Abstract
Background
Patients with inflammatory bowel disease (IBD) have an increased risk of compromised bone mineral density (BMD) and body composition. There are limited data on the physical exercise (PE) habits of patients with childhood-onset IBD and on the associations between PE and BMD and body composition.
Patients and methods
In total, 72 young adults with childhood-onset IBD and 1341 normative young adult controls answered questionnaires regarding PE [hours/week (h/w)] in the last 12 months. BMD and body composition were measured with dual x-ray absorptiometry (DXA) and presented as age- and gender-adjusted Z-scores for BMD, skeletal muscle index (SMI, the weight of lean mass in arms and legs/m2), and percentage body fat (Fat %).
Results
A total of 41 (57%) patients with IBD engaged in PE during the previous 12 months, as compared to 913 (68%) of the controls (p = .053). Sedentary patients had significantly lower median BMD, SMI, and Fat % Z-scores than the controls with corresponding PE habits (all p < .05). In contrast, highly active (>4 h/week) patients had total body BMD, SMI, and Fat % in the same range as the controls with corresponding PE levels (p = .151, p = .992, and p = .189, respectively), albeit with lower BMDs in the spine (p = .007) and femoral neck (p = .015). Using multiple regression analyses, a diagnosis of childhood-onset IBD was independently associated with inferior BMD and body composition, regardless of the amount of PE.
Conclusion
Physical exercise is associated with beneficial bone mineral density and body composition in patients with IBD despite the negative effects of the disease.
Introduction
Young adults with childhood-onset inflammatory bowel disease (IBD) have an increased risk to develop altered body composition traits, i.e. low bone mineral density (BMD), low skeletal muscle mass, and high-percentage body fat (Fat %) [Citation1]. Physical exercise plays a vital role in developing and perpetuating these body composition traits, as is well-documented in healthy individuals [Citation2–4]. However, the relationships between physical exercise, BMD, and body composition components in patients with IBD have been studied only to a limited extent. The few available studies involving pediatric and adult patients with IBD indicate that despite the presence of chronic inflammation, physical exercise has positive effects on BMD, leg lean mass, and fat mass [Citation5,Citation6]. However, to our knowledge, no study has focused on the extent to which physical exercise is associated with body composition traits in young adults with childhood-onset IBD.
Suffering from IBD can have a negative impact on the engagement in physical exercise. Thus, in a study from England, Tew et al. found that less than one-fifth of adult patients with IBD reported a high amount of regular physical exercise, whereas one-third reported being more or less sedentary [Citation7]. For comparison, a physical activity prevalence study from Sweden reported a higher level of activity in about two-fifths of healthy adults who were heavily engaged in physical exercise [Citation8]. In addition, children with IBD appear to be less physically active than age-matched healthy controls [Citation9]. This is somewhat alarming as regular physical exercise in childhood is thought to serve as the basis for establishing health-promoting exercise habits in early adulthood [Citation10].
BMD and body composition (lean mass and fat mass) are usually estimated using dual x-ray absorptiometry (DXA) [Citation11]. Skeletal muscle mass is reliably estimated as the appendicular lean mass (weight of lean mass in both arms and legs) divided by the participants height squared, resulting in a skeletal muscle index (SMI, kg/m2) [Citation11]. A representative fat mass estimation in patients with IBD is achieved using the percentage fat of the total body weight (Fat %) [Citation12].
Our primary aim was to investigate the amount of physical exercise undertaken by young adults with childhood-onset IBD and its associations with BMD, SMI, and Fat %. A secondary aim was to evaluate whether there is a link, at the individual level, between physical exercise habits in adolescence and later in early adulthood.
Patients and methods
Patients and population controls
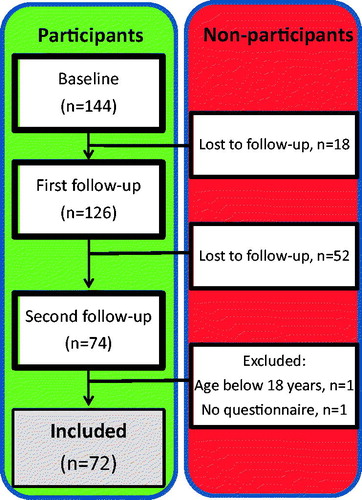
The present study is part of a longitudinal project investigating BMD and body composition in patients with childhood-onset IBD from the Gothenburg region in Sweden. As shown in , at baseline, we initially included 144 patients with childhood-onset IBD, 126 of whom participated in the first follow-up after 2 years. A total of 72 of the 126 participated in the second follow-up in early adulthood (age range, 18–27 years), with a median (IQR) follow-up period of 8.2 (6.9–12) years after initial inclusion (baseline).
