Abstract
Objectives: The effect of pregnaSSncy on the course of inflammatory bowel disease (IBD) remains controversial. We aimed to describe the disease course before and after a first pregnancy in IBD patients.
Methods: We analyzed data from a prospectively followed-up pregnancy cohort (minimal follow-up of 7 years), with clinical, biochemical and endoscopic characteristics obtained pre-pregnancy, during pregnancy and post-pregnancy. Possible factors associated with relapse (disease activity during pregnancy, maternal age, smoking, alcohol use, pre-pregnancy BMI, mode of delivery, thiopurine use during pregnancy, biological use during pregnancy, combination of thiopurine and biological use during pregnancy, breastfeeding, IBD diagnosis, endoscopic scores) were scored.
Results: One hundred twenty six patients (95 Crohn’s Disease [CD; 75%] and 31 Ulcerative Colitis/IBD unclassified [UC/IBD-U; 25%]) were enrolled, with one hundred pregnancies occurring in 100 primigravida patients. All pregnancies resulted in live birth. Twenty patients (20%) had a relapse during pregnancy. The median number of relapses/patient/year was 0.25 (IQR 0.5) and 0 (IQR 0.43) respectively before and after pregnancy (p = .00). For CD patients the median relapses/person/year was 0.25 (IQR 0.5) before and 0 (IQR 0.25) after delivery (p = .00), for UC/IBD-U patients there was no significant difference. In the post-partum period more UC patients relapsed compared to CD patients (68% vs 30.7%, p = .01). Seven-year IBD-course was unchanged in the 26 women who did not become pregnant.
Conclusion: In this prospective observational cohort study, we found a lower rate of relapses in the 4 years after delivery compared to the 3 years prior to a first pregnancy. Post-partum, more UC patients experienced a relapse compared to CD patients.
Introduction
IBD affects many women of childbearing age and gaining information on the effect of IBD on pregnancy and vice versa is of great importance, not only for a successful and healthy pregnancy, but also for the health of mother and child postpartum [Citation1–3]. Nevertheless, the effect of pregnancy on the course of Crohn’s disease (CD) and ulcerative colitis (UC) remains controversial.
During pregnancy hormonal, immunological and microbial changes take place in the female body [Citation4], which together allow for the growth of an MHC-mismatched fetus, suggesting an enhanced immunological tolerance. These changes are also observed in pregnant IBD patients. For instance, we showed that reduced microbial α-diversity present in IBD women at first trimester normalizes to diversity levels seen in healthy pregnancy at trimester 2 and 3 [Citation5]. Furthermore, serum pro-inflammatory cytokine patterns in IBD patients present pre-conception improve during pregnancy, and pregnancy hormones directly strengthen epithelial barrier functions [Citation5,Citation6]. The observation that the microbiome from multiparous IBD women differs from that of nulliparous IBD patients suggests that these changes may have a lasting effect. Nevertheless, studies on the course of IBD during and after pregnancy are limited and contradictory. Pedersen and colleagues demonstrated in a prospective study that pregnant CD patients in remission prior and during pregnancy experienced a similar number of relapses when compared to matched non-pregnant patients. However, while not significant due to low patient numbers, post-partum remission seemed to be achieved more often for patients with active disease during the third trimester as compared to matched non-pregnant IBD women. For pregnant UC patients, a higher risk of relapse during pregnancy as well as post-partum was reported as compared to non-pregnant UC controls [Citation7]. In contrast, Castiglione et al. described less relapses 3 years after childbirth compared to pre-pregnancy, for both UC and CD patients [Citation8]. A 10-year follow-up study described a decrease from 0.34 flares/year pre-pregnancy to 0.18 flares/year post-pregnancy in CD patients, while UC patients decreased from 0.76 flares/year pre-pregnancy to 0.12 flares/year post-pregnancy [Citation9]. In addition, it seems safe to stop anti-TNFα treatment in pregnant IBD patients without increasing the risk of a relapse during pregnancy [Citation10]. Of note, relapse rates are higher when CD patients conceive during active disease [Citation7]. For this reason, it is advised to strive for complete remission a minimum of 6 months prior to conception [Citation11,Citation12].
Thus, while molecular disease parameters appear to be beneficially modulated during pregnancy in IBD, controversy exists regarding their effect on clinical disease course. Therefore, the aim of our study was to assess the effect of pregnancy on the risk of relapse in primigravida IBD patients and to describe the disease course before and after a first pregnancy in patients with IBD.
Method
Study design and population
We analyzed data from our ongoing prospectively followed-up pregnancy cohort, where we collect clinical, biochemical and endoscopic characteristics prior to pregnancy, during pregnancy and post-partum. IBD patients with a pregnancy wish visited our outpatient clinic every 3 months when not pregnant, and every trimester during pregnancy. Patients were selected when the follow-up period was a minimum of 7 years and patients had not conceived before. When clinically necessary, patients were seen more frequently during this period. Not all included patients became pregnant during follow-up. To answer our main question we analyzed the course of the patients who did conceive separately. To assess if there is a difference between the women who did and did not conceive, we also describe the group in which there was no pregnancy.
Outcomes, data measurements and definitions
Relapse was defined as an endoscopic SES-CD score of ≥7 for CD, MAYO endoscopic score ≥ 2 for UC or Rutgeerts’ score ≥ i1 and/or fecal calprotectin >200 µg/g and/or medication adjustment during follow-up. The SES-CD score is displayed as the SES-CD score divided by the number of segments obtained during endoscopy.
Statistical methods
Statistical analyses were performed using IBM SPSS (version 24.0 Chicago III, USA). Descriptive statistics of continuous variables are depicted as medians with interquartile range (IQR) or means with standard deviation (SD) and compared using T-tests or Mann Whitney U tests. Categorical variables are displayed in absolute numbers and percentages and compared using Chi-square or Fisher’s exact tests. All tests were performed using 2-tailed tests. When the univariate analysis was significant we tested these factors in a multivariate analysis.
Ethical consideration
This study was approved by the ethics committee of the Erasmus Medical Center (Rotterdam, The Netherlands, MEC2013-579).
Results
The study population
In total, 126 patients with IBD (95 CD, 28 UC and 3 IBD-unclassified [IBD-U]) were enrolled with a mean follow-up of 7.1 ± 2.7 years. Of these, one hundred patients became pregnant during follow-up (75 CD, 23 UC and 2 IBD-unclassified [IBD-U]) and all pregnancies resulted in live birth. These women had a mean follow up of 7.3 ± 2.9 years (3.5 ± 1.9 years before pregnancy and 3.8 ± 2.0 years post-partum). Twenty-six patients did not conceive. This group consisted of 20 CD patients [76.9%], 5 UC patients [19.2%] and 1 IBD-U patient [3.8%], with a mean follow-up time of 6.3 ± 1.7 years falling within the same decade as the patients who did conceive. Baseline characteristics for the pregnant and non-pregnant patients are displayed in , medication use prior and during pregnancy is indicated in . Age was comparable among the groups (26.9 ± 4.2 vs 26.5 ± 5.6, p = .72, respectively), as was educational level (p = .44). Also BMI, smoking and whether patients underwent IBD surgery did not differ between pregnant and non-pregnant women.
Table 1. Characteristics pregnant and non-pregnant IBD patients.
Relapse per person per year
Overall, 74.6% of patients experienced a relapse during follow up (70.5% CD patients and 87.1% UC/IBD-U patients, p = .06). In the time leading up to pregnancy, 68% of the UC/IBD-U patients experienced a relapse compared to 66.7% of the CD patients (p = .902). Relapse during pregnancy occurred in significantly more UC/IBD-U patients (36%) compared to CD patients (14.7%, p = .021). Within the 4 years post-partum, relapse also occurred more often in the UC/IBD-U patients (68%) compared to the CD patients (30.7%, p = .01). When comparing CD patients pre-pregnancy and post-partum, less patients showed relapses after giving birth (66.7% vs 30.7%, respectively, p = .01, see ). Consistent with the pre-pregnancy data of the conceiving patients, no differences were observed between CD and UC patients who did not become pregnant during the follow-up period.
Figure 2. Disease course of IBD patients before, during and after pregnancy. (A) Significantly more UC/IBD-U patients than CD patients experienced relapses during pregnancy (36% vs 14.7%, respectively, p = .021) and post-partum (68% vs 30.7%, respectively, p = .01). Also, fewer CD patients presented with relapse post-partum as compared to pre-pregnancy (66.7% vs 30.7%, respectively, p = .01). (B) Mean relapses per person per year during the complete follow up time. For the patients who became pregnant this is displayed as 3 years pre-pregnancy and 4 years after pregnancy.
