Abstract
Background: The choice of treatment for Crohn’s disease (CD) and ulcerative colitis (UC) depends among other factors, disease severity. Patients with moderate-to-severe disease should be prescribed biologic response modifiers (biologics), according to guidelines. This study aims to explore the treatment patterns of patients diagnosed with CD and UC between 2003 and 2015 that were treated with biologics in Denmark between the years 2003 and 2016.
Methods: This national register study included patients diagnosed between 2003 and 2015, identified in the Danish National Patient Registry. Biologic therapies available during the study period were infliximab, adalimumab, vedolizumab and golimumab. The share of patients initiating and receiving biologic treatment in each year was estimated. Additionally, the time from IBD diagnosis to first biologic treatment and time between treatments was calculated.
Results: Among 10,302 CD patients and 22,144 UC patients, 28.5% of CD patients and 11.3% of UC patients received treatment with biologics during the study period, with an increasing trend in the number of patients initiating treatment with biologics each year. About 46% of CD patients and 45% of UC patients received their first biologic treatment within the first year after IBD diagnosis. About 57–68% of CD and UC patients received treatment with their second line biologic within 2 months of the last treatment of their first line.
Conclusions: The number of patients initiating biologic treatments after diagnosis increased throughout the study period. Most patients diagnosed with CD and UC are receiving biologic treatments relatively soon after their diagnosis.
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are the two most common types of inflammatory bowel disease (IBD), affecting nearly 2.5–3 million people in Europe [Citation1]. It’s prevalence is highest in regions such as the United States, Scandinavia and the United Kingdom [Citation2]. It’s cause remains unknown, however a genetic predisposition and environmental factors like smoking and diet are believed to be some of the possible contributing factors in their development [Citation3,Citation4]. Disease onset can occur at any age, yet people typically develop CD and UC between the ages of 15 and 40 [Citation3,Citation4].
IBD is a chronic disease that requires medical intervention and sometimes surgery in severe cases. According to the European Crohn’s and Colitis Organization (ECCO), the type of treatment depends on disease severity [Citation5]. Patients with mild UC for instance can begin treatment with aminosalicylates and progress to corticosteroids. Biologic treatments are approved only for patients with moderate-to-severe CD and UC who have failed immunomodulators and corticosteroids [Citation5]. In Denmark, biologic treatments have been available since 1999 and are classified into treatment lines, based on evaluations done by the Danish Medicine Council [Citation6,Citation7]. As more become available, understanding the prevalence of their use, how long a patient is on a treatment, and how soon after diagnosis they begin treatment may help optimize treatment with biologics.
The aim of this study was to investigate the treatment patterns of patients diagnosed with CD and UC between 2003 and 2015 who were treated with biologics in Denmark.
Materials and methods
This retrospective population-based study included all Danish citizens diagnosed with CD and UC in the period of 2003–2015 who were treated with biologics.
Register data sources
Data on all Danish citizens and residents were obtained from several national registries that include the entire population. Using the Danish National Patient Register (NPR), information on all admissions and outpatient visits to hospitals and diagnosis codes according to the International Classification of Diseases, 10th revision (ICD-10) were retrieved. [Citation8]. Codes for treatments according to Danish national classification are also available in this database from 2002 onward and were retrieved for this study [Citation8]. This registry is exhaustive because it is linked to the reimbursement and payment systems. Using the Danish Civil Registration System (CRS), which includes all citizens and residents with a civil personal registration number, an identity-secure linkage between the national registries at an individual level was done [Citation9,Citation10]. As the private healthcare system constitutes for less than 1% of all the healthcare provided in Denmark, only residents treated within the public healthcare system were included.
Selection criteria
The following selection criteria was applied: (1) Individuals living in Denmark with at least two hospital contacts (admission, outpatient or emergency room visit) collected from the NPR, in the period 2003–2015, with a primary or secondary diagnosis of CD or UC using the ICD-10 codes K50 and K51 and at least one of the registrations defined as the primary diagnosis; (2) The patient had no hospital contacts related to CD or UC during 1994–2002 (wash-out period); (3) Incidence date was defined as the first hospital contact—admission, outpatient or emergency room visit—with CD or UC during 2003–2015; (4) Patients diagnosed with UC followed by a CD diagnosis were categorized as diagnosed with CD.