Figure 1. Overview of the longitudinal study of bone mineral density and body composition in the cohort of patients with childhood-onset IBD. The numbers of participating patients at baseline, first follow-up (after 2 years), and second follow-up (about 6 years later) during early adulthood are shown.
In the present study, we focused on the patients’ amount of physical exercise and the association with body composition traits, i.e. BMD, skeletal muscle index (SMI; the sum of arms and legs lean mass/height squared, kg/m2), and Fat %. We have previously published data on the BMD measurements in this patient cohort during childhood/adolescence: at baseline [Citation13] and at the first follow-up after 2 years [Citation14]. In addition, we have recently performed a second follow-up of BMD development in early adulthood [Citation15], reporting that for the cohort of young adults with childhood-onset IBD the BMD Z-scores are < −1 and < −2 in 33.8 and 9.5%, respectively of the patients. We have also published the complete body composition data for these patients [Citation1]. At this second follow-up, the participants also answered a questionnaire regarding their physical exercise habits during the past year ().
Overall, 54 of the 126 patients from the first follow-up did not participate in the second follow-up, due to relocation out of the area, unwillingness to participate, or a failure to establish contact with previous participants. These non-participants did not differ significantly from the participants in the second follow-up regarding age, weight, height, gender, diagnosis, age at diagnosis, disease duration, and pharmacologic treatment (corticosteroid, azathioprine), or with respect to the BMD Z-scores at the first follow-up visit [Citation15].
Three previously published population-based control cohorts from two urban regions in the southwest of Sweden were pooled and used as controls for the measurements of physical exercise habits and body composition. The first cohort included 1068 population-based young adult men in the age range of 18–25 years from the greater Gothenburg area, Sweden (the GOOD study [Citation16,Citation17]). The second cohort entailed normative data collected from 86 young adults (40 men and 46 women) in the age range of 18–30 years from the greater Malmö area, Sweden [Citation18]. The third cohort consisted of 187 young adults (97 men and 90 women) in the age range of 18–25 years, also from the Malmö region [the pediatric osteoporosis prevention (POP) study] [Citation19]. Body composition Z-scores for BMD, SMI, and Fat % were calculated for all the controls (n = 1341, of whom 136 were women) according to our previously published Z-score calculations [Citation1].
Data collection
Demographic and clinical characteristics of the patients
Anthropometric and disease-specific characteristics have previously been published for the patient cohort [Citation15]. In total, 48 of 72 [66.7%] patients included in the study were male and the median (IQR) age was 22.7 (21.3–24.5) years. The vast majority of the patients had Caucasian ethnicity. Anthropometrical data [median (IQR) ] for the patient group were as follows: height, 175.3 (168.1–181.1) cm; weight, 69.5 (61.2–76.4) kg; and BMI, 22.6 (20.9–25.0) kg/m2. Overall, 47 (65%) patients had ulcerative colitis, 24 (33%) had Crohn’s disease, and one patient (1%) was diagnosed with IBD-Unclassified. The median (IQR) age at disease-onset was 11.8 (9.1–13.7) years, and the median (IQR) disease duration was 10.9 (9.4–13.6) years. A previous surgical procedure was reported in 21 patients [29.2%]. The following pharmacologic treatments were prescribed from the time of diagnosis to the time of second follow-up: corticosteroids, n = 67 [93.1%]; 5-aminosalicylic acid (5-ASA), n = 72 [100%]; azathioprine n = 53 [73.6%]; and anti-TNF-α agent as biological therapy, n = 15 [20.8%]. No biological therapies other than TNF-α inhibitors were used in these patients.
Anthropometric and body composition measurements
Height was measured to the nearest 0.5 cm using a wall-mounted stadiometer. Weight was measured to the nearest 0.1 kg using a calibrated standard scale with the participants in light clothing.
BMD (g/cm2), lean mass (kg), and fat mass (kg) were measured using the Lunar Prodigy DXA (GE Medical Systems Lunar, Madison, WI). The GOOD cohort controls were measured with the same Lunar Prodigy DXA apparatus as the IBD patients, while the control populations from the Malmö region were measured using the Lunar DPX-L (ver. 1.3z; GE Medical Systems Lunar) apparatus [Citation18]. Since the correlation of BMD, lean mass, and fat mass between different Lunar machines is strong [Citation20], we used the raw data without adjustment for the used apparatus.
BMD was measured in the total body, spine, and femoral neck using the standard software. We calculated the appendicular SMI as the sum of arms and legs lean mass/height squared (kg/m2), which is a reasonable estimate for total body skeletal muscle mass [Citation21]. Fat mass was measured for the total body and calculated as the percentage fat mass (Fat %) of the total body weight.
