Pancreatic cancer is an exceptionally aggressive tumor that is recalcitrant to most therapies. The 5-year survival rate is below 5% [Citation1]. Metabolic reprogramming is a key feature of pancreatic cancer and appears to contribute to tumor aggressiveness [Citation2]. Pancreatic cancer cells can adapt their metabolism to the specific environmental conditions within the tumor microenvironment and utilize alternative nutrient acquisition pathways [Citation3]. In this way, pancreatic cancer cells can survive under very severe and nutrient-poor conditions. Oncogenic KRAS is a pivotal tumor driver in pancreatic cancer and recent evidence suggests that it governs much of the metabolic rewiring that occurs in this tumor [Citation4].
Metabolic analysis of pancreatic cancer tissue has revealed a depletion of several major nutrients such as glucose, glutamine and serine as compared to non-tumor controls [Citation5]. However, the tumors are still able to accumulate essential amino acids. Macropinocytosis has been proposed as a mechanism for the accumulation of these essential amino acids. Several studies [Citation5–7] have confirmed the presence of active macropinocytosis in pancreatic tumor specimens as measured by intracellular uptake of high molecular weight dextran. This is further supported by experimental data from these studies showing that cultured pancreatic cancer cells can obtain enough amino acids via macropinocytosis, even when albumin is the only amino acid source.
Macropinocytosis is an endocytic mechanism whereby extracellular fluid is internalized into eukaryotic cells through vesicles that are termed ‘macropinosomes’ [Citation8]. This process involves actin-mediated remodeling of the cell membrane to engulf the extracellular contents. The ingested material is degraded in the lysosome to support metabolism and macromolecular synthesis. Macropinocytosis is involved in several physiological processes including nutrient uptake, antigen presentation, as well as cell signaling and migration [Citation9]. Normal macropinocytic function can be subverted in various pathological settings. Bacteria, viruses and prions can use macropinocytosis to invade host cells, and cancer cells are able to use this pathway to scavenge extracellular nutrients [Citation10]. Mutations in oncogenes, as well as tumor suppressors, have been shown to induce macropinocytosis in cancer cells [Citation11]. Growth factors can also stimulate macropinocytosis in cancer [Citation12].
Oncogenic KRAS mutations occur in almost 95% of all pancreatic ductal adenocarcinomas [Citation13]. The KRAS gene encodes the protein KRAS, a small GTPase that acts as a molecular switch for various cellular processes. The majority of pancreatic cancers harbor an activating point mutation of the KRAS oncogene on codon G12 (98%). Several different amino acid substitutions have been identified at G12, mainly represented by G12D, G12V and G12R [Citation14]. These mutations render KRAS persistently GTP-bound and constitutively active, leading to stimulation of KRAS downstream signaling pathways and induction of several phenotypic hallmarks of cancer, such as uncontrolled proliferation, avoidance of apoptosis, tissue invasion and metastasis, as well as altered metabolism. KRASG12D/V has been found to drive macropinocytosis via PI3Kα, while KRASG12R, due to structural alterations in switch II, acts through PI3Kγ () [Citation15,Citation16]. Oncogenic KRAS also drives the cell surface expression of syndecan 1 (SDC1), which upregulates the macropinocytic pathway [Citation17]. The level of macropinocytosis in KRAS mutant pancreatic cancer cells can vary with changes in nutrient supply [Citation18,Citation19]. Thus, the macropinocytic drive is not only dependent on underlying genetic changes but also on the microenvironmental conditions.
Figure 1. Proposed molecular mechanisms by which oncogenic KRAS signaling drives macropinocytosis in pancreatic cancer.
