Abstract
Aim: This Danish national guideline describes the treatment of adult patients with Clostridioides (formerly Clostridium) difficile (CD) infection and the use of faecal microbiota transplantation (FMT). It suggests minimum standard for implementing an FMT service.
Method: Four scientific societies appointed members for a working group which conducted a systematic literature review and agreed on the text and recommendations. All clinical recommendations were evalluated for evidence level and grade of recommendation.
Results: In CD infection, the use of marketed and experimental antibiotics as well as microbiota-based therapies including FMT are described. An algorithm for evaluating treatment effect is suggested. The organisation of FMT, donor recruitment and screening, laboratory preparation, clinical application and follow-up are described.
Conclusion: Updated evidence for the treatment of CD infection and the use of FMT is provided.
Introduction
Clostridioides (formerly Clostridium) difficile (CD) infection (CDI) is a major cause of nosocomial diarrhoea and accounts for 20–30% of cases of antibiotic-associated diarrhoea [Citation1]. The disease poses a persistent health threat, is associated with a high mortality and generates considerable hospital costs [Citation2–5].
Faecal microbiota transplantation (FMT) is the transfer of minimally processed faeces from a healthy donor to a patient in order to treat disease [Citation6]. The method has been used in modern medical science since 1958 [Citation7]. Its clinical effect in recurrent CDI (rCDI) has been documented in observational [Citation8–13] and randomised [Citation14–19] studies. The use of FMT in the treatment of other conditions is being explored in clinical trials. In the future, the use of microbiota-based drugs may potentially replace or supplement FMT. The basis for this clinical guideline is the use of FMT in patients with CDI. Experimental treatments and indications for FMT are briefly discussed.
This Danish national guideline describes the treatment of adult patients with CDI and the use of FMT.
Methods
Guideline development process
The formation of this Danish national guideline followed the proforma for clinical guidelines under the Danish Society for Gastroenterology and Hepatology, including representation in the working group by specialists, doctors in training, university hospitals, regional hospitals, and all geographic regions of Denmark. The process and the final guideline were endorsed by the Danish Society for Gastroenterology and Hepatology, the Danish Society of Infectious Diseases, the Danish Society for Clinical Microbiology, and the Danish Immunology Society for Clinical Immunology, following a hearing process in each scientific society. Each scientific society appointed at least two working group members.
Definitions
Clostridioides difficile infection (CDI)
Presence of diarrhoea AND a stool sample that is positive for Clostridioides difficile (CD) toxin or toxin gene (); in special cases of CDI, also defined as endoscopic/histopathological finding of pseudomembranous colitis. For information about which test is used to detect CD, please consult local guidelines.
Diarrhoea is defined as three or more bowel movements per day with a consistency corresponding to the Bristol Stool Form Scale (Bristol) 6-7.
First CDI
First detection of CDI. Synonymous with initial/index CDI.
Recurrence of CDI
A new case of CDI occurring after discontinuation of treatment for previous CDI cases where clinical resolution was achieved during treatment (absence of diarrhoea).
Mild/moderate CDI
CDI cases not meeting the criteria for being severe or fulminant, see below.
Severe CDI
CDI with the presence of one or more signs of severe colitis (systemic impact): clinic (abdominal pain), biochemistry (hypoalbuminemia (p-albumin < 30 g/l); leukocytosis (leukocytes > 15 × 109/l); leukopenia (leukocytes <2 × 109/l); renal impairment (creatinine above 1.5 × premorbid level).
Fulminant CDI
Severe CDI and one or more of the following: clinic(cognitive affection; fever above 38.5 °C; ileus; hypotension/septic shock), course (development of multi-organ failure; need for intensive therapy), imaging (ileus; toxic megacolon (colon diameter> 6 cm)), endoscopy (pseudomembranous colitis).
Treatment-refractory CDI
Persistent diarrhoea after five days of anti-CD antibiotic treatment, or worsening at any time during treatment. Cessation of diarrhoea is defined by daily bowel movements <3 and Bristol <6. Any differential diagnoses should be considered in the absence of treatment effect. The term treatment refractory is used synonymously with treatment failure, which, however, is used by some in the sense of recurrence and is therefore avoided in this guideline.
Faecal microbiota transplantation (FMT)
Transfer of minimally processed donor faeces from a donor to a patient in order to establish normal bowel function.
Resolution of CDI
Absence of CD-related diarrhoea after conclusion of treatment, i.e. either a maximum of three non-liquid stools (Bristol 5 or lower) daily or, in case of diarrhoea, a negative CD test.
Level of evidence and grade of recommendation
Assessments of evidence level (EL 1–5) and grade of recommendation (RG A-D) follow recommendations established by the Centre for Evidence-Based Medicine, University of Oxford.
Literature search method
The National Library of Medicine, Pubmed, and The Cochrane search databases were used. Free text search was employed, and the following MeSH term was used; fecal microbiota transplantation. The following search string was used to identify clinical trials using FMT in CDI: (fecal OR faecal) AND ((microbiota AND (therapy OR installation OR transplant OR transplant) OR bacterotherapy) OR stool transplant) AND (CDI OR rCDI OR Clostridioides difficile OR Clostridium difficile OR Clostridium infection OR Recurrent Clostridium difficile infection) AND (colonoscopy OR oral OR capsule OR capsules OR nasojejunal tube OR nasoduodenal tube OR nasogastric tube OR enema OR retention enema OR efficacy OR retrospective OR experience). The literature search was concluded on 22 April 2020.
Treatment of C. difficile infection
Metronidazole and vancomycin
Two small non-blinded, randomised trials from 1983 (n = 88) and 1996 (n = 62) compared treatment with metronidazole and vancomycin and found no difference in efficacy (cure rate) within 3–4 weeks [Citation20,Citation21]. Based on these studies and due to the large price difference, the US guidelines from 1997 in place at the time of the studies recommended metronidazole as first-choice treatment [Citation22]. In 2007, the first randomised blinded study stratified by CDI severity in a study including 172 patients and follow-up after three weeks showed a significantly higher cure rate of vancomycin of 97 versus 84% after metronidazole [Citation23]. The difference in effect was observed in the group with severe disease. In another study with data collected from two multinational studies, vancomycin (n = 266) was also superior to metronidazole (n = 289) with an 81 against a 73% cure rate [Citation24]. Furthermore, a retrospective cohort study from 2016 found that 30-day mortality and recurrence risk (evaluated up to 56 days) were lower after vancomycin than after metronidazole [Citation25]. Furthermore, if vancomycin is minimally absorbed from the gastrointestinal tract, the concentration of active substance in the intestine is significantly higher than with metronidazole. The latter is absorbed to a greater extent, which is associated with a risk of several systemic side effects.
In severe or fulminant CDI, increasing the dose of vancomycin from 125 mg × 4 to 500 mg × 4 has been considered. Two studies examined the effect of different doses, finding no effect of a dose increase [Citation26,Citation27]. In contrast, it has been proposed that a dose increase may have a negative effect on the intestinal microbiome.
In fulminant CDI, the addition of intravenous metronidazole to oral vancomycin in a single-centre retrospective study of 88 patients in an intensive care unit showed reduced mortality [Citation28]. However, in a recent two-centre retrospective study including526 patients with CDI of varying severity including fulminant CDI, the addition of intravenous metronidazole had no effect on death, colectomy or recurrence [Citation29]. It is generally agreed that in case of paralytic ileus or threatening toxic megacolon, the addition of intravenous metronidazole to oral or rectal vancomycin improves antibacterial treatment and protection against other anaerobic bacteria in the bloodstream. Intravenous metronidazole cannot be used as monotherapy for the treatment of CDI. If the patient is unable to take vancomycin capsules, administering vancomycin in liquid form (oral solution or infusion solution) is recommended, either by drinking it, through a nasointestinal tube, or rectally by endoscopy.
More than 95% of intravenously administered vancomycin is excreted unchanged through the kidneys. Only a very limited amount is excreted in bile and faeces, so intravenous vancomycin prescribed on another indication has no effect on CDI, and oral or enteral vancomycin must be supplemented according to the above principles for concomitant CDI.
Fidaxomicin and vancomycin
Fidaxomicin is a narrow-spectrum antibiotic against gram-positive aerobic and anaerobic bacteria, which is believed to be microbiotasparing compared with vancomycin. Like vancomycin, it is minimally absorbed from the gastrointestinal tract. In 2011 and 2012, two phase-3 studies were published with 629 and 535 patients, respectively. Both studies showed the same treatment effect for vancomycin and fidaxomicin in relation to treatment of the current infection, but fidaxomicin significantly reduced the recurrence rate compared with vancomycin (vancomycin 25–27% versus fidaxomicin 13–15%) [Citation30,Citation31]. Similar findings were described in a study where fidaxomicin for five days followed by pulse treatment every other day until day 25 was compared with standard vancomycin for ten days [Citation32]. Most trial participants in the first two trials were treated for their first CDI, whereas only a small subset had recurrent CDI. In this group, a similar difference was seen between the recurrence rate of fidaxomicin- and vancomycin-treated patients, but the difference observed in this subgroup was not significant. In a randomized, 3-armed, clinical trial including recurrent CDI – FMT (n = 24), fidaxomicin (n = 24) for ten days or vancomycin (n = 16) for ten days – the difference between fidaxomicin (cure rate 42%) and vancomycin (cure rate 19%) was not statistically significant with cure rate defined as absence of diarrhoea recurrence within eight weeks [Citation19]. Both antibiotics were significantly poorer than FMT, which showed a 92% cure rate. Fidaxomicin has not been compared with FMT in other studies.
Studies on and experience with fidaxomicin treatment in patients with recurrent CDIs, including multiple recurrences, are generally lacking.
Vancomycin pulse-tapering therapy
In recurrent CDI, prolonged treatment with vancomycin with dose reduction or pulse therapy has been studied in few studies. In a retrospective study from 2017, 100 patients with an average of three relapses were treated with vancomycin 125 mg four times daily for ten days, followed by vancomycin 125 mg once daily for one week and then either a 125 mg dose every other or every three days, the latter producing a prolonged regime [Citation33]. Calculated after 90 days, the treatment effect was superior (cure rate 81%) in those who received treatment every three days compared with those who received treatment every other day (cure rate 61%). In a series of 22 patients, most of whom had three or more recurrences, the vancomycin 125% × 4 daily cure rate was reduced to 125 mg × 2 daily for one week, 125 mg × 1 daily for one week and then pulse treatment with 125 mg every two days for a week and lastly pulse treatment with 125 mg every three days for one week [Citation34]. Conversely, a propensity score-matched cohort study with patients with first or second recurrence of CDI found no difference in recurrence rate after vancomycin with (n = 226) or without tapering (n = 678). However, they did observe a difference in mortality after 90 days in favour of the downsizing regime, but not after 180 days [Citation35]. No current randomised trials have investigated different tapered or pulse regimens for vancomycin, but one large, double-blind study is ongoing with standard fidaxomicin for vancomycin decoupling over 31 days (Clinicaltrials.gov registration number NCT02667418). In one study, tapering with vancomycin seemed superior to a single enema FMT, whereas colonoscopic FMT was three times better than vancomycin [Citation15].