Estimation of physical exercise habits
At the second follow-up in early adulthood, we used a standardized physical exercise questionnaire to gather information about present physical exercise patterns in the last 12 months. The questionnaire also included questions regarding participation in sports during childhood and adolescence. Physical exercise was defined as regular training, whereas activities such as leisure walking or bicycling to work were not taken into account. Physical exercise was registered as the time in hours per week (h/w) spent on various training activities. Seasonality was taken into account, and the weekly amount of training was averaged for the entire year. Thus, in this study, we use the term ‘Amount of physical exercise’ to describe the average amount of training over the past year in hours per week. Corresponding data on physical exercise in the control group (n = 1341) were also registered in hours per week (h/w) and averaged over the past year.
Statistics
Statistical analyses were performed with the SPSS ver. 26 software (IBM Corp., Armonk, NY). Continuous variables are presented as median [interquartile range (IQR)]. Age- and gender-specific Z-scores were calculated based on the previously published values in the control cohorts for BMD of total body, spine, and femoral neck, SMI, and Fat % [Citation1]. Differences in variables between the three physical exercise subgroups were tested with the Kruskal-Wallis one-way ANOVA or Extended Fisher’s exact test. The differences between categorical and continuous variables between the two groups were tested with Fisher’s exact test or the Mann-Whitney U-test. The logistic regression analysis had ‘physical exercise, yes/no’ in adulthood as the dependent variable with gender and diagnosis of IBD as covariates. Each multivariable linear regression analysis had one body composition trait Z-score (total body BMD, spine BMD, femoral neck BMD, SMI, or Fat %) as the dependent variable. Covariates were ‘diagnosis of IBD’ and ‘physical exercise subgroup’ (moderate or high compared to the sedentary subgroup). SMI Z-score was also a covariate for the additional femoral neck BMD regression analysis. All tests were 2-tailed and conducted, assuming a significance level of 0.05.
Ethical considerations
Informed written consent was obtained from all young-adult patients with IBD and controls. The study was approved by the Regional Ethical Review Committee of the University of Gothenburg (Sweden) (application numbers: for the IBD cohort 182-02 and 117-11; and for the GOOD study cohort S600-02 and 017-08) and the Regional Ethics Committee at the Lund University, Lund, Sweden (LU 471-95, LU 486-96 and 2015/118).
Results
Physical exercise in young adulthood
Of the 72 young adult patients with childhood-onset IBD, 41 (57%) reported regular physical exercise during the last 12 months. Participation in physical exercise tended to be higher in the control group: n = 913 (68%) (p = .053). In the patient group, no significant gender differences were found, 52% of the male patients and 66% of the female patients were engaged in regular physical exercise (p = .315). In contrast, in the control group, 66% of the males and 86% of the females exercised regularly (p < .001). To account for this gender difference between the two diagnostic groups, we used a logistic regression to adjust for gender. Participation in regular physical exercise was significantly lower in the group of patients with IBD than in the control group [odds ratio (OR) 0.487, 95% CI 0.295–0.805]. Those patients who engaged in physical exercise weekly spent a median [IQR] of 4 (2–8) h/w on physical exercise, which was similar to the active controls at 4 (2–6) h/w (p = .542). In total, 25 (52%) male patients and 16 (67%) female patients exercised. Patients with Crohn’s disease and patients with ulcerative colitis reported regular exercise in the same proportions, 15 (60%) and 25 (53%), respectively. In young adult patients with IBD, training in a gym (n = 22, 54%) was the most common exercise form, followed by running (n = 7, 17%) and floorball (n = 4, 10%). Twelve patients (17%) were active in more than one type of sports activity, whereas another twelve patients (17%) had never participated in any sports activity.
To study the association between the amount of physical exercise and BMD, SMI, and Fat % Z-scores, the study participants (patients and controls separately) were divided into three subgroups (tertiles) of physical exercise based on their grade of physical exercise engagement. The patients, as well as the controls, were proportionally distributed between the physical exercise subgroups (p = .160): Subgroup 1: Sedentary, no regular exercise at all, 0 h/w (patients, n = 31; controls, n = 428); Subgroup 2: Moderate physical exercise, 1–4 h/w (patients, n = 19; controls n = 428); and Subgroup 3: High physical exercise, ≥4 h/w (patients, n = 22; controls, n = 485).
The anthropometric characteristics of the patients and controls in the different physical exercise subgroups are presented in . The three exercise subgroups of patients with IBD had similar clinical characteristics, including diagnosis, age of disease onset, disease duration, treatment history, and smoking habits ().
Table 1. Anthropometric characteristics of the patients and controls. The patients and controls were categorized separately into three subgroups based on the amount of physical exercise.
Table 2. Disease-specific characteristics, treatment history, and lifestyle-related habits of young adults with childhood-onset IBD. The patients were categorized separately into three subgroups based on their amount of physical exercise.