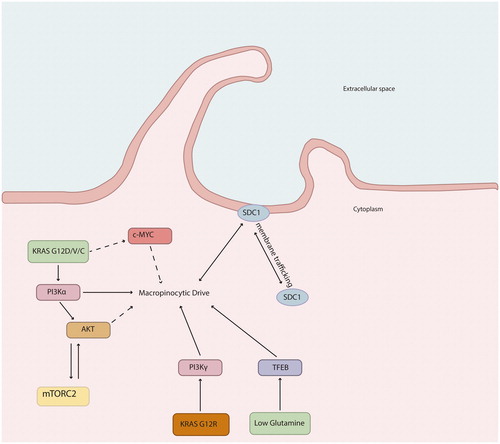
In addition to KRAS, other signaling pathways have also been demonstrated to stimulate macropinocytosis in pancreatic tumor cells. PTEN is a tumor suppressor gene encoding a dual-specificity phosphatase that antagonizes the PI3K signaling pathway and negatively regulates the MAPK pathway. Approximately 10% of pancreatic cancers display PTEN deficiency. In KRAS-driven pancreatic cancer, PTEN deficiency appears to increase macropinocytosis via mTORC2 [Citation20]. The WNT pathway is another driver of macropinocytosis [Citation21]. Enhanced activity of YAP/TAZ has been reported in pancreatic cancer cells under nutrient-deprived conditions. The target of YAP/TAZ transcription, Axl, has been linked to the activation of macropinocytosis under these conditions [Citation22].
Pancreatic cancer cells are greatly dependent on enhanced macropinocytosis for their survival and proliferation. However, it still to be determined whether macropinocytosis can be exploited as a therapeutic target in pancreatic cancer. Several different strategies targeting KRAS activity and macropinocytosis are currently under investigation, including a) direct KRAS targeting, b) indirect KRAS targeting, c) macropinocytosis inhibitors and d) the use of macropinocytosis as a vehicle for drug delivery. KRAS has for many years been considered ‘undruggable’. Only recently have small molecules that directly bind to KRAS and cause perturbations in function been described and characterized. Clinical trials are ongoing of allele-specific mutant KRAS inhibitors in a variety of tumor types [Citation23]. Indirect targeting of KRAS by interrupting upstream regulators or downstream effectors is possible but has proven to be clinically challenging [Citation24]. Given the importance of SDC1 in macropinocytosis, this molecule may represent an attractive drug target. Autologous CAR T-Cells targeting SDC1 is being tested for clinical activity in multiple myeloma (NCT03672318). The substance 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) has been found to be a specific inhibitor of macropinocytosis. It reduces macropinocytic uptake and cancer growth in pancreatic xenograft tumors [Citation6,Citation7]. The effects of EIPA seem to be specific to KRAS mutant tumors, as EIPA treatment did not affect the growth of KRAS-wild-type tumors. Macropinocytosis can also be used as a drug delivery strategy. Albumin is a major component in extracellular fluid taken up by macropinocytosis. The application of albumin or albumin domain-based drug delivery may be an effective approach to improve the efficacy of antitumor drugs [Citation6,Citation25–27]. In addition to fluid-phase nutrients such as proteins, pancreatic cancer cells use macropinocytosis to internalize secreted vesicles (exosomes). Engineered exosomes (iExosomes) have been used for therapeutic targeting of KrasG12D in mouse models of pancreatic cancer [Citation28].
In summary, macropinocytosis has recently emerged as a critical metabolic adaptation process in pancreatic cancer enabling the cancer cells to survive in a harsh tumor microenvironment. Elucidation of the molecular mechanisms that drive macropinocytosis may facilitate the development of new types of intervention, thereby targeting specific metabolic vulnerabilities. In this editorial, we have outlined some of the signaling networks that regulate macropinocytosis in pancreatic tumor cells. Further exploration of molecular pathways and pharmacological inhibition is mandated in order to clarify the true therapeutic benefit of macropinocytosis in pancreatic cancer.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bengtsson A, Andersson R, Ansari D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci Rep. 2020;10(1):16425.
- Biancur DE, Kimmelman AC. The plasticity of pancreatic cancer metabolism in tumor progression and therapeutic resistance. Biochim Biophys Acta Rev Cancer. 2018;1870(1):67–75.
- Derle A, De Santis MC, Gozzelino L, et al. The role of metabolic adaptation to nutrient stress in pancreatic cancer. Cell Stress. 2018;2(12):332–339.
- Pupo E, Avanzato D, Middonti E, et al. KRAS-driven metabolic rewiring reveals novel actionable targets in cancer. Front Oncol. 2019;9:848.