As patients are treated with biologics in the hospital, they were identified in the NPR using the hospitals’ registration of treatment codes plus a CD or UC diagnosis code. Biologic therapies available during the study period and therefore included were infliximab, adalimumab, vedolizumab and golimumab (treatment codes BOHJ18A1, BOHJ18A3, BOHJ19H4 and BOHJ18A4). In Denmark, Infliximab was approved for CD in 2001 and UC in 2006. Adalimumab was approved in 2003 for CD and 2012 for UC. Golimumab is not approved for CD in Denmark, however it was approved in 2013 for UC. Lastly, vedolizumab was approved for both CD and UC in 2014. Several regional and local guidelines have been in place during the study period, but were not officially formalized nationally before 2012. Infliximab has remained the first-choice biologic for CD and UC for most of the years this study was conducted.
Infliximab and vedolizumab are administered intravenously (IV), while adalimumab and golimumab are administered subcutaneously.
Biologic treatment
Biologic therapies are administered at different intervals of time, so we therefore use the term ‘units’ when calculating time on each therapy. One unit is the number of days between treatments, as recommended by the guidelines from the Danish Regions [Citation7]. For each therapy, one unit was defined as: adalimumab: 1 unit = 14 days, infliximab and vedolizumab: 1 unit = 56 days, and golimumab: 1 unit = 28 days.
As switching between treatments is common, we have categorised the biologic therapies by treatment lines, in which treatment line one includes bio-naïve patients, that is bio-naïve to the treatments stated above. Patients treated with other biologics than those above, and not indicated for IBD were not excluded from the analysis. In the analysis, treatment duration was defined as more than 6 months without interruption of the same treatment, with regard to: between two consecutive treatments, between each treatment and the total treatment time within the treatment line.
Statistical analyses
The percentage of patients receiving biologic treatment each year and the total share of patients that received biologic treatment at some point during the study period were estimated. Additionally, the total share of the patient population initiating biologic treatment each year was estimated, along with the time from IBD diagnosis to first biologic treatment. Time between treatments in treatment line one was calculated for the two IV therapies (infliximab and vedolizumab) since they are administered in hospitals and have registrations in the NPR. The number of days between the last treatment in treatment line one and the first treatment in treatment line two was also calculated. Analyses investigating the time between treatments did not include those administered subcutaneously, as their registrations in the NPR might represent a patient picking up the medicine for one or several treatments and are then administered at home at unknown dates. All statistical analyses were conducted in SAS version 9.4 (SAS Institute Inc, Cary, NC) on Statistics Denmark’s research computers via a remote server. Only anonymized data without contact or active participation of research subjects were used. Individuals are not identifiable and all results with fewer than five incidences are reported as <5. This study complies with the regulations of the Danish Data Protection Agency.
Results
The study population consisted of 10,302 incident patients with CD and 22,144 incident patients with UC (). and also present that incidence per year by age and gender at diagnosis.
Table 1. Population of incident patients with Crohn’s disease (CD) and ulcerative colitis (UC) in Denmark, 2003–2015.
Table 2. Incidence per year by age and gender at diagnosis, Crohn’s disease (CD).
Table 3. Incidence per year by age and gender at diagnosis, ulcerative colitis (UC).
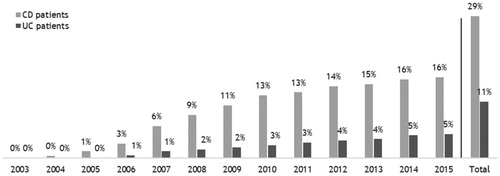
Our results show that 28.5% of CD patients and 11.3% of UC patients received treatment with biologics at any point during the study period (). In 2008, the proportion of incident CD patients receiving treatment with biologics was 9%, meaning that these patients diagnosed between 2003 and 2008 were treated with biologics in 2008. In 2015, it was 16%. For UC, it was 2 and 5%.
Figure 1. Share of population receiving biologic treatment each year among 10,302 incident patients with Crohn’s disease (CD) and 22,144 incident patients with ulcerative colitis (UC) in Denmark, 2003–2015.
Note: The share is calculated as share of cumulated incident patients alive primo in the given year. A person is included as receiving biologics if they received biologic treatment in the given calendar year. The ‘Total’ columns show the share of the patient populations incident in 2003–2015 who received treatment with biologics at some point during 2003–2016.