The association between bone mineral density and the grade of physical exercise
The overall group of patients with childhood-onset IBD had lower median BMD Z-scores [IQR] at all the measured sites than the entire control group: total body, −0.71 [−1.35–0.41] vs. 0.00 [−0.68–0.65]; spine, −0.73 [−1.33– −0.13] vs. −0.08 [−0.73–0.64]; and femoral neck, −0.48 [−1.24–0.18] vs. −0.07 [−0.77–0.68] (all p < .001).
The sedentary patients (Subgroup 1) who were not exercising regularly had significantly lower BMD Z-scores for total body, spine, and femoral neck than the controls with the same sedentary habits (Subgroup 1) (). Comparing the patients with childhood-onset inflammatory bowel disease and controls the BMD Z-scores were lower in patients in the total body (p = .002), spine (p = .001), and femoral neck (p = .012) (). Furthermore, these patients had significantly lower median BMD Z-scores at all locations than the entire control group (see the first paragraph) (all p < .001).
Figure 2. Associations between the amount of physical exercise (PE) and the bone mineral density (BMD) Z-scores in patients with childhood-onset inflammatory bowel disease and controls. The patients and the controls are divided separately into three subgroups (Subgroups 1, 2, and 3) based on the amount of regular PE. BMD Z-scores and each subgroup median values with IQR for total body, spine, and femoral neck are shown. The difference between the subgroups was tested with a Kruskal-Wallis 1-way ANOVA for patients and controls separately. The difference between patients and controls within a PE subgroup was tested with the Mann-Whitney U-test.
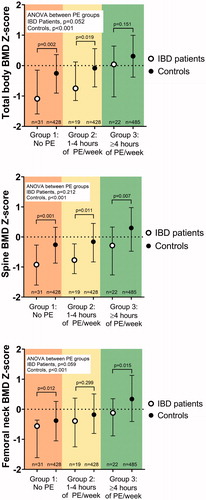
The patients who exercised moderately (Subgroup 2) had significantly lower BMD Z-scores for total body (p = .019) and spine (p = .011) compared with controls who had a corresponding exercise activity (Subgroup 2) (). However, these patients had a median femoral neck BMD Z-score in the same range as these controls (p = .299). Further, the median BMD Z-scores for the patients with moderate physical exercise differed from those of the entire control group (see the first paragraph) at the same locations (total body, p = .011; spine, p = .003; and femoral neck; p = .159)
Patients with high amount of physical exercise (Subgroup 3) did not differ significantly in median total body BMD Z-score compared to that of the corresponding controls (Subgroup 3), p = .151 (). However, these patients had significantly lower median BMD Z-scores than the controls with high physical exercise (Subgroup 3) for the spine (p = .007) and femoral neck (p = .015) (). Notably, when comparing patients with high physical exercise grade (Subgroup 3) and the entire control group (see the first paragraph), we found that they had median BMD Z-scores in the same ranges for the total body, spine, and femoral neck (p = .829, p = .102, and p = .406, respectively).
To determine whether higher levels of physical exercise were associated with better BMD, we used a one-way ANOVA on ranks. In the patients with IBD, such a trend was observed for both the total body and femoral neck (p = .052 and p = .059, respectively). However, such a trend was not seen for the spine (p = .202). For comparison, significant differences between the amount of exercise activity and BMD were seen in the controls (all sites, p < .001) ().
The association between skeletal muscle index and the amount of physical exercise
The overall group of IBD patients had a lower median [IQR] SMI Z-score than the entire control group: −0.34 [−1.28–0.33] vs. −0.09 [−0.8–0.65] (p = .019).
Patients who had no regular training (Subgroup 1) had a lower SMI Z-score than the corresponding control exercise subgroup (p = .024) (). These patients also had a lower median SMI Z-score than the entire control group (p < .001).
Figure 3. Associations between the amount of physical exercise (PE) and skeletal muscle index (SMI) Z-scores in patients with childhood-onset inflammatory bowel disease and controls. The patients and the controls are divided separately into three subgroups (Subgroups 1, 2, and 3) based on the amount of regular PE. SMI Z-scores and each subgroup median values with IQR for Z-scores and median values with IQR for PE subgroups are shown. The difference between subgroups was tested with a Kruskal-Wallis 1-way ANOVA for patients and controls separately. The difference between patients and controls within a PE subgroup was tested with the Mann-Whitney U-test.