Studies of long-term treatment with tapering or pulse treatment were summarized in a recent review [Citation36]. This review also described studies in which vancomycin treatment was combined with probiotics and rifaximin.
Comparisons of metronidazole, vancomycin and fidaxomicin were summarized in a network meta-analysis from 2020. In this meta-analysis, vancomycin and fidaxomicin were effective first-line treatments for mild/moderate CDI as opposed to metronidazole, and fidaxomicin was possibly superior to vancomycin in avoiding CDI recurrence [Citation37]. In addition, a number of overviews and guidelines review the established treatments and new treatments and provide recommendations from the clinical societies SHEA/IDSA, ESCMID, ASID and ACG [Citation38–42].
Other CDI-active antibiotics
In addition to metronidazole, vancomycin and fidaxomicin, a number of other antibiotics are also active against CD. Although the above mentioned three antibiotics predominantly cover the treatment need, the following may be considered, e.g. in the case of significant side effects, allergies or interactions related to the first three.
Nitazoxanide
Nitazoxanide is primarily a anti-parasite drug, but in a single study it has also shown activity against CD. Musher et al. [Citation43] described the results of a small, double-blind, randomised study including 49 patients with CDI who were treated with either vancomycin 125 mg × 4 or nitazoxanide 500 mg × 2, both for ten days. The study did not have the strength to show non-inferiority for nitazoxanide relative to vancomycin but did have comparable results: The response rate was 73% (16/22) in the nitazoxanide group and 67% (18/27) in the vancomycin group (RR 1.09, 95% CI 0.75–1.58). Nitazoxanide is not marketed in Denmark, but may be ordered from a pharmacy that holds a dispensing permit from the Danish Medicines Agency.
Rifaximin
Rifaximin is an orally ingested antibiotic that is poorly absorbed; it belongs to the rifamycin group. Rifaximin is active against CD, but resistance may occur with ribotypes 027/ST1 and 017/ST37 (Unpublished). In a clinical, randomised study from 1990 with a total of 20 patients, Boero [Citation44] found no significant difference in outcome after vancomycin or rifaximin (RR 0.90, 95% CI 0.69–1.18). In a systematic review from 2018 [Citation45], eight studies from 2007–2017 were reviewed, including two randomised studies. One common finding was that rifaximin was used primarily as a relapse prophylaxis immediately after stopping either vancomycin or metronidazole treatment. In a random-effects meta-analysis of the two randomised clinical trials (RCT) included in the meta-analysis, the effect of rifaximin, although not statistically significant, was compared with either placebo or metronidazole when given as recurrence prophylaxis (OR 0.36 (95% CI 0.12–1.10)).
Teicoplanin
Teicoplanin is usually used intravenously. In oral administration, it is absorbed only to a very modest extent from the gastrointestinal tract. Nelson’s 2017 Cochrane review [Citation39] used data from two smaller randomised trials [Citation21,Citation46] that compared teicoplanin withvancomycin. The success rate was higher in the teicoplanin group (87%; 48/55) than in the vancomycin group (73%; 40/55) (relative risk (RR) 1.21, 95% CI 95% 1.00–1.46). In both studies, the duration of treatment was ten days and the dose of vancomycin was 500 mg x 4. In de Lalla’s study, the dose of teicoplanin was 100 mg × 2 and in the Wenish study, the dose was 400 mg × 2. A Serbian study [Citation47] from 2018 compared the effects of teicoplanin to those of vancomycin. Patients with severe CDI were treated with teicoplanin at 100 mg × 2 versus vancomycin 125 mg × 4 for ten days. Patients with severe fulminant CDI were all initially given metronidazole 500 mg × 3 IV together with either teicoplanin 200 mg × 2 or vancomycin 500 mg × 4. The overall clinical cure rate for both groups of severity was higher in the teicoplanin group (97/107; 91%) than in the vancomycin group (143/180; 79%) (odds ratio (OR) 2.51 CI 95% 1.19–5.28). Likewise, clinical cure was higher for both groups separately, but this was not statistically significant. The recurrence rate was significantly lower in patients treated with teicoplanin (9/97; 9.3%) than in patients treated with vancomycin(49/143; 34.3%), p<.001, OR (95% CI) 0.20 (0.09–0.42)). Experience with teicoplanin as a treatment for CDI in Denmark is limited, partly due to the significantly higher price compared with vancomycin.
Bezlotoxumab
Bezlotoxumab is a human monoclonal antibody that binds toxin B produced by CD with high affinity, thereby neutralizing the toxin. Bezlotoxumab is administered intravenously as a single dose and is used as an adjunct to ongoing antibiotic therapy (metronidazole, vancomycin or fidaxomicin). Bezlotoxumab is not a treatment for CDI and has no effect on the current CDI episode, but may be used prophylactically for new CDIs as it provides passive immunity to toxins produced by persistent or newly acquired CD spores. The applicability of bezlotoxumab in clinical practice is limited by a high cost. In two large double-blind multinational phase 3 RCTs, a significant reduction in the recurrence rate was seen after 12 weeks by comparison between bezlotoxumab and placebo given in addition to standard antibiotic treatment (16–17% recurrence rate versus 26–28% in the placebo group, p<.001) [Citation48]. In these studies, the number needed to treat (NNT) was found to be ten; and for the subgroup of patients over 65 years of age or CDI cases within six months, the NNT wassix. Bezlotoxumab has been approved in adults for adjuvant treatment in this particular subgroup. However, experience and evidence concerning prophylactic use of bezlotoxumab against rCDI or as a CDI treatment option are generally lacking. Specifically, knowledge is missing that may define the place of treatment within the treatment algorithm.
Oral vancomycin prophylaxis (OVP) against new CDI by other concomitant antibiotic action
In retrospective case series, a reduced risk of recurrent CDI has been found when vancomycin is given concomitantly with antibiotic treatment for other infections. The studies compared patients who received OVP with patients who did not receive OVP (non-OVP) during other antibiotic treatment. A survey comprising 551 patients was found that OVP (125 mg × 4) reduced the risk of CDI recurrence in patients older than 65 years with two previous CDIs and where OVP was given for more than 50% of the time that another antibiotic treatment was given [Citation49]. In another study counting 203 participants, patients had received OVP (n = 71, such as 125 mg × 2 or 250 mg × 2) for up to one week during other concomitant antibiotic treatment. Here, a reduction in the recurrencerate was seen (27% in the non-OVP group against only 4% in the OVP group, p<.001) [Citation50]. The studies were described in a review [Citation51], which also provided information about the prospective studies to clarify doses and treatment durations, which were reported to www.clinicaltrials.gov.
Long-term or lifelong vancomycin treatment
For patients with persistent, recurrent CDI, where other treatment options seem exhausted or are contraindicated or where the patient does not want, e.g. FMT, empirical long-term treatment with vancomycin is used in the lowest possible dose, possibly for years/lifespan in case the expected remaining life is short, i.e. as secondary prophylaxis also without exposure to other antibiotics. This practice has been sparsely researched and described. A retrospective case series described the use of long-term treatment with vancomycin (defined at 125 mg once daily for at least eight weeks after tapering over 14 days from 125 mg × 4 daily for 14 days) in a group of 20 elderly patients with multiple previous recurrences who were either not candidates for FMT or had not benefitted from multiple FMT treatments. The patients were not exposed to other antibiotics during the period. Only one patient experienced recurrence during long-term treatment. This case was successfully treated with FMT. Some patients were treated for life/during the entire follow-up period (up to 19 months). Recurrence was seen in 31% of patients in whom vancomycin was later discontinued. No side effects were observed. Four patients died during follow-up, but none due to CDI [Citation52].
Intestinal microbiota treatment
In light of the increased incidence of CDIs and with increasing challenges associated with recurrent CDIs over the next 10–15 years, attempts to restore a normal intestinal microbiota using intestinal microbiota treatments have attracted much attention. Both nationally and internationally, the focus has been on FMT.
FMT is the transfer of faeces from a healthy donor to a patient in order to treat disease. FMT is reviewed in this guideline in a separate section, and here only the role of FMT in the treatment of patients with CDI is summarised.
Another approach to restoring normal intestinal microbiota is instillation of a standardised bacterial culture also coined rectal bacterial instillation (RBI)/rectal bacteriotherapy (RBT). RBI differs from FMT in that it does not take into account donor recruitment and screening and it eliminates the, albeit modest, risk of transmitting unknown pathogens. The method is not widespread internationally, but has been used on a smaller scale in Denmark since the 1980s and is therefore also discussed below.
FMT in recurrent CDI
FMT has been described as an effective treatment for recurrent CDI in case series since 1958 [Citation7]. However, the first RCT documenting the beneficial effect compared with standard vancomycin treatment was not published until 2013 [Citation14]. Here, the authors compared FMT administered via a nasoduodenal tube (preceded by 4–5 days of high-dose vancomycin) with oral vancomycin alone and oral vancomycin followed by cleansing. After ten weeks, 81% were non-recurrent in the FMT group against only 31 and 23% in the two vancomycin groups, respectively (p=.008/.003). Two subsequent RCTs have shown a similar effect for FMT administered via colonoscopy over 1–4 times (after three days of vancomycin) versus oral vancomycin for 10 days followed by pulse treatment for a minimum of three weeks (90 versus 26%, p<.0001) [Citation15] and for FMT administered via colonoscopy or naso-jejunal tube (preceded by 4–10 days of vancomycin) versus fidaxomicin for ten days or vancomycin for ten days (92 versus 42 versus 19%, p=.0002/<.0001) [Citation19]. The latter study also showed that FMT was superior to fidaxomicin in preventing new recurrences. A single study was unable to demonstrate the same effect as the others, as no difference in effect was found after 120 days of follow-up between a single FMT given via rectal installation, enema, and oral vancomycin for 14 days followed by tapering over four weeks. Use of single enema administration may explain non-superiority [Citation53].
In American and European guidelines, FMT is now recommended for patients with recurrent CDI who have not had an effect of repeated antibiotic regimens. No clear recommendations exist for when FMT should be offered. The published RCTs have recruited patients with a median of 3–4 CDI relapses, so evidence for the effect of FMT is strongest for patients with multiple relapses. A recent Danish RCT (not yet published) found a total effect of 1–3 rectal FMT infusions of 76%. Subgroup analysis showed that 90% of patients with first recurrence were cured, whereas the proportion was 70% among patients with second recurrence. The results for the limited number of patients in the study with multiple relapses were more mixed. These findings may strengthen an argument for using FMT earlier [Citation54].
FMT for a patient’s first CDI
The above studies examined FMT as a treatment modality exclusively in patients with recurrent CDI. A small proof of concept RCT from Norway randomised 21 adults with first CDI (no previous CDI case) to either ten days of metronidazole or FMT via enema/cleavage. Their results indicated a possible beneficial effect of FMT for this patient group [Citation55]. The same research group is now in the process of conducting a major RCT on the effect of FMT versus vancomycin, this time using regular donor stool. (Clinicaltrials.gov registration number NCT03796650).