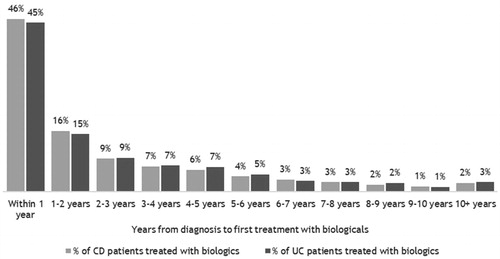
The number of patients initiating treatment with biologics is illustrated in . During the study period, an increasing trend was observed, with 369 CD and 371 UC patients initiating treatment in 2015. The number of patients initiating biologic treatment and the share of the patient populations is shown in Supplementary Table S2. and Supplementary Table S3 present the distribution of time from IBD diagnosis to first biologic treatment among the study populations. Most patients received their first treatment within the first year after IBD diagnosis (46% of CD patients and 45% of UC patients). Additional analyses show that time to first treatment for both CD and UC patients are significantly shorter (p < .0001) for patients diagnosed in 2010–2013 compared to patient diagnosed in 2006–2009.
Figure 2. Number of patients initiating biologic treatment each year among 10,302 incident patients with Crohn’s disease (CD) and 22,144 incident patients with ulcerative colitis (UC) in Denmark, 2003–2016.
Note: The population includes incident patients with CD or UC in the period 2003–2015. A person is counted if they initiated biologic treatment in the given calendar year, but after their IBD diagnosis. As shown in the Supplementary Appendix, there are more than twice as many patients in the incident UC population than in the incident CD population.
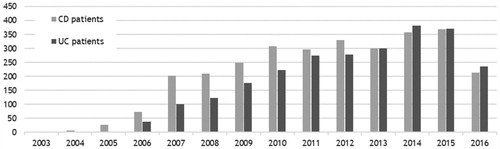
Figure 3. Time from diagnosis to first treatment with biologics among 2,939 incident patients with Crohn’s disease (CD) and 2,504 incident patients with ulcerative colitis (UC) in Denmark treated with biologics in 2003–2016.
Note: The population includes incident patients with CD or UC, who received treatment with biologics after their IBD diagnosis.
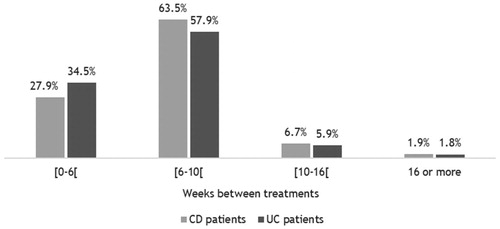
For the IV therapies, according to national guidelines, the recommended time between treatments is 8 weeks. As illustrated in , 27.9 and 34.5% of IV treatments in treatment line one were administered earlier than what is recommended in the guidelines for both patient groups. However, most treatments were administered within an interval that corresponds to the recommendations in the national guidelines (63.5 and 57.9% for CD and UC, respectively).
Figure 4. Time between treatments in treatment line one among 29,294 biologic treatments administered intravenously to patients with Crohn’s disease (CD) and 16,905 biologic treatments administered intravenously to patients with ulcerative colitis (UC) in Denmark in 2003–2016.
Note: The population includes incident patients with CD or UC who received treatment with Infliximab and Vedolizumab as their first biologic treatment. If there were more than 6 months between two consecutive treatments in treatment line one, we assumed a treatment stop.
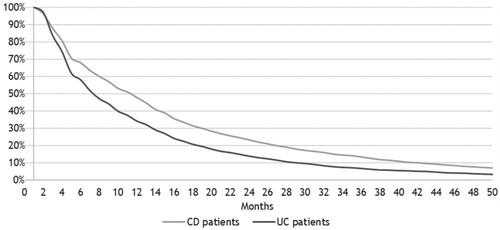
shows the time (in ‘units’) on biologic treatment line one (including all four biologic therapies). Per definition, all patients received biologic treatment for at least one unit (one hospital contact). Among CD patients, 75% received treatment for two or more units and 65% of UC patients. The drug survival is 50% for CD patients at the time of 6 units, and for UC patients it is 50% at 3 units. shows the time in months on treatment line one. After 11 months, CD patient’s drug survival is 50%, and for UC patients it is 50% after 7 months.
Figure 5. Time (in units) on biologic treatment line one among 2,939 incident patients with Crohn’s disease (CD) and 2,504 incident patients with ulcerative colitis (UC) in Denmark treated with biologics in 2003–2016.