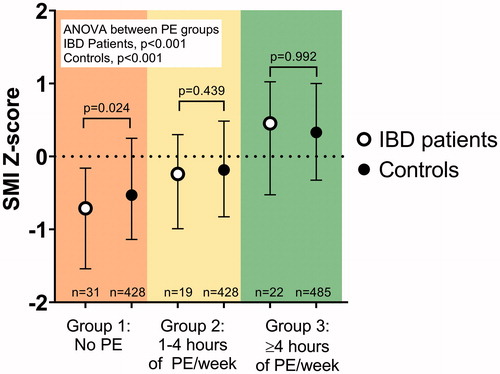
However, the patients with moderate physical exercise (Subgroup 2) had a similar median SMI Z-score to the controls who exercised equally (Subgroup 2) (p = .439) (). These patients also had a similar median SMI Z-score to the entire control group (p = .207).
The patients who exercised the most (Subgroup 3) had the highest median SMI Z-score of the three exercise groups. Their median SMI Z-score was similar to that of the controls with the corresponding amount of exercise (Subgroup 3) (p = .992) (). Furthermore, these patients (Subgroup 3) had a median SMI Z-score comparable to that of the entire control group (p = .087).
We found a significant difference in SMI Z-score between the three patient physical exercise subgroups (ANOVA, p < .001). The same held true when comparing the three control exercise subgroups (ANOVA, p < .001) ().
The association between fat percentage and the grade of physical exercise
The overall group of patients with IBD had a higher median Fat % Z-score than the entire control group: 0.48 [−0.21–1.25] vs. −0.20 [−0.78–0.52] (p < .001).
Those patients with no regular exercise (Subgroup 1) had a significantly higher median Fat % Z-score than the controls with the same sedentary habit (p = .011) (). These patients also had a higher median Fat % Z-score than the whole control group (p < .001).
Figure 4. Associations between the amount of physical exercise (PE) and body fat percentage (Fat %) Z-scores in patients with childhood-onset inflammatory bowel disease and controls. The patients and the controls are divided separately into three subgroups (Subgroups 1, 2, and 3) based on the amount of regular PE. Fat % Z-scores and median values with IQR for physical exercise subgroups are shown. The difference between subgroups was tested with a Kruskal-Wallis 1-way ANOVA for patients and controls separately. The difference between patients and controls within a PE subgroup was tested with the Mann-Whitney U-test.
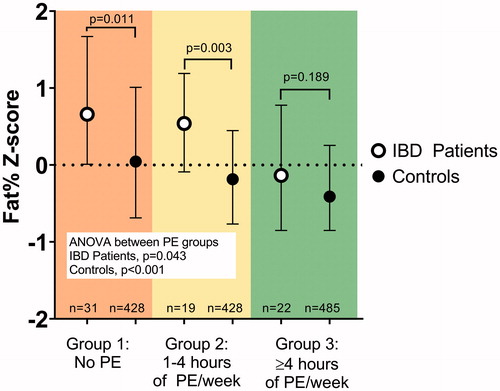
Similarly, patients with moderate training (Subgroup 2) had higher Fat % Z-scores than the corresponding subgroup of controls (p = .003) (). In similarity to the patients with sedentary habits (Subgroup 1), patients with moderate physical exercise (Subgroup 2) had a higher median Fat % Z-score than the entire control group (p = .005).
Patients who engaged in a high level of physical exercise (Subgroup 3) had the lowest median Fat % Z-score, similar to that in controls with high physical exercise (Subgroup 3) (p = .189) (). These patients had a median Fat % Z-score in the same range as the entire control group (p = .656).
A clear trend was seen for both patients and controls: the higher the amount of exercise, the lower the Fat % Z-scores. Thus, the difference in median Fat % Z-score between physical exercise subgroups was significant for the patients as well as the controls (ANOVA, p = .043 and p < .001, respectively) ().
Predictors of bone mineral density and body composition traits
To evaluate whether having childhood-onset IBD constituted an independent association with BMD or body composition measures, we performed a series of multiple regression analyses. Each regression model had either BMD or body composition Z-score (total body BMD, spine BMD, femoral neck BMD, SMI, and Fat %) as the dependent variable, with physical exercise subgroups (Subgroups 2 and 3 compared to Subgroup 1) and diagnosis of IBD as covariates (). We observed an independent negative association between suffering from IBD and all BMD and body composition measurements, regardless of the physical exercise subgroup. Furthermore, to analyze whether the BMD Z-score increase was mediated by SMI and not by physical exercise per se, we performed an additional analysis of BMD in the femoral neck (weight-bearing part) with the SMI Z-score included as a covariate. This regression analysis indicated an independent albeit weaker association between the amount of physical exercise and the diagnosis of IBD for the femoral neck BMD Z-score, when accounting for skeletal muscle mass, highlighting the importance of SMI as a mediator of the association between IBD and BMD ().
Table 3. Predictors of bone mineral density and body composition measures in young adults with childhood-onset IBD and controls.
