Abstract
Objectives
Fecal calprotectin is a valued surrogate marker for intestinal inflammation. It has been argued that calprotectin levels are higher in early age than in later life hampering the use of calprotectin in young children.
Subjects and methods
To study age-related variation, we used data from our laboratory information system on consecutive, unselected fecal calprotectin measurements from 2014 to 2017 in all children aged 0 to 18 years. From each individual, the first measurement was included and repeated measurements were excluded. Fecal calprotectin was quantitated in the major clinical laboratory in southern Finland, HUSLAB with an ELISA kit from Calpro AS (Calpro/Calprolab, Oslo, Norway). Currently, the assay is performed on two automatic pipetting analysers (Dynex DS2, Chantilly, USA) according to the instructions of the manufacturer.
Results
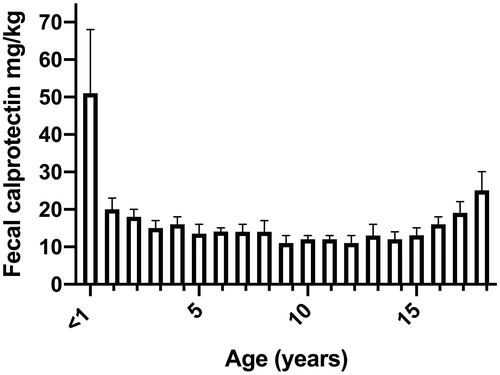
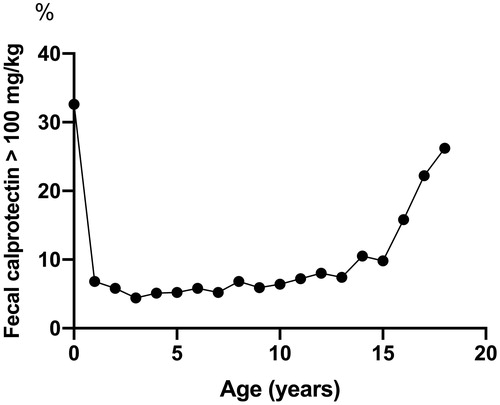
There were altogether 11,255 fecal calprotectin results from as many children. The median level of fecal calprotectin was 51 mg/kg in infants < 1 year of age (95th percentile 648 mg/kg; n = 239). This was 3–4-fold higher when compared to yearly age groups from 1 to 10 years (total number of children included 5,691). Across yearly age groups from 11 to 18, the median values varied from 11 to 19 mg/kg (total number of included children 5,325). The proportion of samples above the routine cut-off for an elevated concentration >100 mg/kg increased with increasing age.
Conclusions
Fecal calprotectin values in children beyond the first year of life are in general low and comparable in children and adolescents.
Introduction
Fecal calprotectin measurement is mostly used to screen for the possibility of inflammatory bowel disease, IBD. There is no general consensus of the most accurate cut-off for a raised values and cut-offs of > 50 mg/kg, ≥ 100 mg/kg, ≥ 250 mg/kg are most frequently used [Citation1,Citation2]. It is important to keep in mind that the performance of the different calprotectin ELISA assays varies regarding specificity and absolute values [Citation3]. There is also some individual variation on a daily basis but this rarely hampers the diagnostic utility of the measurement [Citation4]. Bowel preparation for endoscopy may affect the levels even for several days after the procedure and therefore it is advised to take a sample if needed either before bowel cleansing has started or not earlier than one week after endoscopy [Citation5].
