. Pain. 2013;154:S63–S70.
- Hamrefors V, Spahic JM, Nilsson D, et al. Syndromes of orthostatic intolerance and syncope in young adults. Open Heart. 2017;4(1):e000585.
- Goodman BP. Evaluation of postural tachycardia syndrome (POTS). Auton Neurosci. 2018;215:12–19.
- Manabe N, Tanaka T, Hata J, et al. Pathophysiology underlying irritable bowel syndrome - From the viewpoint of dysfunction of autonomic nervous system activity. J Smooth Muscle Res. 2009;45(1):15–23.
- Brock C, Brock B, Pedersen AG, et al. Assessment of the cardiovascular and gastrointestinal autonomic complications of diabetes. World J Diabetes. 2016;7(16):321–332.
- Trahair LG, Kimber TE, Flabouris K, et al. Gastric emptying, postprandial blood pressure, glycaemia and splanchnic flow in Parkinson's disease. World J Gasroenterol. 2016;22(20):4860–4867.
- Brown TP. Pure autonomic failure. Pract Neurol. 2017;7:341–348.
- Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. 2019;285(4):352–366.
- Salvioli B, Pellegatta G, Malacarne M, et al. Autonomic nervous system dysregulation in irritable bowel syndrome. Neurogastroenterol Motil. 2015;27(3):423–430.
- Yildirim AE, Korkmaz M, Altun R, et al. Is there any association between irritable bowel syndrome subgroups an autonomous dysfunction. Eur Rev Med Pharmacol Sci. 2016;20(7):1315–1322.
- Bonfiglio F, Zheng T, Garcia-Etxebarria K, et al. Female-specific association between variants on chromosome 9 and self-reported diagnosis of irritable bowel syndrome. Gastroenterology. 2018;155(1):168–179.
- Axelrod FB. Familial dysautonomia. Muscle Nerve. 2004;29(3):352–363.
- Manjer J, Carlsson S, Elmståhl S, et al. The Malmö Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev. 2001;10(6):489–499.
- Brignole M, Moya A, de Lange FJ, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39(21):1883–1948.
- Ricci F, Wollmer P, Engström G, et al. Markers of cardiovascular autonomic dysfunction predict COPD in middle-aged subjects. Eur Respir J. 2018;51(3):1702481.
- Drossman DA. Rome III: the new criteria. Chin Dig Dis. 2006;7(4):181–185.
- Bengtsson M, Ohlsson B, Ulander K. Development and psychometric testing of the visual analogue scale for irritable bowel syndrome (VAS-IBS). BMC Gastroenterol. 2007;7:16.
- Bengtsson M, Persson J, Sjölund K, et al. Further validation of the visual analogue scale for irritable bowel syndrome after use in clinical practice. Gastroenterol Nurs. 2013;36(3):188–198.
- Bengtsson M, Ohlsson B. The brief Visual Analogue Scale for Irritable Bowel Syndrome Questionnaire can be used to evaluate psychological well-being in patients with irritable bowel syndrome. Eur J Intern Med. 2013;24(7):e82–e83.
- Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Alimentary Pharmacology & Therapeutics. 1997;11:395–402.
- Mazurak N, Seredyuk N, Sauer H, et al. Heart rate variability in the irritable bowel syndrome: a review of the literature. Neurogastroenterol Motil. 2012;24(3):206–216.
- Cheng P, Shih W, Alberto M, et al. Autonomic response to a visceral stressor is dysregulated in irritable bowel syndrome and correlates with duration of disease. Neurogastroenterol Motil. 2013;25(10):e650–e659.
- Heitkemper M, Jarrett M, Cain KC, et al. Autonomic nervous system function in women with irritable bowel syndrome. Dig Dis Sci. 2001;46(6):1276–1284.
- Polster AV, Palsson OS, Tornblom H, et al. Subgroups of IBS patients are characterized by specific, reproducible profiles of GI and non-GI symptoms and report differences in healthcare utilization: a population-based study. Neurogastroenterol Motil. 2019;31(1):e13483.
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926.
- Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol. 2014;4(4):1339–1368.
- Norcliffe-Kaufmann L, Slaugenhaupt SA, Kaufmann H. Familial dysautonomia: history, genotype, phenotype and translational research. Prog Neurobiol. 2017;152:131–148.
- Vork L, Weerts Z, Mujagic Z, et al. Rome III vs Rome IV criteria for irritable bowel syndrome: a comparison of clinical characteristics in a large cohort study. Neurogastroenterol Motil. 2018;30(2):e13189.