Another small randomised pilot study (n = 16) compared vancomycin and donor faeces mixed from multiple donors to hospitalized patients with first CDI [Citation56]. The study found that 8/9 patients (89%) in the vancomycin group achieved symptom resolution within three days, whereas only 4/7 (57%) patients achieved resolution after the first FMT (57%) and 5/7 (71%) patients achieved resolution after the second FMT (71%). These did not record vancomycin pre-treatment before FMT.
At the end of the literature search, no published results from randomised studies reported on the effect of FMT on the first CDI.
FMT in treatment-refractory/severe/fulminant CDI
FMT can have a beneficial effect in CDI when CDI is medically refractory or develops into severe/fulminant infection during treatment. In 2015, a cohort study described 29 patients with severe or fulminant CDI who were treatment refractory, i.e. without effect of five days of vancomycin treatment. Patients received colonoscopic FMT with repeated FMT every third, fourth or fifth day if CDI symptoms persisted [Citation57]. Overall, 93% (27/29) of patients were cured, of whom all (10/10) had severe CDI and 89% (17/19) fulminant CDI. A single FMT was given to 18 (62%), two FMTs to nine (31%) and three FMTs to two (9%) of the patients.
An RCT from 2018 compared a single versus two or more FMT treatments in 56 admitted CDI patients (12% with their first CDI) with severe/complicated treatment-refractory (for vancomycin or fidaxomicin) disease. Here, the cure rate was 75% after one FMT (21/28) and 100% (28/28) after two or more FMT treatments. Thirty-three patients (59%) had severe-complicated (fulminant) CDI (17 in the single FMT group; 16 in the multiple FMT group) and 36 patients (64%) had pseudomembranous colitis (17 in the single FMT group; 19 in the multiple FMT group).
In a retrospective cohort study with analysis of three-month mortality in 111 patients admitted with CDI (66 in the FMT group and 45 in the non-FMT group), a pronounced reduction in mortality was found after FMT [Citation58]. Mortality was 24% (27/11) three months after diagnosis of CDI among which 12% (8/66) occurred in the FMT group versus 42% (19/45) in the non-FMT group. NNT was only two for patients with severe CDI who needed FMT treatment to save one life. A similar NNT of three for FMT to prevent death from severe/fulminant CDI was found in a retrospective, matched cohort study of 48 CDI patients admitted to the intensive care unit (including 15 patients (31%) with severe CDI and 33 (69%) with fulminant CDI), among whom 16 received FMT and 32 non-FMT [Citation59].
In a subgroup analysis of clinical trials before and after FMT implementation, comprising 199 patients with fulminant CDI and 110 patients with treatment-refractory (i.e. without improvement after five days of anti-CDI antibiotic treatment) severe/fulminant CDI, the investigators reported a significantly lower CDI-related mortality and colectomy rate after introduction of FMT in relation to earlier. For fulminant CDI, mortality was 21% before FMT and 9% after FMT; for refractory severe/fulminant CDI, it was 42% before FMT versus 12% after FMT. Implementation of FMT in treatment produced a significant reduction in the colectomy rate from 16% before FMT to 6% after FMT in fulminant CDI and from 32% before FMT to 8% after FMT was used in refractory severe/fulminant CDI [Citation60].
RBI in recurrent CDI
Internationally, only one Canadian study has been published using a bacterial culture rather than donor faeces. In this study, two patients with rCDI were treated successfully [Citation61]. In Denmark, the bacterial culture for RBI was originally developed in the 1980s at the Copenhagen University Hospital (Rigshospitalet) by Tvede et al. [Citation62]. Until 2014, the bacterial culture was produced on a smaller scale at Rigshospitalet and used for a total of around 200 patients. In 2016, the bacterial culture was approved by the Danish Medicines Agency as a drug for use in recurrent rCDI. Today, the bacterial culture is manufactured on a larger scale. The original work [Citation62] included six patients who had been successfully treated for rCDI. In a case series of 55 patients treated with RBI in the 2000–2012 period, clinical success was recorded for 35 (64%). In the subgroup of patients who had their first CDI episode <6 months before RBI, the success rate was 75% (25 of 32 patients) [Citation63]. In a Danish RCT completed in March 2019 and currently only published in abstract form, patients with rCDI were randomised to three arms: Vancomycin + FMT, vancomycin + RBI, or vancomycin monotherapy with tapering. The study included a total of 96 patients. In the RBI group, 16/31 (52%) were cured against 19/34 (56%) in the FMT group (OR 1.2 95% CI 0.4–3.2) and 14/31 (45%) in the vancomycin group. The RBI treatment consisted of three treatments for three consecutive days, whereas in the study and following clinical assessment of the treatment effect, it was possible to repeat FMT up to two times with a different donor within 14 days after the first FMT. In FMT given 1–3 times (24 patients received one, seven received two and one received three FTM) within 14 days, FMT was significantly superior to RBI, with success rates of 26/34 (76%) and 16/31 (52%), respectively (OR 3.0, 95% CI 1.1–8.8). RBI and vancomycin were equally effective (p = .61) [Citation54].
When assessing the overall clinical evidence for RBI, no clinical recommendation can be made for its use.
Surgery for CDI
In fulminant refractory CDI, mortality can reach 80–90% [Citation64]. Patients with fulminant CDI should therefore undergo close multidisciplinary monitoring and assessment in the absence of a treatment response for surgical intervention.
Colectomy
Surgical intervention with colectomy appears to be able to improve survival in fulminant CDI. In a study of 165 patients with fulminant CDI admitted to the intensive care unit, the mortality rate with continued medical treatment was found to be 65%, whereas the mortality was 36% after colectomy in patients over 65 years [Citation65]. It is well documented that timely surgery may save lives, but no objective clinical and paraclinical parameters currently predict the course of CDI after starting treatment with antibiotics or FMT. With premature surgery, the possibility of healing through medical treatment alone is lost, and the patient is subjected to surgical risk and ileostomy. In the event of late surgery, the risk of postoperative complications and death increases.
Loop ileostomy
An alternative to total colectomy or subtotal colectomy with blind closure of the rectum is the presentation of a loop ileostomy with the possibility of antegrade lavage with fluid, vancomycin or possibly FMT. The procedure of laparoscopic loop ileostomy, perioperative rinsing with fluid followed by rinsing with vancomycin is not inferior to colectomy in terms of mortality [Citation66–72]. To date, antegrade lavage with donor faeces has not been described in the literature. Loop ileostomy is thus a recognized option but should be chosen over colectomy only in the absence of signs of perforation or toxic megacolon.
Endoscopic desufflation and retrograde installation of vancomycin or FMT
May be considered in patients with colon dilatation and without upper access, but is not recommended in patients with fulminant CDI.
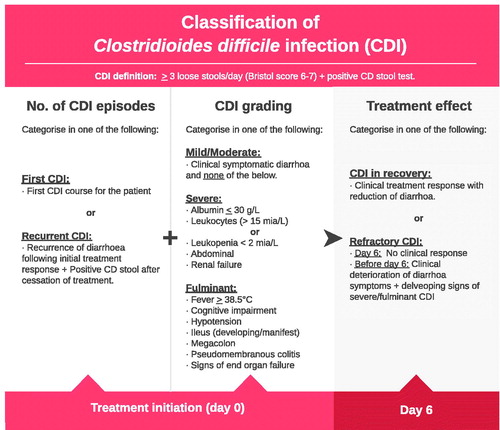
Assessment of treatment effect in CDI
A treatment algorithm for CDI is presented in . The treatment effect is assessed continuously based on the clinical picture (). Clinical recommendations including assessment of evidence level and grade of recommendation are presented in .
Table 1. Treatment algorithm in patients with Clostridioides difficile infection (CDI).
Table 2. Evaluation of treatment effects in patients with Clostridioides difficile infection (CDI).
Table 3. Clinical recommendations for the treatment of patients with Clostridioides difficile infection (CDI).
Persistent or worsening diarrhoea during antibiotic treatment indicates refractory disease, in which case treatment is escalated (). If an effect is observed during antibiotic treatment, the total treatment effect may be assessed one week after treatment cessation. The clinical effect is primarily assessed, i.e. resolution of diarrhoea. Diarrhoea assessment is supplemented with CD testing, as approximately 2% of the healthy population have positive CD test results without experiencing clinical disease [Citation73]. The carrier frequency is higher during hospitalisation and in cases with high comorbidity and age. It may take weeks before the toxin test becomes negative after a clinical treatment effect was observed.
CD testing during ongoing treatment with vancomycin/fidaxomixin/metronidazole cannot be used as the sample may be falsely negative due to poor excretion of toxins. Thus, testing cannot be used to distinguish between refractory disease and non-CDI/late effects of CDI [Citation74]. This is assessed based on the clinical picture. Repetition of tests for double determination is not recommended, as the method is very sensitive [Citation75]. Genetic testing at intervals of less than a week will generally not be meaningful.
Persistent bowel symptoms in combination with a negative CD test after treatment may be due to post-infectious irritable bowel syndrome [Citation76,Citation77]. In case of new-onset diarrhoea, CD testing may help to distinguish between recurrent CDI, treatment-requiring diarrhoeal disease or post-infectious irritable bowel syndrome.
Faecal microbiota transplantation (FMT) – clinical application
Treatment responsibility
Clinical application and follow-up are performed by a specialist in gastroenterology/hepatology or infectious medicine under the auspices of a medical hospital department, or by delegation from a specialist to another doctor/other professional group, and in accordance with agreed clinical guidelines.
Treatment responsibility and responsibility for clinical follow-up rests with the attending physician. Traceability for at least 30 years between donor and recipient must be ensured in accordance with the Tissue Act. Responsibility for traceability rests with the stool bank.
Indications and contraindications to FMT
The use of FMT in patients with CDI is described above, including clinical recommendations.
In ulcerative colitis, FMT has been studied in four RCTs with inconsistent results and without long-term follow-up. Therefore, FMT is currently not recommended in ulcerative colitis outside clinical trials [Citation78–81].
In Crohn’s disease, clinical studies have not yet been performed to assess the potential effect of FMT, but a pilot study suggests a beneficial effect in patients where donor microbiota colonizes in the recipient [Citation82]. The treatment is currently not recommended outside of clinical trials.
Irritable bowel syndrome (IBS)
Five RCTs describe conflicting results [Citation83–88]. Divergent findings and great heterogeneity between the studies challenge the clinical application (different study populations, multiple treatments applied, varying application form, uncertainty about donor selection, unclear risk profile, occurrence of side effects, loss of effect during long-term follow-up). FMT is currently not recommended for IBS.
Carrier status with multi-resistant bacteria
Eradication of carrier condition with multi-resistant bacteria has been sporadically reported after FMT [Citation89–92]. A randomised study did not find that FMT was better than placebo, but did not include a sufficient number of patients to confirm or disprove the null hypothesis [Citation93]. The treatment is recommended for use in clinical trials only.
Other conditions
FMT is being investigated for a future role in the treatment of, e.g. hepatic encephalopathy [Citation94], pouchitis [Citation95–99] and psoriatic arthritis [Citation100]. The clinical experiences are too few or contradictory for a treatment recommendation to be given at present. When using FMT for new indications, treatment should be initiated only after appropriate contact with the authorities.