Recently, normal values for fecal calprotectin were described in a longitudinal cohort of infants for neonatal, 6 months, 12 months and 24 months old infants using an in-house turbidimetric immunoassay [Citation6]. Fecal calprotectin values were high during the first year of life and declined thereafter. The authors suggested an upper reference limit of 57 mg/kg for an increased concentration at 2 years of age. The study cohort, however, included only 84 infants whose health status was followed up by parental questionnaires. Another recent report concluded that as fecal calprotectin vary according to age, there should be different cut-offs for young age groups and suggestions of 910 mg/kg for 0–12 months, 286 mg/kg for >1 to 4 years and 54.4 mg/kg <4 to 12 years were made. This was based on a study cohort of 174 included children. Samples were measured with EliA 2 Calprotectin Assay (Phadia, Sweden) [Citation7]. Although fecal calprotectin values seem to decrease along increasing age, there is no consensus on whether different cut-offs should be used for children or not. However, most authors promote separate consideration for children younger than four years of age compared to older children [Citation8,Citation9]. In Finland, fecal calprotectin measurement was adopted early and it has been in clinical use for more than 15 years. Our laboratory has been responsible for the majority of the analyses. This prompted us to look for the distribution of fecal calprotectin values in a large cohort of children of more than 10,000 assessments during a four-year period.
Methods
Fecal calprotectin measurement
Fecal calprotectin has been quantitated in the major clinical laboratory in southern Finland, HUSLAB, with an ELISA kit from Calpro AS (Calpro/Calprolab, Oslo, Norway) since 2008. Currently, the assay is performed on two automatic pipetting analysers (Dynex DS2, Chantilly, USA) according to the instructions of the manufacturer. In brief, feces was manually weighed (100 mg) and extracted with a 50-fold excess of extraction buffer. After centrifugation of the extract, samples and reagents were loaded on the analyser and the assay performed according to the instructions.
Study cohort
We used data from our laboratory information system (LIS) on consecutive, unselected fecal calprotectin measurements during four subsequent years from 2014 to 2017 in all children aged 0 to 18 years. In case of follow-up samples from the same patient, only the first sample taken during this time period was included in the study. However, as the data are based on the laboratory files (including encrypted patient number, date, age, sex, requestor and the result from fecal calprotectin assay), we had no access to the diagnoses or symptoms of the children, whose samples were sent to the laboratory.
Our routine cut-off for an elevated value is ≥ 100 mg/kg [Citation10]. Thus, we also assessed the proportion of elevated values at each age group according to this cut-off.
Ethics
This was a register-based study and, according to Finnish legislation, ethical approval or informed consents were not needed as the patients were not contacted.
Statistical analysis
Data are presented as median and interquartile range (IQR) unless otherwise stated. If the fecal calprotectin value was <5 mg/kg, we gave a value 5 for these samples for statistical analyses. The samples were diluted to reach exact numerical results. Thus, there was no upper cut-off value. We used Fisher’s exact test to determine differences in binary variables. Non-parametric Mann-Whitney test was used to compare continuous variables between the groups as appropriate. The software used for the analysis was Graph Pad Prism version 8.0 (GraphPad Software, San Diego, CA). The level of significance was set at a p value of < .05.
Results
Fecal calprotectin values according to age
In total, there were 11,255 samples from equally many children aged 0 to 18 years (females/males n = 5520/5735) analyzed for fecal calprotectin. The results according to age groups are shown in . During the latter two-year period the total number of fecal calprotectin measurements had increased by 1.5% but there was no major change in the values or their distribution as such when statistically tested (data not shown). About 80% of the samples were obtained from the county of Uusimaa, including the capital area.
Table 1. Fecal calprotectin values measured in a cohort of 11,255 children during 2014–2017.
The age group younger than one year of age differed from the other age groups in that the median concentration of fecal calprotectin in this group was 3–4-fold higher than in the other groups ( and and Supplemental Figure). In infants less than 6 months of age, the median calprotectin was 129 (IQR 51–268, n = 98), and in infants aged 6 to <12 months 29 mg/kg (IQR 13–75, n = 141), respectively. The difference between these age groups was statistically significant (p < .0001).
Fecal calprotectin values exceeding the routine cut-off
The proportion of values exceeding 100 mg/kg, the cut-off of our clinical laboratory, changed according to age (). In the age interval 1–10 yrs the proportion was fairly stable at about 5% but started to increase thereafter being about 26% in those aged 18 yrs. Upper 95% values increased accordingly (), most likely reflecting the number of patients with IBD.