Note: The population includes incident patients with CD or UC who received treatment with biologics after their IBD diagnosis. One unit is the number of days between treatments for the different therapies as recommended by national guidelines. One unit was defined as follows: adalimumab: 1 unit = 14 days, infliximab and vedolizumab: 1 unit = 56 days, and golimumab: 1 unit = 28 days. If there are more than 6 months between two consecutive treatments in treatment line one, we assume a treatment stop.
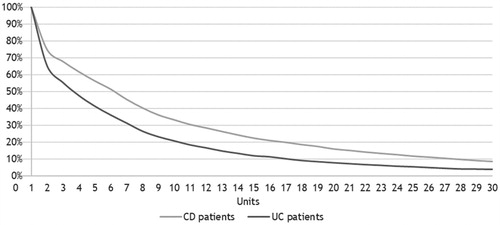
Figure 6. Time (in months) on biologic treatment line one among 2,939 incident patients with Crohn’s disease (CD) and 2,504 incident patients with ulcerative colitis (UC) in Denmark treated with biologics in 2003–2016.
Note: The population includes incident patients with CD or UC who received treatment with biologics after their IBD diagnosis. If there are more than 6 months between two consecutive treatments in treatment line one, we assume a treatment stop.
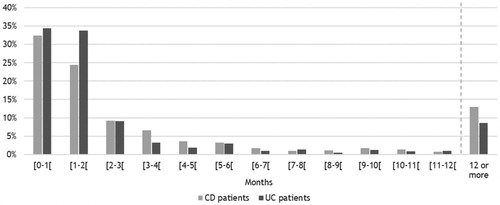
For patients who received treatment with more than one biologic therapy during the study period, the number of months between the last treatment in treatment line one and the first treatment in treatment line two is presented in . Approximately one-third of CD and UC patients received treatment with their second line biologic less than 1 month after the last treatment of their first line. About 57–68% of patients received treatment with their second-line therapy within 2 months of their last treatment in treatment line one. For 13% of CD patients and 8.6% of UC patients, there is a span of 12 months or more between the last treatment in treatment line one and the first treatment in treatment line two. More detailed information can be found in Supplementary Table S4.
Figure 7. Months between last treatment in treatment line one and first treatment in treatment line two among 1,017 incident patients with Crohn’s disease (CD) and 581 incident patients with ulcerative colitis (UC) in Denmark treated with more than one biologic therapy in 2003–2016.
Note: The population includes incident patients with CD or UC who received treatment with more than one biologic therapy.
Among all CD and UC patients receiving biologics, 43.1% (N = 1,266) and 59.4% (N = 1,488), respectively, discontinued treatment with biologics more than 1 year before the end of follow-up or death.
Discussion
In this study, we investigated the treatment patterns of IBD patients taking biologic therapies, including when treatment started after diagnosis, the share of the population initiating biologic treatment at any point during the study period, and how long patients remained on each treatment. The study discovered that the number of patients initiating biologic treatments after diagnosis increased throughout the study period. Additionally, approximately one-third of CD and UC patients treated with more than one biologic received treatment with their second line therapy less than 1 month after the last treatment of their first-line biologic therapy.
For the two IV therapies, the second and third treatments are administered 2 and 6 weeks after the first treatment, respectively. Considering this, it appears that the guidelines regarding duration between treatments are followed relatively closely. However, if treatment within the induction period accounts for less than one-third of all treatments a patient receives, dose escalation can be another explanation of the findings.
Among 13% of CD and 8.6% of UC patients, there is a span of 12 months or more between the last treatment in treatment line one and the first treatment in treatment line two. Generally, if a patient responded well to treatment and consequently discontinued, it would be expected that the patient would continue treatment with the same biologic therapy, in the case of relapse. It is thus rather unexpected to find patients beginning treatment with a second type of therapy after a treatment break of 12 months or more. However, a possible explanation might be that these patients had participated in clinical trials. Clinical trials of biologics for IBD increased over the study period, but we do not expect that many patients were switched to another biologic for non-medical reasons. Five out of 21 IBD clinics in Denmark were involved with trials of adalimumab for CD and two sites for UC. Four were involved with trials of golimumab for UC. Two clinics were included for trials of vedolizumab for UC and none for CD (clinicaltrials.gov).
Our study also observed a decreasing incidence of CD and UC in the later years. This may be related to the selection criteria of two or more contacts regarding the disease. Comparing patients diagnosed earlier to patients with their first contact at the end of the study period, those diagnosed later have a lesser chance of fulfilling this criterion. Thus, the incidence in the later years is probably underestimated, contrasting the increase found by Lophaven et al. [Citation11].