Contraindications and precautions
No absolute contraindications to FMT have been described. FMT can be applied by rectal access in patients with ileus. Food items associated with serious food allergies, such as peanuts, should be used with caution by donors due to the risk of food allergens being transmitted. Pronounced leukopenia and other signs of severe immune incompetence should also lead to increased caution and heightened attention and may possibly place special demands on faecal suspensions.
Patient preparation before FMT
Ensuring indication
In patients with CDI, other causes of chronic diarrhoea may occur and should be investigated legeartis.
Patient guidance
FMT is a new treatment principle with unknown mechanisms of action and long-term consequences [Citation6]. Thorough oral and written patient guidance should therefore be given before treatment. The guidance should take into account that the treatment principle is undergoing development and that aesthetic and ethical reservations may be associated with receiving the treatment. Patients should be informed of possible side effects and their frequency. Examples of written patient guidance are available as appendices.
Planning the application
It is clarified in collaboration with the patient how the treatment will be given (see below).
Patient consent
Transmission of health information to the database and medical records for long-term follow-up when the patient has completed active treatment requires written patient consent. Examples of information and declarations of consent are available as appendices.
Minimisation of risk factors
Patients with CDI are often elderly, multimorbid patients with many medical issues [Citation1]. Risk factors for recurrence of CDI are age above 65 years, high comorbidity, use of concomitant antibiotics and use of proton pump inhibitor [Citation101–103]. Prior to FMT it is therefore recommended to revise medicines, in particular by discontinuing antibiotics and proton pump inhibitors, as well as optimizing the patient’s nutritional and hydration status. Anaemia has been found in a small study to be associated with failure of FMT, but it remains unclear whether correction of anaemia reduces the risk of recurrence after FMT.
Antibiotic treatment before FMT for rCDI is included as part of the overall treatment. In most clinical trials on FMT, a regimen of 4-10 days of vancomycin treatment before FMT and discontinuation of vancomycin 24–48 h before FMT is practiced [Citation40,Citation104]. In practice, FMT therefore means FMT preceded by vancomycin 125 mg × 4 for 4–10 days. Vancomycin is discontinued 1-2 days before administration of FMT, depending on the method of application and the frequency of the patient’s bowel movements. In case of intolerance or lack of effect of vancomycin, fidaxomicin may be given as a pre-treatment. The effect of FMT without prior antibiotics is unknown and is currently being studied in clinical trials [Citation55,Citation105].
Cleansing before FMT depends on the application method and is not a prerequisite for the FMT application itself. Cleansing without FMT hardly has an independent effect on rCDI [Citation14]. However, in an observational study with colonoscopically applied FMT, it was found that inadequate cleansing was related to FMT treatment failure [Citation106]. Colonoscopy cleansing is performed according to usual guidelines.
Other medical pre-treatment
In some studies, a proton pump inhibitor, motility-promoting treatment, etc. were used as accompanying treatments. The significance of this has not been clarified. For capsule-based treatment, discontinuation of an acid pump inhibitor three days before FMT is recommended to reduce the dissolution of the acid-resistant capsules in the stomach.
Clinical application and follow-up
The application method is the single factor that influence the FMT effect rate most. FMT may be given as an infusion with rectal catheters, via colonoscopy, nasojejunal tube, gastroscopy, nasogastric tube or PEG, or as capsule treatment with glycerol-based capsules or lyophilized capsules. Colonoscopy has a high effect rate in all studies. In a meta-analysis, a 78% effect rate was reported after one application and a 98% effect rate was reported after several applications (typically a total of two) [Citation107]. In a meta-analysis, nasogastric and nasoduodenal tube application has been shown to have equal effect in line with that of colonoscopy after one treatment (79%) and 88% after several treatments [Citation107]. The method involves a risk of aspiration, which was observed in three patients (8%) in a Dutch observational study including 39 treatments [Citation108]. When applied through nasojejunal tube with the tube tip distal to the Treitz’ ligament, no aspiration was observed in a single study reporting on the treatment of 11 patients with nasojejunal tube [Citation19]. In a meta-analysis, infusion on rectal catheters had an 56%effect after a single infusion and 92after repeated (typically up to three) infusions [Citation107]. In a randomised study with 30 patients, one infusion was not statistically significantly better than the tapered regimen with vancomycin [Citation53]. However, in a study with 219 patients and the possibility of multiple treatments, an effect of 91% was achieved in the modified intention-to-treat population [Citation18]. Capsules are increasingly used; in particular, a randomised study of 116 patients [Citation109] together with observational studies suggest an effect that is in line with that of FMT by colonoscopy [Citation110–112]. The greatest evidence for FMT still rests on colonoscopic application, which at the same time can contribute differential diagnostics. A systematic review of treatment modalities found that the different modes of administration were equivalent with the exception of rectal infusion, which was associated with a lower effect which was, however, offset by repetitive treatment [Citation107].
Possibility of repetitive treatment
When using rectal infusion and in patients with refractory or fulminant CDI, planning repetitive treatments can be beneficial, because the effect of one FMT is lower than the effect when using other application methods of lower degrees of severity [Citation18,Citation53]. Primary clinical effect is evaluated after 3–7 days. In case of continued diarrhoea, FMT may be repeated after 7–14 days. In case of repetitive administration of FMT, vancomycin is not administered in the period between individual applications [Citation15,Citation18].
Planned serial FMT
In patients with a high risk of recurrence, multiple treatments with FMT may be effective [Citation113]. Possible risk factors for recurrence include severe infection [Citation106], anaemia [Citation19] and liver cirrhosis [Citation114]. Treatments with serial FMT can be given at shorter intervals (e.g. three days), as the decision does not depend on clinical evaluation like in repetitive treatment. The optimal treatment regimen has not been determined and the specific treatment is planned with the patient. In case of continued symptoms after FMT, testing for CD should be repeated as a negative toxin test will suggest a cause other than CDI. However, this should not delay repetition in the above cases.
Amount of donor stool per treatment
In the published studies, down to 3 grams [Citation115] was successfully used for whole donor stools, but most commonly 30–50 grams of donor stool was used [Citation11,Citation116–118]. A cohort study found that effects after a low dose (7.5 grams) and a high dose (45 grams) of donor faeces were comparable [Citation119]. A systematic review indicated a lower treatment effect when using less than 50 grams [Citation107]. In most studies, at least 50 grams of donor faeces are used, and it is recommended that 50 grams of donor faeces be transferred at one FMT. Although a dose-response effect likely exists, a lower dose can be defended.
Post-treatment observation
The clinical significance of post-treatment observation and the duration of observation are unclear. It is recommended that patients remain in observation for 30 min after colonoscopic application lying in a right-sided position. After rectal infusion, the patient is observed lying in a left-sided position for one hour. The recommendation for fulminant CDI patients is in-hospital treatment.
Efficacy measures
All patients who have received FMT should be evaluated clinically to confirm lasting treatment effect. Impact measures may vary depending on the purpose. In randomised studies, different times of primary effect have been used, from 8 to 12 weeks. For consistent assessment of effect, this guideline recommends that clinical effect be assessed eight weeks after treatment, which is in line with international guidelines for CDI [Citation40]. In case of diarrhoea, monitoring should be supplement with a CD test. When evaluating FMT for rCDI, a primary effect measure is proposed in the form of clinical resolution, defined as the absence of CDI-associated diarrhoea after eight weeks or as normal bowel movements (three or fewer bowel movements per day and Bristol Stool Scale score of 5 or lower), or by diarrhoea (more than three thin (Bristol 6–7) stools daily) or a negative CD test.
Recurrence after primary resolution
With recurrence of diarrhoea in a patient who has previously received FMT and experienced a clinical effect, a CD test can distinguish between CD-related disease (recurrence) and non-CD-related diarrhoea. It is therefore recommended that patients with recurrence of diarrhoea after an otherwise successful treatment be evaluated with CD testing. In case of a positive CD test, FMT preceded by vancomycin pre-treatment may be offered again. It remains unclear whether an effect may be achieved by changing the donor or the application method.
Long-term follow-up
The long-term consequences of FMT are unknown. Treatment should therefore be performed with registration that allows for long-term follow-up. The attending physician plans and handles follow-up. The working group recommends a minimum one-year follow-up period.
Side effects and complications
All patients offered FMT should be informed in person and in writing about any risks of the treatment. The patient’s consent to treatment is oral, but follow-up for quality monitoring purposes after the patient’s course has been completed and for dissemination of health information can take place only after prior written patient consent. Adverse events and serious adverse events are documented by the attending physician. The individual FMT service defines how serious adverse events are registered and evaluated. Immediate side effects to FMT are seen in approximately one-third of patients [Citation19]or more, as systematic reviews have found that adverse events after FMT are underreported [Citation120,Citation121]. Adverse events include abdominal pain, diarrhoea and rumbling. The patient should be informed of this prior to treatment. Long-term side effects are consequences that extend beyond the first 1–3 days after treatment. Serious adverse events are recorded in the patient record. When describing serious adverse events, the severity of the events and the causality of FMT are described [Citation122].
Special situations
CDI in patients with inflammatory bowel disease
For treatment of CDI with FMT in patients with IBD, the focus has been on whether the effect of FMT is less in IBD patients than non-IBD patients, whether there is a risk of exacerbation of IBD after FMT and whether IBD disease activity has an effect on the effect of FMT. FMT among IBD patients with CDI has been elucidated in systematic reviews and meta-analyses of cohort studies [Citation123,Citation124] and in comparative studies of IBD patients versus non-IBD patients [Citation125,Citation126]. Several of the considered studies included relatively few patients, and the largest cohort studies included 67 patients with IBD. Overall, although evidence that FMT is effective among patients with IBD and concomitant CDI varied, no difference in treatment efficacy between IBD and non-IBD patients was reported [Citation127–129]. Moreover, a comparable recurrence rate of CDI in IBD and non-IBD patients indicates that there is a similar effect in Crohn’s disease and ulcerative colitis. In a meta-analysis of 29 studies, worsening of IBD was found in 23% after FMT for CDI, whereas the risk of worsening was 11% in patients treated with FMT for IBD [Citation124]. Treatment was also found to be cost-effective compared with vancomycin and fidaxomicin [Citation130].
Severe immune incompetence
Treatment of patients with severe immune incompetence places particular demands on the balance of risk and benefit of FMT, as severe immune incompetence involves a theoretical risk of bacterial translocation following FMT. Two cohort studies [Citation123,Citation131] and a systematic review [Citation132] have found that FMT used in patients with severe immune incompetence does not increase the incidence of complications [Citation131], and treatment can generally be recommended [Citation132,Citation133]. Possible complications include bacteraemia, and the transmission of multi-resistant microorganisms is sought avoided through strict donor screening [Citation134]. Despite thorough donor screening, the increased risk of transmission of potential unknown pathogens should be considered and the patient should be informed about this. As a definition of severe immune incompetence, the following is proposed: Current or predicted severe neutropenia (<0.5 billion/L) OR planned or recent (<100 days) allogeneic stem cell transplantation OR active graft-versus-host disease (GVHD) in need of immunosuppressive therapy. The significance of less pronounced degrees of immune incompetence for outcomes and complications after FMT remains unclear as is the case for patients with a reduced CD4 + cell count, patients on other immunosuppressive therapy such as biological therapy, azathioprine, methotrexate, nucleoside analogues, and patients with congenital or acquired primary immunodeficiency. In such patients, extended follow-up and monitoring may be planned. In patients with organ transplantation, FMT can be used, and a retrospective review does not indicate an increased incidence of complications in organ transplant recipients [Citation123].