The relationship between physical exercise habits in late adolescence and young adulthood
To analyze the possible relationships between physical exercise habits in late adolescence and early adulthood, we analyzed patients aged >21 years. A total of 56 out of 72 patients (78%) was included in this analysis, of whom 34 (61%) exercised regularly and 22 (39%) were sedentary. Those who had regular physical exercise in adulthood reported higher rates of sports participation in late adolescence (16–17 years of age) than those who were sedentary (74 vs. 59%, respectively) (p = .049).
Discussion
In the present study, we focused on the amount of physical exercise and its associations with BMD and body composition in young adults with childhood-onset IBD. For comparison, we used a large normative control cohort from approximately the same geographic area. Our main finding is that for patients with IBD, regular physical exercise may help to counteract the detrimental effects of IBD on BMD and body composition traits. A greater amount of physical exercise is linked to higher BMD, higher skeletal muscle index, and a lower body-fat percentage.
We revealed that young adult patients with childhood-onset IBD who are sedentary are prone to have low BMD and SMI, in combination with a high Fat %. In contrast, patients with a high level of physical exercise, i.e. four or more hours each week, have median values of BMD, SMI, and Fat % that lie in the same range as those of the controls. In line with our findings, Cronin and colleagues have reported an increase in lean muscle mass and a decreased body Fat % after 8 weeks of combined aerobic and resistance training in adult patients with IBD [Citation22]. Furthermore, physical exercise has been reported to be positively associated with BMD in adult patients with IBD [Citation5]. Our approach to evaluate BMD and body composition components simultaneously and to relate these measures to the patients’ amount of exercise has, to our knowledge, not been reported previously.
In a multivariable regression model, we show that the diagnosis of childhood-onset IBD is an individual risk factor for compromised BMD, SMI, and Fat %, regardless of the amount of physical exercise undertaken by these patients. Having IBD implicates several factors that potentially affect BMD and body composition, such as inflammation per se [Citation23,Citation24], nutritional deficits [Citation25], and medical treatment side-effects [Citation26]. It has to be considered that disease-related factors, such as disease duration and the grade of chronic inflammation, may vary between the patient physical exercise subgroups, which might affect the measures of BMD and body composition in parallel with the amount of physical exercise. However, attempts to define and estimate the long-term disease course severity have encountered major methodologic difficulties [Citation27]. Nevertheless, in an attempt to address this issue, we compared the three physical exercise subgroups regarding different clinical parameters as potential surrogate markers for disease severity. However, we found no differences between the physical exercise subgroups in terms of diagnosis, age of diagnosis, elapsed time with a diagnosis of IBD, previously received medical treatment (including corticosteroid use, azathioprine, or biologic treatment), or surgical therapy (). Therefore, we believe that the differences in BMD and body composition observed between the physical exercise subgroups can be attributed in large part to the amount of exercise undertaken.
Interestingly, the associations between the individuals’ amount of physical exercise and values for SMI and the Fat % were more pronounced than those for BMD. This weaker association between physical exercise and BMD might be attributable in part to the fact that most of the bone mineralization process already occurred during adolescence [Citation28]. However, spine BMD was not associated with the amount of physical exercise, possibly because training can affect BMD differently in the axial and appendicular skeleton [Citation2]. Our research group has previously shown that young adults with childhood-onset IBD have the opportunity to increase their BMD beyond the conventional estimated time for peak bone mass [Citation15], indicating an extended time window for optimization of bone mass in early adulthood. In accordance with this, Nilsson and colleagues found physical activity in early adulthood to be important for optimizing peak bone mass in males [Citation2].
The proportion of young adult patients with IBD who exercised regularly were lower than that of controls, roughly 60 vs. 70%. Such a difference remained after adjusting for the difference in gender distribution between the patient group and controls. In a somewhat older cohort of adults with IBD (mean age, 42 years; range 19–87 years), DeFilippis and colleagues [Citation29] have reported an even lower proportion of patients engaged in physical exercise, i.e. only one-third were physically active. Taken together, these lines of evidence indicate that younger adults with IBD engage in more physical exercise than older patients, a trend that has also been reported for healthy individuals [Citation8].
Furthermore, we show that patients engaged in physical exercise during late adolescence are likely to continue with physical exercise in early adulthood. This indication that exercise habits seem to persist during the transition from childhood to early adulthood is supported by Lahti and colleagues in their long-term intervention study of normative children up to early adulthood [Citation19]. Furthermore, physical exercise in childhood has been associated with improved bone mineralization and bone structure in adulthood [Citation30]. To summarize, this indicates a window of opportunity for pediatricians to promote sports participation during patient adolescence, so as to foster a more prolonged relationship to physical exercise.