Strengths and limitations
This study has several strengths. The identification of CD and UC patients and their biologic treatment relies on the ICD-10 coding and treatment codes entered in the NPR. The reliability and validity of this register is in general considered to be high, however there are currently no studies explicitly validating the registration of CD and UC diagnosis codes [Citation8,Citation12,Citation13]. As this study is based on national registers, selection and information bias are limited as all Danish residents are included, the data are prospectively collected, and the quality of data is generally considered to be good. This also means that the generalizability of the results is high, as the study covers all incident cases of CD and UC in Denmark between 2003 and 2015.
This study also has some limitations. The first biologic therapy for CD and UC patients, infliximab, was introduced to the European market in 1999 followed by adalimumab in 2003 [Citation6,Citation14], implying that the biologic treatment options in the first years of our study period are limited. Given the differences in how studied treatments are administered in clinical practice, it was not possible to document the exact date of administration and dosing frequency of subcutaneous treatments, thus affecting the results of the analyses. Treatment with biologics is administered within the hospital sector, therefore treatment with IV therapies like infliximab and vedolizumab always takes place there, usually during an outpatient visit. Subcutaneous treatments like adalimumab and golimumab may be administered in the hospital or the patient may take the medicine at home. Therefore, if the medicine is taken at home, we do not know the exact date of administration, affecting the results of the analyses regarding time on biologic treatment line and months between last treatment in treatment line one and first treatment in treatment line two.
Conclusion
The findings of this study show an increasing trend in the percentage of patients receiving a biologic treatment over the course of the study period and that most CD and UC patients diagnosed receive biologic treatments relatively soon after diagnosis, within the first year. As more biologic treatments become available for IBD patients, future studies should focus on identifying disease markers to provide earlier diagnosis and treatment and thereby provide the patients earlier improved health.
Author contributions
The authors have contributed as follows: KV, TRJ, NQ and PM, contributed to the concept and design of the study, review and interpretation of the data, drafting and critical review of the manuscript, and approval of the final draft. SA and AB contributed to the review and interpretation of the data, drafting and critical review of the manuscript and approval of the final draft. AN contributed to the concept and design of the study, data acquisition, extraction and analysis, review and interpretation of the data, drafting and critical review of the manuscript and approval of the final draft.
Supplemental Material
Download PDF (96 KB)Acknowledgements
The authors thank Christina Wennerström, Janssen, for valuable critique of the manuscript.
Disclosure statement
The funder Janssen Pharmaceuticals provided support in the form of salaries for authors KV, SA and AB. TRJ is a former employee of Janssen Pharmaceuticals. AN is a paid employee at Incentive. NQ and PM have no conflict of interest to declare.
Additional information
Funding
References
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778.
- Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12:S3–S9.
- Torres J, Mehandru S, Colombel J-F, et al. Crohn’s disease. Lancet Lond Engl. 2017;389:1741–1755.
- Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet Lond Engl. 2017;389:1756–1770.
- European Crohn’s and Colitis Organization (ECCO). IBD treatments. Available from: https://www.ecco-ibd.eu/images/1_About_ECCO/1_8_ECCO/IBDTreatments.pdf
- European Medicines Agency. Summary of product characteristics - Remicade. Available from: https://www.ema.europa.eu/en/documents/product-information/remicade-epar-product-information_en.pdf
- RADS. Laegemiddelrekommandation med dyre laegemidler til behandling af kroniske inflammatoriske tarmsygdomme (IBD). 2017.
- Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490.
- Statistics Denmark. Register- og variabeloversigter. [cited 2018 Sep 27]. Available from: https://www.dst.dk/da/TilSalg/Forskningsservice/Data/Register_Variabeloversigter
- Sundhedsdatastyrelsen. Registre [Registers]. [cited 2018 Sep 27]. Available from: https://www.esundhed.dk/Registre
- Lophaven SN, Lynge E, Burisch J. The incidence of inflammatory bowel disease in Denmark 1980-2013: a nationwide cohort study. Aliment Pharmacol Ther. 2017;45:961–972. Apr
- Thygesen LC, Daasnes C, Thaulow I, et al. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39:12–16.
- Kruse M, Christiansen T. Register-based studies of healthcare costs. Scand J Public Health. 2011;39:206–209.
- European Medicines Agency. Summary of product characteristics - Humira. Available from: https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf