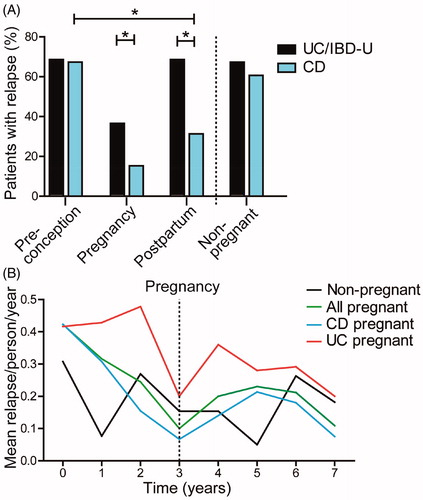
The number of relapses/patient/year in the total IBD group was 0.25 (IQR 0.5) in the time leading up to pregnancy and 0 (IQR 0.43) in the years after (p = .00). For CD patients (N = 75) the median relapses/person/year was 0.25 (IQR 0.5) before pregnancy and 0 (IQR 0.25) after delivery (p = .00). For UC/IBD-U patients (N = 25) there was no significant difference in the median relapses/person/year before and after pregnancy (0.25 [IQR 1.0] vs 0.25 [IQR 0.5], p = .57). The relapses/person/year during follow up time are displayed in , which shows that a decreasing trend in flare rate was already seen in the period leading up to pregnancy. When comparing the patients who became pregnant with those who did not, there was no significant difference in relapses/person/year in either the period prior to pregnancy (0.25 [IQR 0.5] for patients who became pregnant vs 0.18 [IQR 0.33] for those who did not, p = .15) or the post-partum period of the IBD patients (0 [IQR 0.43] vs 0.18 [IQR 0.33], p = .26, respectively).
Endoscopic scores
In total, 63 endoscopies were undergone by 45 patients in the 4 years prior to pregnancy, and 40 endoscopies were performed on 30 women in the 3 years post pregnancy. Endoscopies were performed for a suspected flare. For non-pregnant patients, 28 endoscopies were performed in 19 women during follow-up. Mean endoscopic SES-CD score for CD did not differ pre-conception compared to post-partum. Also when comparing post-partum with non-pregnant group there was no significant difference (see ). MAYO score of endoscopic severity of disease for UC also did not differ between pre-conceptional, post-partum and non-pregnant patients (see ).
Figure 3. Endoscopy scores of IBD patients before and after pregnancy. (A) Comparison of the median SES-CD endoscopy scores divided by segments obtained during endoscopy. No significant difference in score between preconception and post-partum was seen (1.4 [IQR, 0.5] vs 1.4 [IQR, 0.6], respectively, p = .56). Furthermore no significant difference was found when post-partum score of pregnant patients was compared to non-pregnant patients (1.4 [IQR, 0.6] vs 2.2 [IQR, 2.2], respectively, p = .15). (B) Comparison of MAYO scores for UC obtained during endoscopy. We did not find a significant difference in score between preconception and post-partum (2 [IQR, 0] vs 2 [IQR, 0.15], respectively, p = .94). Also no significant difference was found when post-partum was compared to non-pregnant patients (2 [IQR, 0.15] vs 2 [IQR, 0.38], respectively, p = .52).
![Figure 3. Endoscopy scores of IBD patients before and after pregnancy. (A) Comparison of the median SES-CD endoscopy scores divided by segments obtained during endoscopy. No significant difference in score between preconception and post-partum was seen (1.4 [IQR, 0.5] vs 1.4 [IQR, 0.6], respectively, p = .56). Furthermore no significant difference was found when post-partum score of pregnant patients was compared to non-pregnant patients (1.4 [IQR, 0.6] vs 2.2 [IQR, 2.2], respectively, p = .15). (B) Comparison of MAYO scores for UC obtained during endoscopy. We did not find a significant difference in score between preconception and post-partum (2 [IQR, 0] vs 2 [IQR, 0.15], respectively, p = .94). Also no significant difference was found when post-partum was compared to non-pregnant patients (2 [IQR, 0.15] vs 2 [IQR, 0.38], respectively, p = .52).](/cms/asset/3558bfdb-5386-4598-b4c7-6c768646052d/igas_a_1910996_f0002_b.jpg)
Possible patient-related factors associated with relapse rate
Factors as age, BMI, smoking, alcohol use, IBD related surgery and educational level did not differ significantly between patients who relapsed compared to those that did not. Similarly, mode of delivery and whether or not the patient had breastfed her child was not associated with relapsing post-partum.
In we display the percentage of patients on anti-TNFα therapy alone versus other IBD monotherapy experiencing a relapse per period. Patients who were on anti-TNFα post-partum seemed to have a better disease course (p = .03). Patients who were on a combination of thiopurine and anti-TNFα also experienced less relapses than patients who were on another combination therapy, in particular in the post-partum period (p = .01). For thiopurine therapy alone no significant difference was found (see ).