Fecal calprotectin values according to sex
The results were comparable between boys and girls when the whole study population was compared (). However, in the age groups of 17 and 18 years, boys had significantly higher calprotectin values (medians 25 mg/kg (n = 298) and 15 mg/kg (girls n = 414) p < .0024 and 47 mg/kg (n = 312) and 19 mg/kg (girls n = 492) p < .0001, respectively).
Discussion
Fecal calprotectin is widely used as a surrogate marker for intestinal inflammation in adults and as well in children. The main indication for its measurement is to screen for the possibility of IBD or to follow up disease activity in diagnosed patients with IBD [Citation2,Citation11–13]. Fecal calprotectin is non-specific and raised values may be found e.g. in juvenile polyps [Citation14], bacterial and viral gastroenteritis [Citation15], NSAID enteropathy or carcinoma [Citation1]. There is no clear consensus for a definite cut-off for a raised value. Most manufacturers of immunoassays suggest a cut-off of > 50 mg/kg. In our clinical routine we use a cut-off of ≥ 100 mg/kg [Citation10]. However, there are published reports suggesting that in young children fecal calprotectin values are higher than in adolescents and adults, and different cut-offs should be used for various age groups [Citation7,Citation9,Citation16,Citation17]. Here we showed in a cohort of 11,255 pediatric samples that beyond the first year of life, the median values of fecal calprotectin were comparable across different age groups.
Our clinical laboratory has performed analyses of fecal calprotectin since 2008. From the start until now, the assay has remained the same. For this there are two main reasons. Firstly, the clinical performance of the assay is reliable with high throughput. This is important as currently the total annual number of fecal calprotectin analyses is 29,500 with an average yearly increase of about 8%. Secondly, the performance of the assay is stable with no major fluctuation in the results. This is especially important when fecal calprotectin values are followed up and used in clinical decision-making.
In previous studies, it has been suggested that fecal calprotectin is not as reliable surrogate marker in children younger than four years of age compared to adults. The reason for this conclusion was that values are higher in young children [Citation9,Citation16,Citation17]. Recently, it was stated that due to variable levels, fecal calprotectin should be used with caution in children younger than 10 years of age [Citation8]. Our results do not support these conclusions as we found no significant difference in median calprotectin values in pediatric samples beyond one year of age. Our cohort is the largest in sample size reported so far and all the previous studies included considerably lower number of samples.
On contrary, fecal calprotectin was higher during the first year of life as we observed here and as has been reported [Citation6,Citation7,Citation17–19]. It has been suggested that the increased permeability of the gut might increase the influx of inflammatory cells in the gut in babies and thus, have an impact on the higher fecal calprotectin values. Sex has no effect on fecal calprotectin values [Citation16]. In our large study cohort, fecal calprotectin values were in general comparable between boys and girls.
We compared the fecal calprotectin test results in pediatric samples in two sequential two-year periods and found no major differences in the absolute values but there was an increase of 1.5% in the total number of analyses. In adolescents beyond 15 years of age, the proportion of fecal calprotectin values above the clinical cut-off started to increase. Also, among the 17 and 18 -year-olds, boys showed higher calprotectin values. These findings may reflect the overall increase in the incidence of pediatric IBD among adolescents in our country as in IBD fecal calprotectin values are much higher than in healthy children or children with other diseases [Citation20,Citation21]. However, as this was a register-based study, we did not have access to the clinical indications or diagnoses of the patients whose samples were included.
In conclusion, in a large group of pediatric samples, we observed no major differences in the median values of fecal calprotectin in yearly age groups beyond one year of age. During the first year of life, fecal calprotectin values were clearly higher than in later life. The reasons for this are yet undefined.
Supplemental Material
Download Zip (524.4 KB)Acknowledgements
We would like to thank PhD Tuula Metso who run the electronic search for the fecal calprotectin values from the laboratory files.
Disclosure statement
The authors report no conflict of interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Manceau H, Chicha-Cattoir V, Puy H, et al. Fecal calprotectin in inflammatory bowel diseases: update and perspectives. Clin Chem Lab Med. 2017;55(4):474–483.