Liver cirrhosis
A retrospective cohort study found that patients with liver cirrhosis and CDI needed a larger dose of donor stool to achieve the same treatment effect as other patients with CDI. FMT can be used in patients with liver cirrhosis, but more treatments may be needed. The optimal treatment algorithm has not been clarified.
Condition with colectomy
Patients with ileostomy or ileorectal anastomosis may develop CDI, and the effect of FMT is case sensitive and should be considered [Citation135].
Clinical recommendations
Clinical recommendations for the use of FMT are presented in .
Table 4. Clinical recommendations for the use of faecal microbiota transplantation (FMT).
Organisation of FMT, donor recruitment and screening, laboratory preparation
Organisation of an FMT service
Hospital-based non-commercial use
The principle of this guideline is that faecal suspensions are not subject to sale or marketing and are used only in hospital settings on established indications or as part of clinical trials.
Infrastructure
Routine use of FMT takes place as part of a complex treatment that involves both clinical and paraclinical specialties. A complete FMT service includes a formalised system, which can generally be divided into three domains: (1) donor recruitment and donor screening, (2) laboratory preparation and storage of faeces, and (3) clinical application, follow-up and management of complications. The first two domains may be organised in a centralised stool bank that handles the donor-related matters and preparation of donor faeces. A description of the responsible staff should be in place, including a description specifyingwho holds the paraclinical and clinical responsibilities, and the distribution of areas of work and responsibilities. Treatment may take place at the clinical ward of the stool bank or at a separate clinical ward (treatment ward) in collaboration with the stool bank. Treatment that takes place at a local hospital following supply of faeces from a stool bank should take place as part of a formalised collaboration agreement with and clinical medical support provided by the faeces bank’s staff. Clinical responsibility for the application of FMT lies with the attending physician. A clinical department where FMT is performed should have access to medical clinical, clinical immunological and clinical microbiological expertise in order to deal with issues and complications. Treatment can be done according to consensus-based, published protocols [Citation136–139] and international guidelines [Citation140,Citation141]. General principles for donor recruitment, screening, preparation, release and clinical application are described in the Danish Tissue Act with accompanying technical guidance.
Regulation
The general structure of a stool bank and a clinical treatment department may be established according to the principles described in the Council of Europe Guideline on the Safety and Quality of Tissues and Cells for human application [Citation140]. At the European level, however, the EU Commission currently finds that donor faeces is currently not covered by the Tissue and Cell Directive [Citation142] and leaves it up to individual Member States to decide whether treatment should be regulated under this Directive [Citation143] or in pursuance of the Medicines Act [Citation144]. A process is underway to incorporate and classify donor faeces in the most appropriate legislation. Until FMT-specific legislation is available, the working group finds that the most suitable material is the standards established in the Danish Tissue Act for safe handling of donated human material, which ensures donor as well as recipient and which can be applied to donor faeces. The Tissues Act’s standards for selection of donors, donor screening, preparation and storage of tissue suspensions as well as general quality assurance are already established practice in all blood banks. These standards should guide the general structure of an FMT service’s donor handling and donation. In addition, the Council of Europe’s guideline on the safety and quality of human use of tissues and cells contains a separate chapter on FMT, which may further guide clinical management [Citation140].
Documentation and quality assurance
The Danish Tissue Act, derived from the European Tissue and Cells directive [Citation143], describes principles for establishing donor anonymity, traceability, storage of samples and other documentation. Guidelines for medical records are described in the Record Keeping Order (https://www.retsinformation.dk/Forms/R0710.aspx?id=201378). Registration of patients’ health information in the database may take place following registration of the data-responsible authority’s project overview database and requires written patient consent. Documentation of clinical activity and registration of adverse events may be done in accordance with the recommendations provided below. Examples of patient guidance, information material and a consent statement are available as appendices.
Donor recruitment
Faeces donation is voluntary and unpaid in accordance with the Danish Tissue Act. This increases the security of the donation material, as no financial incentive exists to donate; this also ensures that human material does not become the subject of trade, which would violate ethical standards [Citation140,Citation145].
Recruitment
Faeces donors may be recruited through promotion in blood banks or by personal contact. The general population’s suitability as a faeces donor is around 2–10% [Citation146–148]. In order to achieve a cost-effective donor recruitment with the highest possible security and to ensure supply stability, recruitment via the public blood banks is recommended [Citation139,Citation147–153].
Anonymity
Faeces donors must be ensured anonymity in accordance with the Danish Tissue Act. This means that no dedicated donors are used, i.e. donors remain unknown to the recipient. It is recommended to archive and store all documents related to traceability for a minimum of 30 years in accordance with the Danish Tissue Act [Citation143].
Donor consent
Prior to assessment as a faeces donor, the candidate donor should be informed in person and in writing about the course and about any potential risks. The candidate donor should also be examined for any carrier status of multi-resistant microorganisms. If conditions arise during the screening that require further investigation and/or treatment, the donor will be referred hereto. The responsible physician of the stool bank or FMT service is responsible for this. No inconveniences are associated with donating faeces. As personally identifiable data are being stored about the donor, written consent to the registration of health information in the database is required in accordance with applicable law. An agreement is reached on screening, number of donations and possible participation in a research project. An example of a declaration of consent is given in the appendix.
Donor screening
Donor screening includes pre-screening based on general criteria, detailed questionnaires and analysis of blood and faecal samples before commencing and after completingthe donation round () [Citation107,Citation137–139,Citation148,Citation151,Citation153]. The donor screening program is updated continuously, and exchange of experience through national and international networks may be expedient.
Table 5. Donor screening in faecal microbiota transplantation (FMT).
Approval of faeces donors
The overall review of donor screening is handled by a physician with the necessary qualifications who is affiliated with the stool bank or equivalent. Quarantine provisions following travel abroad, blood transfusion, antibiotic treatment, etc. are applied in accordance with the principles in the Danish Transfusion Medicine Standards (https://dski.dk/gaeldende-version-rejse/).
Stool donation
Donation of faeces takes place in donation rounds, which can last up to 30 days. Before the start and after the conclusion of any donation period, donor screening is completed, and any donated faeces are quarantined until the results of both screenings have been approved. The material can then be released for patient treatment.
Donation
Donor faeces are handed in by the donor in the stool bank or transported directly by a third party to the stool bank in sealed packaging. Each faecal donation should be accompanied by a written statement stating the donor time of donation, confirming that the donor has not been ill or exposed to infection since the latest donation and that the faeces were donated by the donor. Using a dedicated laboratory information system throughout the process, the donor’s statement will be an electronic questionnaire, developed specifically for faeces donors, similar to the questionnaire on attendance used for blood donation.
Storage during transport
During transport, donations must be stored in transport containers validated for transport of human material. Storage at room temperature for up to two hours does not appear to affect the bacterial composition [Citation154]. Depending on local logistics, donations may be placed in a cooler bag with cooling elements during transport.
Personnel, physical environment, critical equipment, quality assurance and document management
In the stool bank, an organisation plan must be in place, all staff must have a job description and a competence management system must be in place.
The laboratory where preparation is performed should be established in connection with the specialties of clinical immunology or clinical microbiology to ensure sufficient professional competencies. Standard procedures, detailed laboratory instructions for all parts of faecal sample handling, manufacture and testing are developed and used. Document management is ensured by the faeces bank using version-controlled documents which are regularly reviewed as part of the general quality management system [Citation136,Citation139,Citation155]. Critical equipment must be defined and its use must be logged.
Quality assurance is achieved in accordance with the general principles presented in the Council of Europe’s guide [Citation140] and in the Danish Tissue Act with the accompanying executive order and guidance.
Preparation of donor faeces
Donor faeces are processed minimally and solely for the purpose of conserving and preparing the donation for clinical administration.
Preparation for donation
Prior to each donation, facilities and remedies such as scales and workstations must be cleaned and disinfected with a chlorine product (1,000 ppm), and all material (recyclable) cleaned and chlorinated-disinfected to avoid potential cross-contamination, including with spores. As an alternative to chlorine disinfection, sterilization may be used. All critical equipment used is disposable. In the absence of CE approval, used utensils should be approved for food use.
Duration of preparation
In most studies where FMT has been used, faecal preparation has been completed within six hours without loss of clinical effect. Preparation should begin no more than two hours after donation and be completed no more than six hours after donation. When storing donor faeces before preparation, donor faeces are stored at 4 °C [Citation156].
Aerobic/anaerobic preparation
Although preservation of the intestinal anaerobic bacteria is theoretically desirable, special anaerobic technique in preparation is not documented as necessary for achieving a clinical response after FMT for rCDI [Citation157,Citation158], possibly owed to the high incidence of viable spores in the intestinal microbiota of healthy individuals [Citation159].
Donor-specific preparation
Donor faeces are processed without mixing faeces from several donors, i.e. each faeces suspension for treatment consists of faeces from one donor. This is done to maintain traceability between donor and recipient and to ensure the concept of transfer of one single microbiota.
Homogenisation
Homogenisation may be done using a disposable stick blender, smasher, or stomacher bag [Citation160]. No differences in clinical effect for degree or method of homogenization have been described.
Preparation for faecal suspension or capsules
Standardised protocols for preparation and freezing of donor faeces have been published [Citation100,Citation136,Citation139] and validated with clinical endpoints for both liquid faecal suspensions [Citation147], glycerol-preserved and encapsulated faeces [Citation105,Citation161] and freeze-dried donor faeces [Citation115]. In preparation without freeze-drying, 10% glycerol is usually added to the donor faeces as a cryoprotectant [Citation111,Citation157]. The faeces suspension is homogenized in sterile isotonic sodium chloride [Citation162]. None of the added adjuvants have therapeutic effects. For one treatment, 50 grams of donor stool is recommended [Citation138].
Freezing of donor faeces
Cryopreservation is necessary for storing donor faeces until use. Frozen and fresh donor faeces have the same treatment effect [Citation13,Citation18,Citation116,Citation157]. Because post-donation donor screening is not possible with fresh donations, only frozen-thawed faeces should be used. In addition, this facilitates practical handling and reduces the risks associated with handling.
Archive samples (safety samples)
Archive samples are taken from all donations. Samples are stored at −80 °C for at least two years after the donor faeces is used.
Labelling of faeces suspensions
When marking and supplying suspensions to FMT, the stool bank must comply with the conditions described in the Danish Tissue Order (Executive Order no. 58 of 18/01/2019, Order on quality and safety in the handling of human tissues and cells, Appendix 2, Sections D and E and Annex 8, Section 1.6 and Section 1.7). All FMT suspensions must be marked at the time of removal. As a minimum, the primary container must be marked with donation identification data/code and tissue type.
The container with the FMT suspension must be marked with:
Tissue type, tissue identification number/code and lot or batch number, tissue centre identification and expiration date.
For recipient-specific donations, the label must state for whom the donation is intended.
FMT suspensions must be labelled with a common European code (SEC), e.g. the ISBT 128 coding system, if the suspension is distributed for human use by another legal entity (hospital unit) other than the tissue centre.