Figure 4. Relapse rate before, during and after pregnancy of IBD patients stratified by treatment regimen. (A) Comparison of anti-TNFα treatment only versus other monotherapy (thiopurine, systemic corticosteroids, 5-ASA or Naltrexone). Post-partum relapse rate was lower in patients using anti-TNFα. Data during pregnancy is not shown since in our center, women discontinue anti-TNFα use from week 22 onwards. (B) Patients using anti-TNFα and thiopurine as combination therapy post-partum experienced less relapses when compared to patients using other combination therapy (anti-TNFα and steroids, thiopurine and 5-ASA, thiopurine and steroids, 5-ASA and steroids). (C) Thiopurine use only did not affect relapse rate from IBD patients as compared to other single treatment. Thio: Thiopurine.
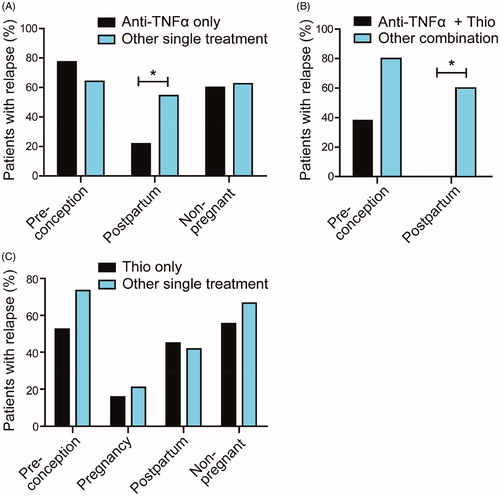
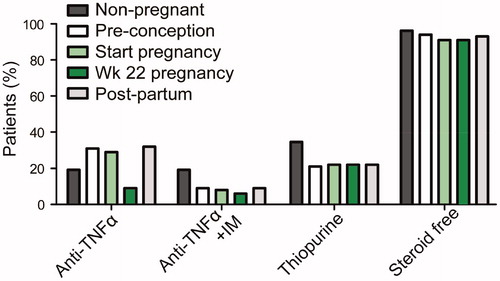
Figure 1. Medication regimen of non-pregnant and pregnant patients. For pregnant patients, pre-pregnancy, start of pregnancy, week 22 of pregnancy and post-partum medication use is indicated. Percentage of patients using anti-TNFα, using anti-TNFα plus immunomodulators (IM), using thiopurines and those that are steroid free are indicated.
To test if IBD diagnosis or the use of anti-TNFα treatment (single or in combination with thiopurine), both significant in univariate analysis, are independent predictors of relapsing post-partum, we performed logistic regression on these factors. For IBD diagnosis, the odds of experiencing a relapse post-partum are 88% higher in UC patients than CD patients (p = .006). Anti-TNFα single treatment or in combination with thiopurine did not significantly affect the relapse rate in post-partum IBD patients.
Discussion
In this prospective observational cohort study, we assessed the disease course before and after a first pregnancy in patients with IBD. We also describe the course of IBD in a group of women who did not conceive during follow up.
We found a lower number of relapses per person per year in the 4 years after delivery compared to the 3 years prior to a first pregnancy. Specifically, CD patients were less prone to experience a relapse after pregnancy than pre-pregnancy and we speculate that pregnancy has a positive influence on the course of disease in CD patients. Logistic regression identified UC patients more at risk for post-partum relapse compared to CD patients, while we also demonstrate that UC patients are more likely to relapse during pregnancy than CD patients. It has been speculated that differences in disease course between CD and UC during pregnancy might be explained by differences in the immunological pathways governing each disease. During pregnancy there is a shift towards a predominantly Th2 phenotype and therefore patients with Th2 dominant diseases, such as UC, might experience more relapses while those with Th1-driven disease, like CD, may benefit [Citation4].
Unlike Pedersen et al. who showed a higher relapse rate of UC patients during pregnancy and post-partum compared to non-pregnant patients, our study did not reveal an increased risk of flaring of UC patients compared to pre-pregnancy or non-pregnant controls [Citation7]. This discrepancy may potentially be explained by the longer follow-up time of 4 years in our study, in contrast to the 6 months of Pedersen et al.
Another contributing factor to the lower relapse rates could be adequate preconceptional counseling. Over the last years the importance of pregnancy counseling for women with IBD has gained attention. Adequate counseling resulted in less relapses during pregnancy, mainly due to drug adherence during pregnancy [Citation1]. Indeed our current study indicates that relapse rates already decline prior to conception. It is advised to strive for a sustained remission of at least 6 months prior to conception and it is conceivable that this may partly be responsible for a more stable disease from pregnancy onwards [Citation11,Citation12]. However, it should be noted that non-pregnant IBD patients, who received the same care and counseling, did not show a reduction in relapses/person/year in this study. Furthermore, the effect was more pronounced for pregnant CD patients than for pregnant UC patients, suggesting that biological differences account for at least part of this effect. For future studies it would be informative to describe the effect of optimization of IBD medication prior to pregnancy on relapse rates.