There are several clinical implications of our observation that physical exercise is associated with beneficial BMD and body composition in young adults with IBD. Primarily, treatment strategies may include efforts to promote increased physical exercise. Most adult patients with IBD, around 90%, consider physical activity to be an important part of daily life [Citation31]. Despite this, only 37% of patients had discussed physical exercise with a physician [Citation32]. When achieved, training is reported to be generally well-tolerated in patients with chronic IBD and may also increase their quality of life [Citation33,Citation34]. Despite this, a substantial proportion of adult patients report that their IBD symptoms limit their possibilities for exercise, approximately one-third of patients reduce their activity levels following the diagnosis of IBD [Citation7,Citation31]. We would like to suggest that physicians repeatedly discuss physical exercise habits with their patients. Studies are needed to investigate how best to stimulate patients with IBD to exercise regularly. In our opinion, a physiotherapist may add valuable skills to the multidisciplinary IBD team, e.g. in developing individual training programs and encouraging patients to exercise regularly.
The main strength of the present study is that the cohort of young adult patients covers the whole clinical spectrum of childhood-onset IBD. Furthermore, we had access to a large normative control cohort from the same region, which allowed for a reliable comparison of our results. One weakness of the study is that we cannot exclude recall bias, as the participants and controls were required to answer questionnaires regarding physical exercise in the past year. This should, however, apply to both groups equally. The patient group’s recollection of childhood sports activities may be subject to an even greater recall bias. We acknowledge that with our study design, we can draw no causal conclusions regarding the effect of physical exercise on measured BMD or body composition.
To conclude, a diagnosis of childhood-onset IBD is independently associated with inferior BMD and body composition in early adulthood. Despite this, those patients who exercise regularly have the capacity to reach similar levels of BMD and body composition as healthy controls.
Summary
Physical exercise is associated with beneficial bone mineral density and body composition in young adult patients with childhood-onset IBD despite the negative effects of the disease.
Disclosures statement
The authors have no conflicts of interest to disclose.
Author contributions
G.V. Sigurdsson, R. Saalman, and M. Lorentzon contributed to the study design, data collection, data analysis, and manuscript writing and review. S. Schmidt, D. Mellström, C. Ohlsson, and M. Karlsson contributed to the study design, data collection, and manuscript review. All the authors approved the final draft of the manuscript.
Acknowledgments
The authors thank all our patients for participation in this study, Britt-Marie Käck (RN) and the staff at the Geriatric Medicine Clinic in Gothenburg, especially Senada Catic, Ulrika Hjertonsson, and Vera Gligoric for the measurements of our patients. Jan-Åke Nilsson at Lund University is thanked for statistical advice.
Additional information
Funding
References
- Sigurdsson GV, Schmidt S, Mellstrom D, et al. Altered body composition profiles in young adults with childhood-onset inflammatory bowel disease. Scandi J Gastroenterol. 2020;55(2):169–169.
- Nilsson M, Ohlsson C, Oden A, et al. Increased physical activity is associated with enhanced development of peak bone mass in men: a five-year longitudinal study. J Bone Miner Res. 2012;27(5):1206–1214.
- Karlsson MK, Rosengren BE. Exercise and peak bone mass. Curr Osteoporos Rep. 2020;18(3):285–290.
- Frost HM. Bone's mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275(2):1081–1101.
- Lee N, Radford-Smith G, Taaffe DR. Bone loss in Crohn’s disease: exercise as a potential countermeasure. Inflammatory Bowel Dis. 2005;11(12):1108–1118.
- Bilski J, Mazur-Bialy A, Brzozowski B, et al. Can exercise affect the course of inflammatory bowel disease? Experimental and clinical evidence. Pharmacol Rep. 2016;68(4):827–836.
- Tew GA, Jones K, Mikocka-Walus A. Physical activity habits, limitations, and predictors in people with inflammatory bowel disease: a large cross-sectional online survey. Inflamm Bowel Dis. 2016;22(12):2933–2942.
- Bauman A, IPS Group, Bull F, Chey T, et al. The International Prevalence Study on Physical Activity: results from 20 countries. Int J Behav Nutr Phys Act. 2009;6:21.
- Werkstetter KJ, Ullrich J, Schatz SB, et al. Lean body mass, physical activity and quality of life in paediatric patients with inflammatory bowel disease and in healthy controls. J Crohns Colitis. 2012;6(6):665–673.
- Telama R, Yang X, Viikari J, et al. Physical activity from childhood to adulthood: a 21-year tracking study. Am J Prev Med. 2005;28(3):267–273.
- Kim J, Wang ZM, Heymsfield SB, et al. Total-body skeletal muscle mass: estimation by new dual-energy X-ray absorptiometry method. Faseb J. 2002;16(4):A230–A230.
- Bryant RV, Trott MJ, Bartholomeusz FD, et al. Systematic review: body composition in adults with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38(3):213–225.