If no space is available for the information on the container’s label, it must be indicated on a separate delivery note accompanying the container. The delivery note must be packed together with the container in a manner that ensures that they remain together at all times. In addition to the above, the label or delivery note must contain the following information:
Description (definition) and, if applicable, the size of the tissue or cell product
Date of tissue distribution
Selected biological determinations performed on the donor and the results
Recommendations regarding storage
Instructions for opening the container, packing and any handling/reconstitution required
Shelf life after opening/handling
Instructions for reporting serious side effects and adverse events
When supplying FMT suspensions, the procedures of the approved tissue centre must meet the following criteria:
Critical transport conditions, e.g. temperature and time limit, are determined so that the required tissue and cell properties are maintained
The container/package must be secure and ensure that the tissue and cells are preserved under the conditions specified. All containers and packages must be validated as being fit for purpose
If FMT suspensions are distributed by a third party under contract, a documented agreement ensuring that the required conditions are present must be produced
Tissue establishments must have approved staff who can assess the need for recall and implement and coordinate the necessary measures
An effective recall procedure must be in place that includes a description of the division of responsibilities and measures to be taken. The procedure must include notification to the Danish Agency for Patient Safety
A documented system must be in place for handling returned products, including criteria for including them in the inventory.
Storage, quarantine, release and distribution
Storage
Stools are stored suspended in 10% glycerol at −80 °C; tissue suspensions must be stored at −80 °C or cooler. After storage, clinical effect has been documented for 6–10 months, and shelf life may be longer [Citation163]. Storage at −20 °C is possible for up to one month [Citation164].
Quarantine
After preparation, stool suspensions must be placed in a separate section for quarantined suspensions until all release criteria are met. There should be clear markings and a control system to handle and separate released faecal suspensions from quarantined ones. Marking follows local instructions or standard operating procedures (SOP).
Release of donor faeces
Before the FMT suspension can be released, ensure that: (a) The donor is suitably assessed on the basis of initial donor screening. (b) A signed consent form exists. (c) Signed delivery notes from all faecal donations are available. (d) All analysis results are available and well known, both from the initial screening and from the first and last donation within the donation round. (e) The FMT suspension was processed and frozen no later than six hours after the donation time.
Distribution
As the use of FMT is developed and established as a clinical treatment option, simultaneous treatment is made possible locally in specialist departments by qualified specialists. The fact that the treatment can be performed locally has benefits for patients and therapists alike. It also places demands on logistics, quality assurance and documentation at the local treatment centre. Distribution may take place as part of a formalised collaboration between the stool bank and the treatment site. The conditions needed to establish FMT at the clinical treatment department are established in dialogue with the distributing stool bank. As the treatment principle is under development, any application is subject to registration and annual evaluation of clinical activity, effect, adverse events, etc. [Citation165].
Preparation before treatment
The day before its clinical use, donor faeces stored in cryotubes, cryobags, or capsules may be transferred to −20 °C. Generally, storage at −20 °C is acceptable for up to one month [Citation163]. During distribution to another location donor faeces is stored on dry ice. Defrosting is done according to local instructions. Thawed donor faeces should be used within two hours and should not be re-frozen, as repeated freeze-thaw cycles may reduce viability [Citation166].
Clinical application and safety parameters
Clinical application follows the general descriptions above. Clinical responsibility lies with the treating specialist.
Deviations
There must be local procedures for reporting deviations (e.g. risk of infection or possible unwanted side effects) to the physician in charge. Stool banks must ensure communication channels before commencing faeces distribution to clinical departments and the clinical departments must be able to handle any clinical complications.
Traceability
Traceability must be ensured for each FMT suspension. Traceability includes unique identification allocated to each donation, traceability between donor and suspension, suspension records, suspension status and recipient, as well as critical equipment used in preparation. Stool banks must register data on the patient for whom the FMT suspension is handed out for treatment so as to ensure traceability between donor and recipient.
Look-back
If it is suspected that a patient may have become infected with pathogenic microorganisms through FMT, look-back should be initiated. An archive sample (described above) is stored from each faecal donation, which is used for look-back and, if relevant, for testing for a specific pathogen.
Follow-up and registration of adverse events must be done as described above.
Internal audits must be conducted in accordance with the Danish Tissue Act, Appendix 1, Section F. Internal audits must be conducted at least every two years in order to verify that the approved internal instructions (SOPs) are complied with.
Minimum standards
Suggestion for minimum standards for implementing an FMT service are presented in .
Table 6. Mininum standards for implementing a faecal microbiota transplantation (FMT) service.
The emergence of societal and health-related patterns, exemplified by the recent COVID-19 pandemic, will likely require a dynamic adaptation of stool bank and FMT services in the future [Citation167].
| Abbreviations | ||
| CD | = | Clostridioides difficile |
| EL | = | evidence level |
| FMT | = | faecal microbiota transplantation |
| IBS | = | irritable bowel syndrome |
| NNT | = | number needed to treat |
| OVP | = | oral vancomycin prophylaxis |
| RBI | = | rectal bacterial instillation |
| RBT | = | rectal bacteriotherapy |
| rCDI | = | recurrent Clostridioides difficile infection |
| RCT | = | randomised clinical trial |
| RG | = | recommendation grade |
| SOP | = | standard operating procedure. |
Acknowledgements
This Danish national guideline was endorsed by the Danish Society for Gastroenterology and Hepatology, the Danish Society of Infectious Diseases, the Danish Society for Clinical Microbiology, and the Danish Immunology Society for Clinical Immunology.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539–1548.
- Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999–2004. Emerg Infect Dis. 2007;13(9):1417–1419.
- Le Monnier A, , Duburcq A, Zahar JR, GMC Study Group, et al. Hospital cost of Clostridium difficile infection including the contribution of recurrences in French acute-care hospitals. J Hosp Infect. 2015;91(2):117–122.
- Magee G, Strauss ME, Thomas SM, et al. Impact of Clostridium difficile-associated diarrhea on acute care length of stay, hospital costs, and readmission: a multicenter retrospective study of inpatients, 2009-2011. Am J Infect Control. 2015;43(11):1148–1153.
- Zhang S, Palazuelos-Munoz S, Balsells EM, et al. Cost of hospital management of Clostridium difficile infection in United States-a meta-analysis and modelling study. BMC Infect Dis. 2016;16(1):447.
- Allegretti JR, Mullish BH, Kelly C, et al. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet. 2019;394(10196):420–431.
- Eiseman B, Silen W, Bascom GS, et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–859.
- Garborg K, Waagsbo B, Stallemo A, et al. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis. 2010;42(11–12):857–861.
- Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(7):1079–1087.
- Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol. 2012;46(2):145–149.
- Mattila E, Uusitalo-Seppala R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142(3):490–496.
- Rubin TA, Gessert CE, Aas J, et al. Fecal microbiome transplantation for recurrent Clostridium difficile infection: report on a case series. Anaerobe. 2013;19:22–26.
- Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58(11):1515–1522.
- van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415.
- Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41(9):835–843.
- Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med. 2016;165(9):609–616.
- Jiang ZD, Ajami NJ, Petrosino JF, et al. Randomised clinical trial: faecal microbiota transplantation for recurrent Clostridum difficile infection – fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment Pharmacol Ther. 2017;45(7):899–908.
- Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. Jama. 2016;315(2):142–149.
- Hvas CL, Jørgensen SMD, Jørgensen SP, et al. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent Clostridium difficile infection. Gastroenterology. 2019;156(5):1324–1332.
- Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2(8358):1043–1046.
- Wenisch C, Parschalk B, Hasenhundl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22(5):813–818.
- Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 1997;92(5):739–750.
- Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302–307.
- Johnson S, Louie TJ, Gerding DN, for the Polymer Alternative for CDI Treatment (PACT) investigators, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345–354.
- Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med. 2017;177(4):546–553.
- Fekety R, Silva J, Kauffman C, et al. Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens. Am J Med. 1989;86(1):15–19.
- Lam SW, Bass SN, Neuner EA, et al. Effect of vancomycin dose on treatment outcomes in severe Clostridium difficile infection. Int J Antimicrob Agents. 2013;42(6):553–558.
- Rokas KE, Johnson JW, Beardsley JR, et al. The addition of intravenous metronidazole to oral vancomycin is associated with improved mortality in critically ill patients with Clostridium difficile infection. Clin Infect Dis. 2015;61(6):934–941.
- Wang Y, Schluger A, Li J, et al. Does addition of intravenous metronidazole to oral vancomycin improve outcomes in Clostridioides difficile infection? Clin Infect Dis. 2020;71(9):2414–2420.
- Mullane KM, Miller MA, Weiss K, et al. Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clin Infect Dis. 2011;53(5):440–447.
- Cornely OA, Crook DW, Esposito R, OPT-80-004 Clinical Study Group, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12(4):281–289.
- Guery B, Menichetti F, Anttila VJ, et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect Dis. 2018;18(3):296–307.
- Sirbu BD, Soriano MM, Manzo C, Lum J, et al. Vancomycin taper and pulse regimen with careful follow-up for patients Wwth recurrent Clostridium difficile infection. Clin Infect Dis. 2017;65(8):1396–1399.
- Tedesco FJ, Gordon D, Fortson WC. Approach to patients with multiple relapses of antibiotic-associated pseudomembranous colitis. Am J Gastroenterol. 1985;80(11):867–868.
- Gentry CA, Giancola SE, Thind S, et al. 2nd: A propensity-matched analysis between standard versus tapered oral vancomycin courses for themanagement of recurrent Clostridium difficile infection. Open Forum Infect Dis. 2017;4:ofx235.
- Murphy MM, Patatanian E, Gales MA. Extended duration vancomycin in recurrent Clostridium difficile infection: a systematic review. Ther Adv Infect Dis. 2018;5(6):111–119.
- Okumura H, Fukushima A, Taieb V, et al. Fidaxomicin compared with vancomycin and metronidazole for the treatment of Clostridioides (Clostridium) difficile infection: a network meta-analysis. J Infect Chemother. 2020;26(1):43–50.
- Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(2):1–26.
- Nelson RL, Suda KJ, Evans CT. Antibiotic treatment for Clostridium difficile-associated diarrhoea in adults. Cochrane Database Syst Rev. 2017;3:CD004610.
- McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987–994.
- Guery B, Galperine T, Barbut F. Clostridioides difficile: diagnosis and treatments. BMJ. 2019;366:l4609.
- Cheng YW, Fischer M. Clinical management of severe, fulminant, and refractory Clostridioides difficile infection. Expert Rev anti Infect Ther. 2020;18(4):323–333.
- Musher DM, Logan N, Bressler AM, et al. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clin Infect Dis. 2009;48(4):e41-6–e46.
- Boero M, Berti E, Morgando A, et al. Treatement of pseudomembranous colitis: a randomized controlled trial with rifaximine vs. vancomycin. Microbiologia Medica. 1990;5:74–77.
- Ng QX, Loke W, Foo NX, et al. A systematic review of the use of rifaximin for Clostridium difficile infections. Anaerobe. 2019;55:35–39.