Over the last years there is less reluctance to actively treat IBD patients during pregnancy. For instance, in this study 79% of the patients received IBD-related medication during pregnancy, compared to 81% before pregnancy. The exception are biologicals, treatment of which is often ceased at the end of second trimester. We showed a better disease course after pregnancy in patients using anti-TNF-α (with or without thiopurine). Patients restarted their anti-TNF-α within 6 weeks after delivery in a same dose as used previously and therefore an ‘induction therapy’ effect is not likely to contribute to the lower relapse rate. In addition to our findings, Rottenstreich et al. found that biologic therapy is an independent protective factor against relapse already during pregnancy [Citation13]. This study has several strengths. First, the IBD patients were followed for a mean period of 7.1 years. In this period the patients visited the outpatient clinic on a regular and comparable basis. All patients received the same care and counseling. Secondly, our pregnant and non-pregnant groups were followed in the same era, as development of knowledge around medication is changing quickly. As shown in , pregnant and non-pregnant patients in our group were comparable. In our study we also took severity of relapse into account by including endoscopy scores.
This study also has some limitations that need to be addressed. First, our cohort consists of a tertiary patient population who received specialized care during their visits to the outpatient clinic. However, over the last years this approach has become standard of care in many hospitals. Secondly, we designed a prospective observational cohort study and therefore were not able to match the cases and controls described in our study, which may lead to confounding. To eliminate confounding effects, a matched case-control study needs to be conducted. Thirdly, we did not perform a sample size calculation prior to the study and our study populations might be underpowered to make comparisons between groups, however the aim of our study was to describe the course of IBD around pregnancy in our total group.
In conclusion, these data are in favor of pregnancy having an effect on disease course, with in particular CD patients showing a natural decrease in disease activity over time. Larger case-control studies of pregnant and not pregnant women are needed to further investigate the course of IBD and its molecular consequences.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Lima A. d, Zelinkova Z, Mulders A, et al. Preconception care reduces relapse of inflammatory bowel disease during pregnancy. Clin Gastroenterol Hepatol. 2016;14(9):1285–1292.e1.
- Reddy D, Murphy SJ, Kane SV, et al. Relapses of inflammatory bowel disease during pregnancy: in-hospital management and birth outcomes. Am J Gastroenterology. 2008;103(5):1203–1209.
- Bröms G, Granath F, Linder M, et al. Birth outcomes in women with inflammatory bowel disease: effects of disease activity and drug exposure. Inflamm Bowel Dis. 2014;20(6):1091–1098.
- Giessen JVD, Huang VW, Woude CJVD, et al. Modulatory effects of pregnancy on inflammatory bowel disease. Clin Transl Gastroenterol. 2019;10(3):e00009.
- Giessen JVD, Binyamin D, Belogolovski A, et al. Modulation of cytokine patterns and microbiome during pregnancy in IBD. Gut. 2020;69:473–486.
- J van der G, C van der W, Peppelenbosch M, et al. A direct effect of sex hormones on epithelial barrier function in inflammatory bowel disease models. Cells. 2019;8(3):261.
- Pedersen N, Bortoli A, Duricova D, et al. The course of inflammatory bowel disease during pregnancy and postpartum: a prospective European ECCO-EpiCom Study of 209 pregnant women. Aliment Pharmacol Ther. 2013;38(5):501–512.
- Castiglione F, Pignata S, Morace F, et al. Effect of pregnancy on the clinical course of a cohort of women with inflammatory bowel disease. Ital J Gastroenterol. 1996;28:199–204.
- Riis L, Vind I, Politi P, et al. Does pregnancy change the disease course? A study in a European cohort of patients with inflammatory bowel disease. Am J Gastroenterol. 2006;101(7):1539–1545.
- Lima AD, Zelinkova Z, Ent CVD, et al. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut. 2016;65(8):1261–1268.
- Woude CVD, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohn’s Colitis. 2015;9(2):107–124.
- Nguyen GC, Seow CH, Maxwell C, et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150(3):734–757e1.
- Rottenstreich A, Shifman Z, Grisaru-Granovksy S, et al. Factors associated with inflammatory bowel disease flare during pregnancy among women with preconception remission. Dig Dis Sci. 2021;66:1189–1194.