- Kamphorst JJ, Nofal M, Commisso C, et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75(3):544–553.
- Davidson SM, Jonas O, Keibler MA, et al. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat Med. 2017;23(2):235–241.
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497(7451):633–637.
- Commisso C. The pervasiveness of macropinocytosis in oncological malignancies. Philos Trans R Soc Lond B Biol Sci. 2019;374(1765):20180153.
- Lin XP, Mintern JD, Gleeson PA. Macropinocytosis in different cell types: similarities and differences. Membranes. 2020;10(8):177.
- Bloomfield G, Kay RR. Uses and abuses of macropinocytosis. J Cell Sci. 2016;129(14):2697–2705.
- Zhang Y, Commisso C. Macropinocytosis in cancer: a complex signaling network. Trends Cancer. 2019;5(6):332–334.
- Palm W. Metabolic functions of macropinocytosis. Philos Trans R Soc Lond B Biol Sci. 2019;374(1765):20180285.
- Bryant KL, Mancias JD, Kimmelman AC, et al. KRAS: feeding pancreatic cancer proliferation. Trends Biochem Sci. 2014;39(2):91–100.
- Buscail L, Bournet B, Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2020;17(3):153–168.
- Cayron C, Guillermet-Guibert J. The type of KRAS mutation drives PI3Kalpha/gamma signalling dependency: implication for the choice of targeted therapy in pancreatic adenocarcinoma patients. Clin Res Hepatol Gastroenterol. 2020. DOI:10.1016/j.clinre.2020.05.021
- Hobbs GA, Baker NM, Miermont AM, et al. Atypical KRASG12R mutant is impaired in PI3K signaling and macropinocytosis in pancreatic cancer. Cancer Discov. 2020;10(1):104–123.
- Yao W, Rose JL, Wang W, et al. Syndecan 1 is a critical mediator of macropinocytosis in pancreatic cancer. Nature. 2019;568(7752):410–414.
- Nofal M, Zhang K, Han S, et al. mTOR inhibition restores amino acid balance in cells dependent on catabolism of extracellular protein. Mol Cell. 2017;67(6):936–946.
- Lee SW, Zhang Y, Jung M, et al. EGFR-Pak signaling selectively regulates glutamine deprivation-induced macropinocytosis. Dev Cell. 2019;50(3):381–392.
- Michalopoulou E, Auciello FR, Bulusu V, et al. Macropinocytosis renders a subset of pancreatic tumor cells resistant to mTOR inhibition. Cell Rep. 2020;30(8):2729–2742.
- Redelman-Sidi G, Binyamin A, Gaeta I, et al. The canonical Wnt pathway drives macropinocytosis in cancer. Cancer Res. 2018;78(16):4658–4670.
- King B, Araki J, Palm W, et al. Yap/Taz promote the scavenging of extracellular nutrients through macropinocytosis. Genes Dev. 2020;34(19–20):1345–1358.
- Moore AR, Rosenberg SC, McCormick F, et al. RAS-targeted therapies: is the undruggable drugged? Nat Rev Drug Discov. 2020;19(8):533–552.
- Nollmann F, Ruess D. Targeting mutant KRAS in pancreatic cancer: futile or promising? Biomedicines. 2020;8(8):281.
- Liu H, Sun M, Liu Z, et al. KRAS-enhanced macropinocytosis and reduced FcRn-mediated recycling sensitize pancreatic cancer to albumin-conjugated drugs. J Control Release. 2019;296:40–53.
- Sheng W, Geng J, Li L, et al. An albuminbinding domain and targeting peptidebased recombinant protein and its enediyneintegrated analogue exhibit directional delivery and potent inhibitory activity on pancreatic cancer with Kras mutation. Oncol Rep. 2020;43(3):851–863.
- Wang X, Sheng W, Wang Y, et al. A macropinocytosis-intensifying albumin domain-based scFv antibody and its conjugate directed against K-Ras mutant pancreatic cancer. Mol Pharm. 2018;15(6):2403–2412.
- Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503.