- de Lalla F, Nicolin R, Rinaldi E, et al. Prospective study of oral teicoplanin versus oral vancomycin for therapy of pseudomembranous colitis and Clostridium difficile-associated diarrhea. Antimicrob Agents Chemother. 1992;36(10):2192–2196.
- Popovic N, Korac M, Nesic Z, et al. Oral teicoplanin versus oral vancomycin for the treatment of severe Clostridium difficile infection: a prospective observational study. Eur J Clin Microbiol Infect Dis. 2018;37(4):745–754.
- Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376(4):305–317.
- Carignan A, Poulin S, Martin P, et al. Efficacy of secondary prophylaxis with vancomycin for preventing recurrent Clostridium difficile infections. Am J Gastroenterol. 2016;111(12):1834–1840.
- Van Hise NW, Bryant AM, Hennessey EK, et al. Efficacy of oral vancomycin in preventing recurrent Clostridium difficile infection in patients treated with systemic antimicrobial agents. Clin Infect Dis. 2016;63(5):651–653.
- Brown CC, Manis MM, Bohm NM, et al. Oral vancomycin for secondary prophylaxis of Clostridium difficile infection. Ann Pharmacother. 2019;53(4):396–401.
- Zhang K, Beckett P, Abouanaser S, et al. Prolonged oral vancomycin for secondary prophylaxis of relapsing Clostridium difficile infection. BMC Infect Dis. 2019;19(1):51.
- Hota SS, Sales V, Tomlinson G, Salpeter MJ, et al. Oral vancomycin followed by fecal transplantation versus tapering oral vancomycin treatment for recurrent Clostridium difficile infection: an open-label, randomized controlled trial. Clin Infect Dis. 2017;64(3):265–271.
- Rode AA, Chehri M, Krogsgaard L, et al. Cure of recurrent Clostridium difficile infection with a mix of 12 gut bacteria, faecal microbiota transplantation or oral vancomycin: results from an open-label multicentre randomised controlled trial. Aliment Pharmacol Ther. 2021;53(9):999–1009.
- Juul FE, Garborg K, Bretthauer M, et al. Fecal microbiota transplantation for primary Clostridium difficileinfection. N Engl J Med. 2018;378(26):2535–2536.
- Camacho-Ortiz A, Gutierrez-Delgado EM, Garcia-Mazcorro JF, et al. Randomized clinical trial to evaluate the effect of fecal microbiota transplant for initial Clostridium difficile infection in intestinal microbiome. PLOS One. 2017;12(12):e0189768.
- Fischer M, Sipe B, Cheng YW, et al. Fecal microbiota transplant in severe and severe-complicated Clostridium difficile: a promising treatment approach. Gut Microbes. 2017;8(3):289–302.
- Hocquart M, Lagier JC, Cassir N, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66(5):645–650.
- Tixier EN, Verheyen E, Ungaro RC, et al. Faecal microbiota transplant decreases mortality in severe and fulminant Clostridioides difficile infection in critically ill patients. Aliment Pharmacol Ther. 2019;50(10):1094–1099.
- Cheng YW, Phelps E, Nemes S, et al. Fecal microbiota transplant decreases mortality in patients with refractory severe or fulminant Clostridioides difficile infection. Clin Gastroenterol Hepatol. 2020;18(10):2234–2243.
- Petrof EO, Gloor GB, Vanner SJ, et al. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome. 2013;1(1):3.
- Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet. 1989;1(8648):1156–1160.
- Tvede M, Tinggaard M, Helms M. Rectal bacteriotherapy for recurrent Clostridium difficile-associated diarrhoea: results from a case series of 55 patients in Denmark 2000–2012. Clin Microbiol Infect. 2015;21(1):48–53.
- Bhangu S, Bhangu A, Nightingale P, et al. Mortality and risk stratification in patients with Clostridium difficile-associated diarrhoea. Colorectal Dis. 2010;12(3):241–246.
- Lamontagne F, Labbe AC, Haeck O, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007;245(2):267–272.
- Neal MD, Alverdy JC, Hall DE, et al. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann Surg. 2011;254(3):423–427.
- Ferrada P, Callcut R, Zielinski MD, EAST Multi-Institutional Trials Committee, et al. Loop ileostomy versus total colectomy as surgical treatment for Clostridium difficile-associated disease: an Eastern Association for the surgery of trauma multicenter trial. J Trauma Acute Care Surg. 2017;83(1):36–40.
- Kulaylat AS, Kassam Z, Hollenbeak CS, et al. Surgical Clostridium-associated risk of death score predicts mortality after colectomy for Clostridium difficile. Dis Colon Rectum. 2017;60(12):1285–1290.
- McKechnie T, Lee Y, Springer JE, et al. Diverting loop ileostomy with colonic lavage as an alternative to colectomy for fulminant Clostridioides difficile: a systematic review and meta-analysis. Int J Colorectal Dis. 2020;35(1):1–8.
- Vely A, Ferrada P. Role of surgery in Clostridium difficile infection. Clin Colon Rectal Surg. 2020;33(2):87–91.
- Juo YY, Sanaiha Y, Jabaji Z, et al. Trends in diverting loop ileostomy vs total abdominal colectomy as surgical management for Clostridium difficile colitis. JAMA Surg. 2019;154(10):899–906.
- Bowman JA, Utter GH. Evolving strategies to manage Clostridium difficile colitis. J Gastrointest Surg. 2020;24(2):484–491.
- Green DA, Stotler B, Jackman D, et al. Clinical characteristics of patients who test positive for Clostridium difficile by repeat PCR. J Clin Microbiol. 2014;52(11):3853–3855.
- Sunkesula VC, Kundrapu S, Muganda C, et al. Does empirical Clostridium difficile infection (CDI) therapy result in false-negative CDI diagnostic test results? Clin Infect Dis. 2013;57(4):494–500.
- Aichinger E, Schleck CD, Harmsen WS, et al. Nonutility of repeat laboratory testing for detection of Clostridium difficile by use of PCR or enzyme immunoassay. J Clin Microbiol. 2008;46(11):3795–3797.
- Wadhwa A, Al Nahhas MF, Dierkhising RA, et al. High risk of post-infectious irritable bowel syndrome in patients with Clostridium difficile infection. Aliment Pharmacol Ther. 2016;44(6):576–582.
- Svendsen AT, Bytzer P, Engsbro AL. Systematic review with meta-analyses: does the pathogen matter in post-infectious irritable bowel syndrome? Scand J Gastroenterol. 2019;54(5):546–562.
- Costello SP, Hughes PA, Waters O, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321(2):156–164.
- Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149(1):102–109.
- Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389(10075):1218–1228.
- Rossen NG, Fuentes S, van der Spek MJ, et al. Findings rom a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015;149(1):110–118.
- Sokol H, Landman C, Seksik P, Saint-Antoine IBD Network, et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: a pilot randomized controlled study. Microbiome. 2020;8(1):12.
- Halkjaer SI, Christensen AH, Lo BZS, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. 2018;67(12):2107–2115.
- Johnsen PH, Hilpusch F, Cavanagh JP, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018;3(1):17–24.
- Aroniadis OC, Brandt LJ, Oneto C, et al. Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: a double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2019;4(9):675–685.
- El-Salhy M, Hatlebakk JG, Gilja OH, et al. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. 2020;69(5):859–867.
- Holster S, Lindqvist CM, Repsilber D, et al. The Effect of allogenic versus autologous fecal microbiota transfer on symptoms, visceral perception and fecal and mucosal microbiota in irritable bowel syndrome: a randomized controlled study. Clin Transl Gastroenterol. 2019;10(4):e00034.
- Ianiro G, Eusebi LH, Black CJ, et al. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50(3):240–248.
- Manges AR, Steiner TS, Wright AJ. Fecal microbiota transplantation for the intestinal decolonization of extensively antimicrobial-resistant opportunistic pathogens: a review. Infect Dis. 2016;48(8):587–592.
- Stalenhoef JE, Terveer EM, Knetsch CW, et al. Fecal microbiota transfer for multidrug-resistant Gram-negatives: a clinical success combined with microbiological failure. Open Forum Infect Dis. 2017;4(2):ofx047.
- Singh R, de Groot PF, Geerlings SE, et al. Fecal microbiota transplantation against intestinal colonization by extended spectrum beta-lactamase producing Enterobacteriaceae: a proof of principle study. BMC Res Notes. 2018;11(1):190.
- Grosen AK, Povlsen JV, Lemming L, et al. Faecal microbiota transplantation eradicated extended-spectrum beta-lactamase-producing Klebsiella pneumoniae from a renal transplant recipient with recurrent urinary tract infections. Case Rep Nephrol Dial. 2019;9(2):102–107.
- Huttner BD, de Lastours V, Wassenberg M, et al. A five-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae: a randomized clinical trial. Clin Microbiol Infect. 2019;25(7):830–838.
- Bajaj JS, Kassam Z, Fagan A, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology. 2017;66(6):1727–1738.
- Landy J, Walker AW, Li JV, et al. Variable alterations of the microbiota, without metabolic or immunological change, following faecal microbiota transplantation in patients with chronic pouchitis. Sci Rep. 2015;5:12955.
- Fang S, Kraft CS, Dhere T, et al. Successful treatment of chronic Pouchitis utilizing fecal microbiota transplantation (FMT): a case report. Int J Colorectal Dis. 2016;31(5):1093–1094.
- Herfarth H, Barnes EL, Long MD, et al. Combined endoscopic and oral fecal microbiota transplantation in patients with antibiotic-dependent pouchitis: low clinical efficacy due to low donor microbial engraftment. Inflamm Intest Dis. 2019;4(1):1–6.
- Nishida A, Imaeda H, Inatomi O, et al. The efficacy of fecal microbiota transplantation for patients with chronic pouchitis: a case series. Clin Case Rep. 2019;7(4):782–788.
- Selvig D, Piceno Y, Terdiman J, et al. Fecal microbiota transplantation in pouchitis: clinical, endoscopic, histologic, and microbiota results from a pilot study. Dig Dis Sci. 2020;65(4):1099–1106.
- Kragsnaes MS, Kjeldsen J, Horn HC, et al. Efficacy and safety of faecal microbiota transplantation in patients with psoriatic arthritis: protocol for a 6-month, double-blind, randomised, placebo-controlled trial. BMJ Open. 2018;8(4):e019231.
- Arora V, Kachroo S, Ghantoji SS, et al. High Horn’s index score predicts poor outcomes in patients with Clostridium difficile infection. J Hosp Infect. 2011;79(1):23–26.
- Garey KW, Sethi S, Yadav Y, et al. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70(4):298–304.
- Kyne L, Warny M, Qamar A, et al. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251):189–193.
- Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–498.
- Peri R, Aguilar RC, Tuffers K, German Clinical Microbiome Study Group (GCMSG), et al. The impact of technical and clinical factors on fecal microbiota transfer outcomes for the treatment of recurrent Clostridioides difficile infections in Germany. United Eur Gastroenterol J. 2019;7(5):716–722.
- Ianiro G, Valerio L, Masucci L, et al. Predictors of failure after single faecal microbiota transplantation in patients with recurrent Clostridium difficile infection: results from a 3-year, single-centre cohort study. Clin Microbiol Infect. 2017;23(5):337.e1–e3.