- Sipponen T, Kolho KL. Fecal calprotectin in diagnosis and clinical assessment of inflammatory bowel disease. Scand J Gastroenterol. 2015;50(1):74–80.
- Prell C, Nagel D, Freudenberg F, et al. Comparison of three tests for faecal calprotectin in children and young adults: a retrospective monocentric study. BMJ Open. 2014;4(5):e004558.
- Kristensen V, Malmstrøm GH, Skar V, et al. Clinical importance of faecal calprotectin variability in inflammatory bowel disease: intra-individual variability and standardisation of sampling procedure. Scand J Gastroenterol. 2016;51(5):548–555.
- Kolho KL, Alfthan H, Hamalainen E. Effect of bowel cleansing for colonoscopy on fecal calprotectin levels in pediatric patients. J Pediatr Gastroenterol Nutr. 2012;55(6):751–753.
- Peura S, Fall T, Almqvist C, et al. Normal values for calprotectin in stool samples of infants from the population-based longitudinal born into life study. Scand J Clin Lab Invest. 2018;78(1–2):120–124.
- Roca M, Rodriguez Varela A, Donat E, et al. Fecal calprotectin and eosinophil-derived neurotoxin in healthy children between 0 and 12 years. J Pediatr Gastroenterol Nutr. 2017;65:394–398.
- Joshi S, Lewis SJ, Creanor S, et al. Age-related faecal calprotectin, lactoferrin and tumour M2-PK concentrations in healthy volunteers. Ann Clin Biochem. 2010;47(3):259–263.
- Davidson F, Lock RJ. Paediatric reference ranges for faecal calprotectin: a UK study. Ann Clin Biochem. 2017;54(2):214–218.
- Kolho KL, Raivio T, Lindahl H, et al. Fecal calprotectin remains high during glucocorticoid therapy in children with inflammatory bowel disease. Scand J Gastroenterol. 2006;41(6):720–725.
- Bunn SK, Bisset WM, Main MJ, et al. Fecal calprotectin: validation as a noninvasive measure of bowel inflammation in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;33:14–22.
- Heida A, Park KT, van Rheenen PF. Clinical utility of fecal calprotectin monitoring in asymptomatic patients with inflammatory bowel disease: a systematic review and practical guide. Inflamm Bowel Dis. 2017;23(6):894–902.
- Haisma SM, Verkade HJ, Scheenstra R, et al. Time-to-reach target calprotectin level in newly diagnosed patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2019;69(4):466–473.
- Olafsdottir I, Nemeth A, Lörinc E, et al. Value of fecal calprotectin as a biomarker for juvenile polyps in children investigated with colonoscopy. J Pediatr Gastroenterol Nutr. 2016;62:43–46.
- Chen CC, Huang JL, Chang CJ, et al. Fecal calprotectin as a correlative marker in clinical severity of infectious diarrhea and usefulness in evaluating bacterial or viral pathogens in children. J Pediatr Gastroenterol Nutr. 2012;55:541–547.
- Fagerberg UL, Loof L, Merzoug RD, et al. Fecal calprotectin levels in healthy children studied with an improved assay. J Pediatr Gastroenterol Nutr. 2003;37(468):468–472.
- Oord T, Hornung N. Fecal calprotectin in healthy children. Scand J Clin Lab Invest. 2014;74(3):254–258.
- Song JY, Lee YM, Choi YJ, et al. Fecal calprotectin level in healthy children aged less than 4 years in South Korea. J Clin Lab Anal. 2017;31(6):e22113.
- Zhu Q, Li F, Wang J, et al. Fecal calprotectin in healthy children aged 1–4 years. PLoS One. 2016;11(3):e0150725.
- Virta LJ, Saarinen MM, Kolho KL. Inflammatory bowel disease incidence is on the continuous rise among all paediatric patients except for the very young: a nationwide registry-based study on 28-year follow-up. J Crohns Colitis. 2017;11(2):150–156.
- Olafsdottir E, Aksnes L, Fluge G, et al. Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Acta Paediatr. 2007;91(1):45–50.