- Schmidt S, Mellstrom D, Norjavaara E, et al. Low bone mineral density in children and adolescents with inflammatory bowel disease: a population-based study from Western Sweden. Inflammatory Bowel Dis. 2009;15(12):1844–1850.
- Schmidt S, Mellstrom D, Norjavaara E, et al. Longitudinal assessment of bone mineral density in children and adolescents with inflammatory bowel disease. J Pediatric Gastroenterol Nutr. 2012;55(5):511–518.
- Sigurdsson GV, Schmidt S, Mellstrom D, et al. Bone mass development from childhood into young adulthood in patients with childhood-onset inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(12):2215–2226.
- Darelid A, Nilsson M, Kindblom JM, et al. Bone turnover markers predict bone mass development in young adult men: A five-year longitudinal study. J Clin Endocrinol Metab. 2015;100(4):1460–1468.
- Lorentzon M, Mellstrom D, Ohlsson C. Age of attainment of peak bone mass is site specific in Swedish men-The GOOD study. J Bone Miner Res. 2005;20(7):1223–1227.
- Alwis G, Rosengren B, Stenevi-Lundgren S, et al. Normative dual energy X-ray absorptiometry data in Swedish children and adolescents. Acta Paediatr. 2010;99(7):1091–1099.
- Lahti A, Rosengren BE, Nilsson JA, et al. Long-term effects of daily physical education throughout compulsory school on duration of physical activity in young adulthood: an 11-year prospective controlled study. BMJ Open Sport Exerc Med. 2018;4(1):e000360.
- Mazess RB, Hanson JA, Payne R, et al. Axial and total-body bone densitometry using a narrow-angle fan-beam. Osteoporosis Inter. 2000;11(2):158–166.
- Guo B, Wu Q, Gong J, et al. Relationships between the lean mass index and bone mass and reference values of muscular status in healthy Chinese children and adolescents. J Bone Miner Metab. 2016;34(6):703–713.
- Cronin O, Barton W, Moran C, et al. Moderate-intensity aerobic and resistance exercise is safe and favorably influences body composition in patients with quiescent Inflammatory Bowel Disease: a randomized controlled cross-over trial. BMC Gastroenterol. 2019;19(1):29.
- Paganelli M, Albanese C, Borrelli O, et al. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(4):416–423.
- Talathi S, Nagaraj P, Jester T, et al. Relations between disease status and body composition in pediatric inflammatory bowel disease. Eur J Pediatr. 2020;179(10):1499–1505.
- Lopes LH, Sdepanian VL, Szejnfeld VL, et al. Risk factors for low bone mineral density in children and adolescents with inflammatory bowel disease. Dig Dis Sci. 2008;53(10):2746–2753.
- LeBlanc CM, Ma J, Taljaard M, et al.; Canadian STeroid-Associated Osteoporosis in Pediatric Population (STOPP) Consortium. Incident Vertebral Fractures and Risk Factors in the First Three Years Following Glucocorticoid Initiation Among Pediatric Patients With Rheumatic Disorders. J Bone Miner Res. 2015;30(9):1667–1675.
- Peyrin-Biroulet L, Panes J, Sandborn WJ, et al. Defining Disease Severity in Inflammatory Bowel Diseases: Current and Future Directions. Clinical gastroenterology and hepatology: the official clinical practice journal of the. Clin Gastroenterol Hepatol. 2016;14(3):348–354 e317.
- Baxter-Jones AD, Faulkner RA, Forwood MR, et al. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011;26(8):1729–1739.
- DeFilippis EM, Tabani S, Warren RU, et al. Exercise and Self-Reported Limitations in Patients with Inflammatory Bowel Disease. Dig Dis Sci. 2016;61(1):215–220.
- Gunter KB, Almstedt HC, Janz KF. Physical activity in childhood may be the key to optimizing lifespan skeletal health. Exerc Sport Sci Rev. 2012;40(1):13–21.
- Gatt K, Schembri J, Katsanos KH, et al. Inflammatory Bowel Disease [IBD] and physical activity: a study on the impact of diagnosis on the level of exercise amongst patients with IBD. J Crohns Colitis. 2019;13(6):686–692.
- Lambdin J, Rowe A, Borum M. Physical activity and inflammatory bowel disease. J Crohns Colitis. 2018;12(7):880.
- Taylor K, Scruggs PW, Balemba OB, et al. Associations between physical activity, resilience, and quality of life in people with inflammatory bowel disease. Eur J Appl Physiol. 2018;118(4):829–836.
- Chan D, Robbins H, Rogers S, et al. Inflammatory bowel disease and exercise: results of a Crohn's and Colitis UK survey. Frontline Gastroenterol. 2014;5(1):44–48.