- Ianiro G, Maida M, Burisch J, et al. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: a systematic review and meta-analysis. United Eur Gastroenterol J. 2018;6(8):1232–1244.
- van Beurden YH, de Groot PF, van Nood E, et al. Goorhuis A: complications, effectiveness, and long term follow-up of fecal microbiota transfer by nasoduodenal tube for treatment of recurrent Clostridium difficile infection. United European Gastroenterol J. 2017;5(6):868–879.
- Kao D, Roach B, Silva M, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2017;318(20):1985–1993.
- Hirsch BE, Saraiya N, Poeth K, et al. Effectiveness of fecal-derived microbiota transfer using orally administered capsules for recurrent Clostridium difficile infection. BMC Infect Dis. 2015;15:191.
- Youngster I, Mahabamunuge J, Systrom HK, et al. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016;14(1):134.
- Iqbal U, Anwar H, Karim MA. Safety and efficacy of encapsulated fecal microbiota transplantation for recurrent Clostridium difficile infection: a systematic review. Eur J Gastroenterol Hepatol. 2018;30(7):730–734.
- Ianiro G, Masucci L, Quaranta G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy plus vancomycin for the treatment of severe refractory Clostridium difficile infection-single versus multiple infusions. Aliment Pharmacol Ther. 2018;48(2):152–159.
- Pringle PL, Soto MT, Chung RT, et al. Patients with cirrhosis require more fecal microbiota capsules to cure refractory and recurrent Clostridium difficile infections. Clin Gastroenterol Hepatol. 2019;17(4):791–793.
- Staley C, Hamilton MJ, Vaughn BP, et al. Successful resolution of recurrent Clostridium difficile infection using freeze-dried, encapsulated fecal microbiota; pragmatic cohort study. Am J Gastroenterol. 2017;112(6):940–947.
- Satokari R, Mattila E, Kainulainen V, et al. Simple faecal preparation and efficacy of frozen inoculum in faecal microbiota transplantation for recurrent Clostridium difficile infection–an observational cohort study. Aliment Pharmacol Ther. 2015;41(1):46–53.
- Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53(10):994–1002.
- Costello SP, Tucker EC, La Brooy J, et al. Establishing a fecal microbiota transplant service for the treatment of Clostridium difficile infection. Clin Infect Dis. 2016;62(7):908–914.
- Allegretti JR, Fischer M, Sagi SV, et al. Fecal microbiota transplantation capsules with targeted colonic versus gastric delivery in recurrent Clostridium difficile infection: a comparative cohort analysis of high and lose dose. Dig Dis Sci. 2019;64(6):1672–1678.
- Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLOS One. 2016;11(8):e0161174.
- Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect. 2016;92(2):117–127.
- Kelly CR, Kunde SS, Khoruts A. Guidance on preparing an investigational new drug application for fecal microbiota transplantation studies. Clin Gastroenterol Hepatol. 2014;12(2):283–288.
- Cheng YW, Phelps E, Ganapini V, et al. Fecal microbiota transplantation for the treatment of recurrent and severe Clostridium difficile infection in solid organ transplant recipients: a multicenter experience. Am J Transplant. 2019;19(2):501–511.
- Qazi T, Amaratunga T, Barnes EL, et al. The risk of inflammatory bowel disease flares after fecal microbiota transplantation: systematic review and meta-analysis. Gut Microbes. 2017;8(6):574–588.
- Tabbaa OM, Aboelsoud MM, Mattar MC. Long-term safety and efficacy of fecal microbiota transplantation in the treatment of Clostridium difficile infection in patients with and without inflammatory bowel disease: a tertiary care center’s experience. Gastroenterol Res. 2018;11(6):397–403.
- Hirten RP, Grinspan A, Fu SC, et al. Microbial engraftment and efficacy of fecal microbiota transplant for Clostridium difficile in patients with and without inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(6):969–979.
- Meighani A, Hart BR, Bourgi K, et al. Outcomes of fecal microbiota transplantation for Clostridium difficile infection in patients with inflammatory bowel disease. Dig Dis Sci. 2017;62(10):2870–2875.
- Chen T, Zhou Q, Zhang D, et al. Effect of Faecal microbiota transplantation fortreatment of Clostridium difficile infection in patients with inflammatory bowel disease: a systematic review and meta-analysis of cohort studies. J Crohns Colitis. 2018;12(6):710–717.
- Allegretti JR, Kelly CR, Grinspan A, et al. Outcomes of fecal microbiota transplantation in patients with inflammatory bowel diseases and recurrent Clostridioides difficile infection. Gastroenterology. 2020;159(5):1982–1984.
- You JHS, Jiang X, Lee WH, et al. Cost-effectiveness analysis of fecal microbiota transplantation for recurrent Clostridium difficile infection in patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2020;35(9):1515–1523.
- Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–1071.
- Shogbesan O, Poudel DR, Victor S, et al. A systematic review of the efficacy and safety of fecal microbiota transplant for Clostridium difficile infection in immunocompromised patients. Can J Gastroenterol Hepatol. 2018; 2018:1–10.
- Abu-Sbeih H, Ali FS, Wang Y. Clinical review on the utility of fecal microbiota transplantation in immunocompromised patients. Curr Gastroenterol Rep. 2019;21(4):8.
- DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. colibacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043–2050.
- Oppfeldt AM, Dahlerup JF, Christensen LA, et al. Faecal microbiota transplantation for recurring Clostridium difficile infection in a patient with Crohn’s disease and ileorectal anastomosis. BMJ Case Rep. 2016;217209.
- Jørgensen SMD, Hansen MM, Erikstrup C, et al. Faecal microbiota transplantation: establishment of a clinical application framework. Eur J Gastroenterol Hepatol. 2017;29:36–45.
- Cammarota G, Ianiro G, Tilg H, European FMT Working Group, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66(4):569–580.
- Cammarota G, Ianiro G, Kelly CR, et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019;68(12):2111–2121.
- Rode AA, Bytzer P, Pedersen OB, et al. Establishing a donor stool bank for faecal microbiota transplantation: methods and feasibility. Eur J Clin Microbiol Infect Dis. 2019;38(10):1837–1847.
- Guide to the quality and safety of tissues and cells for human application. 4th ed. Strasbourg: European Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM); 2019.
- Mullish BH, Quraishi MN, Segal JP, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut. 2018;67(11):1920–1941.
- Competent Authorities on Substances of Human Origin Expert Group (CASoHO E01718). Meeting of the competent authorities for tissues and cells, 3–4 December 2014. Summary Report https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/ev_20141203_sr_en.pdf.
- Directive 2004/23/ec of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, preparation, preservation, storage and distribution of human tissues and cells. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:102:0048:0058:en:PDF.
- EU. EU directive relating to medicinal products for human use (2001/83/EC): European Union; 2001. https://ec.europa.eu/health/sites/default/files/files/eudralex/vol-1/dir_2001_83_consol_2012/dir_2001_83_cons_2012_en.pdf
- Walsh JH, Purcell RH, Morrow AG, et al. Post transfusion hepatitis after open-heart operations. Incidence after the administration of blood from commercial and volunteer donor populations. JAMA. 1970;211(2):261–265.
- Paramsothy S, Borody TJ, Lin E, et al. Donor recruitment for fecal microbiota transplantation. Inflamm Bowel Dis. 2015;21(7):1600–1606.
- Terveer EM, van Beurden YH, Goorhuis A, et al. How to: establish and run a stool bank. Clin Microbiol Infect. 2017;23(12):924–930.
- Kassam Z, Dubois N, Ramakrishna B, et al. Donor screening for fecal microbiota transplantation. N Engl J Med. 2019;381(21):2070–2072.
- Kazerouni A, Burgess J, Burns LJ, et al. Optimal screening and donor management in a public stool bank. Microbiome. 2015;3(1):75.
- Woodworth MH, Carpentieri C, Sitchenko KL, Kraft CS. Challenges in fecal donor selection and screening for fecal microbiota transplantation: a review. Gut Microbes. 2017;8(3):225–237.
- Jørgensen SMD, Erikstrup C, Dinh KM, et al. Recruitment of feces donors among blood donors: Results from an observational cohort study. Gut Microbes. 2018;9(6):540–550.
- Tariq R, Weatherly R, Kammer P, et al. Donor screening experience for fecal microbiota transplantation in patients with recurrent C. difficile infection. J Clin Gastroenterol. 2018;52(2):146–150.
- Lai CY, Sung J, Cheng F, et al. Systematic review with meta-analysis: review of donor features, procedures and outcomes in 168 clinical studies of faecal microbiota transplantation. Aliment Pharmacol Ther. 2019;49(4):354–363.
- Guo Y, Li SH, Kuang YS, He JR, et al. Effect of short-term room temperature storage on the microbial community in infant fecal samples. Sci Rep. 2016;6:26648.
- Kragsnaes MS, Nilsson AC, Kjeldsen J, et al. How do I establish a stool bank for fecal microbiota transplantation within the blood- and tissue transplant service? Transfusion. 2020;60(6):1135–1141.
- Ott SJ, Musfeldt M, Timmis KN, et al. In vitro alterations of intestinal bacterial microbiota in fecal samples during storage. Diagn Microbiol Infect Dis. 2004;50(4):237–245.
- Hamilton MJ, Weingarden AR, Sadowsky MJ, et al. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(5):761–767.
- Papanicolas LE, Choo JM, Wang Y, et al. Bacterial viability in faecal transplants: Which bacteria survive? EBioMedicine. 2019;41:509–516.
- Browne HP, Forster SC, Anonye BO, et al. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature. 2016;533(7604):543–546.
- McCune VL, Quraishi MN, Manzoor S, et al. Results from the first English stool bank using faecal microbiota transplant as a medicinal product for the treatment of Clostridioides difficile infection. EClinical Medicine. 2020;20:100301.
- Thornit DN, Sander B, la Cour M, et al. The effects of peroral glycerol on plasma osmolarity in diabetic patients and healthy individuals. Basic Clin Pharmacol Toxicol. 2009;105(5):289–293.
- Liao CH, Shollenberger LM. Survivability and long-term preservation of bacteria in water and in phosphate-buffered saline. Lett Appl Microbiol. 2003;37(1):45–50.
- Terveer EM, Vendrik KE, Ooijevaar RE, et al. Faecal microbiota transplantation for Clostridioides difficile infection: four years’ experience of the Netherlands Donor Feces Bank. United European Gastroenterol J. 2020;8(10):1236–1247.
- Takahashi M, Ishikawa D, Sasaki T, et al. Faecal freezing preservation period influences colonization ability for faecal microbiota transplantation. J Appl Microbiol. 2019;126(3):973–984.
- Detailed guidance on the collection, verification and presentation of adverse event/reaction reports arising from clinical trials on medicinal products for human use (‘CT-3’). https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2011:172:0001:0013:EN:PDF
- Sleight SC, Wigginton NS, Lenski RE. Increased susceptibility to repeated freeze-thaw cycles in Escherichia coli following long-term evolution in a benign environment. BMC Evol Biol. 2006;6(1):104.
- Ianiro G, Mullish BH, Kelly CR, et al. Reorganisation of faecal microbiota transplant services during the covid-19 pandemic. Gut. 2020;69(9):1555–1563.